Abstract
Sexual intercourse is the major means of HIV transmission, yet the impact of semen on HIV infection of CD4+ T cells remains unclear. To resolve this conundrum, we measured CD4+ target cell infection with X4 tropic HIV IIIB and HC4 and R5 tropic HIV BaL and SF162 after incubation with centrifuged seminal plasma (SP) from HIV-negative donors and assessed the impact of SP on critical determinants of target cell susceptibility to HIV infection. We found that SP potently protects CD4+ T cells from infection with X4 and R5 tropic HIV in a dose- and time-dependent manner. SP caused a diminution in CD4+ T cell surface expression of the HIVR CD4 and enhanced surface expression of the HIV coreceptor CCR5. Consequently, SP protected CD4+ T cells from infection with R5 tropic HIV less potently than it protected CD4+ T cells from infection with X4 tropic HIV. SP also reduced CD4+ T cell activation and proliferation, and the magnitude of SP-mediated suppression of target cell CD4 expression, activation, and proliferation correlated closely with the magnitude of the protection of CD4+ T cells from infection with HIV. Taken together, these data show that semen protects CD4+ T cells from HIV infection by restricting critical determinants of CD4+ target cell susceptibility to HIV infection. Further, semen contributes to the selective transmission of R5 tropic HIV to CD4+ target cells.
Two million people are infected with HIV every year, most through sexual intercourse (1, 2). Yet, the development of strategies to prevent the sexual transmission of HIV infection has been hampered by an incomplete understanding of the earliest events in HIV transmission. One major unresolved question is how semen influences the mucosal transmission of HIV infection. Because semen contains infectious HIV at all stages of HIV infection, including during fully suppressive antiretroviral therapy (3–7), it is imperative to clarify the impact of semen on target cell susceptibility to HIV infection.
HIV infects CD4+ T cells, dendritic cells, and macrophages at mucosal surfaces (8–12). The impact of semen on HIV infection of these target cells, however, remains controversial. Mucin-6 in semen reduces target cell infection with HIV via abrogation of DC-SIGN–mediated transfer of HIV from dendritic cells to CD4+ T cells (13, 14), and semenogelin-1 in semen also mediates target cell protection from HIV infection (15). By contrast, semen-derived enhancer of viral infection (SEVI) facilitates HIV infection of CD4+ T cells and other target cell types (16), and heparan sulfate on spermatozoa captures and transmits HIV to dendritic cells, macrophages, and CD4 T cells (17). It is therefore unclear whether semen chiefly exacerbates or ameliorates HIV infection of CD4+ T cells.
In this study, we characterize the impact of semen on CD4+ T cell infection with HIV, as well as CD4+ T cell expression of critical markers of susceptibility to HIV infection. Identifying the mechanisms through which semen modulates HIV infection of target cells has the potential to lead to the development of novel means of preventing the sexual transmission of HIV infection.
Materials and Methods
Semen collection and processing
We collected semen from 20 HIV-negative donors who gave consent in a research protocol approved by the Dartmouth Medical School Committee for the Protection of Human Subjects. All semen donors were asymptomatic for sexually transmitted infections, and no lubricants were used during semen sample collection. Each semen sample had total white cell count below the standard World Health Organization threshold of 1 million cells/ml (18), and Chlamydia trachomatis and Neisseria gonorrhoeae gene amplification tests were negative for all samples (Aptima Combo 2 assay; Gen-Probe, San Diego, CA). Samples were mixed 1:3 with PBS (Mediatech, Manassas, VA), centrifuged at 544 × g for 10 min at room temperature using a Sorvall Legend RT+ centrifuge (Thermo Scientific, Braunschweig, Germany), and seminal plasma (SP) harvested from the supernatant for use fresh or after storage at −°C. SP samples were used separately in each experiment; the number and concentration of donor SP samples used for each experiment is indicated in the text and the figure legends of this article.
HIV infection of CD4+ T cells
We isolated PBMCs by Ficoll centrifugation of whole blood from consenting HIV-negative donors different from SP donors followed by culture in RPMI supplemented with 10% FCS (Mediatech) and penicillin, streptomycin, and amphotericin B (Mediatech). After activation with PHA (Sigma-Aldrich, St. Louis, MO) and human recombinant IL-2, we infected PBMCs for 3 d with X4 tropic HIV (IIIB or HC4) or R5 tropic HIV (BaL or SF162) at a multiplicity of infection (MOI) of 0.1 with or without SP. HIV MOI was determined using the 50% infectivity end-point method of Reed and Muench (19) after quantifying HIV using HIV-1 p24 ELISA (PerkinElmer, Waltham, MA). IL-2 and HIV strains were provided courtesy of the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (Germantown, MD). We measured the percentage of CD4+ T cells expressing intracellular HIV p24 (KC57; Beckman Coulter, Brea, CA) using multi-parameter flow cytometry (FACS Canto; Becton Dickinson, Sparks, MD).
HIV infection of TZM-bl cells
Transformation zone metaplasia cells expressing an HIV Tat-driven luciferase reporter gene [TZM-bl (20)] were used as a second HIV target cell model (obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH, from Dr. John C. Kappes, Dr. Xiaoyun Wu, and Tranzyme, Durham, NC). TZM-bl cells were cultured in DMEM/F-12 (Mediatech) supplemented with 10% FBS, penicillin, streptomycin, and amphotericin B (Mediatech) and then infected with HIV IIIB or HIV BaL with or without SP at the indicated concentrations for 24 h before the assessment of infection via β-Glo–induced luminescence using the manufacturer's protocol (Promega, Madison, WI). In time-course experiments, TZM-bl cells were infected with HIV BaL for 24 h with or without coincubation with 0.5% SP for 2, 4, and 24 h before assessment of infection via β-Glo–induced luminescence.
Cell-free versus cell-associated infection
PBMCs were cultured in RPMI with 10% FBS, penicillin, streptomycin, and amphotericin B in the presence of PHA and IL-2, then infected with HIV IIIB or HIV BaL for 3 d to generate a pool of HIV-infected PBMCs. We cocultured 100,000 of these HIV-infected PBMCs, or the supernatant from an identical number of simultaneously HIV-infected and identical number of PBMCs from the same donor, with 25,000 TZM-bl cells on a 96-well tissue culture plate for 24 h with or without SP before measuring TZM-bl infection. Negative controls included the same number of TZM-bl cells cultured with an identical number of uninfected PBMCs from the same donor or their supernatant.
Assessment of CD4+ T cell CD4, CXCR4, CCR5 expression, activation, and apoptosis
PBMCs were cultured using RPMI with 10% FBS, penicillin, streptomycin, and amphotericin B with or without SP for 1, 2, 4, 8, 24, or 72 h and then stained with monoclonal Abs against CD3, CD4, CXCR4, and CCR5 (BioLegend, San Diego, CA), the activation marker CD38, and the apoptosis marker intracellular activated caspase-3 (both from BD Biosciences, San Jose, CA). In separate experiments comparing the impact of SP on surface versus intracellular expression of CCR5, cells were split after incubation with medium or SP and then either surface stained for CCR5 or fixed and permeabilized using the Fix & Perm cell permeabilization reagents (Invitrogen, Carlsbad, CA), followed by intracellular staining with the same mAb against CCR5 (BioLegend, San Diego, CA). Data was acquired using multiparameter flow cytometry.
Assessment of T cell CD4 expression by confocal microscopy
CD4+ T cells were isolated from whole blood using the RosetteSep human CD4+ T cell enrichment mixture (StemCell Technologies, Vancouver, Canada), cultured in RPMI with 10% FBS, penicillin, streptomycin, and amphotericin B (Mediatech), and incubated for 24 h with or without 0.5% SP. Cells were stained with anti-human CD4 Ab or its isotype control (BioLegend), fixed, and mounted onto a chamber slide (Nunc-Thermo Fisher Scientific, Rochester, NY), and expression of the HIVR CD4 was assessed using a laser scanning confocal microscope (LSM510 Meta; Carl Zeiss, Thornwood, NY).
Assessment of T cell CD4, CXCR4, and CCR5 expression by RT-PCR
Freshly purified CD4+ T cells were cultured in RPMI with 10% FBS, penicillin, streptomycin, and amphotericin B (Mediatech) alone or with 0.5% SP for 24 h, after which total RNA was isolated using the QIAsh-redder and RNeasy Mini Kit (Qiagen, Valencia, CA). Reverse transcription was accomplished using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), and real-time PCR was done using the MicroAmp Optical 96-well reaction plates, CD4, CXCR4, and CCR5 gene primers, SYBR Green PCR master mix, and the 7000 Real-time PCR system (Applied Bio-systems, Foster City, CA). CD71 primers (Applied Biosystems) were used as an endogenous mRNA expression control. Fold change was calculated via the 2−ΔΔCT method (21) using the difference in CD4, CXCR4, and CCR5 mRNA threshold cycle (Ct) values between the SP-treated and media controls after normalization of CD4, CXCR4, and CCR5 mRNA expression using CD71 mRNA levels.
Assessment of TZM-bl cell viability
SP and HIV IIIB or BaL were added onto TZM-bl cell monolayer in a 96-well plate, the mixture was incubated for 24 h, and TZM-bl cell viability was assessed using the CellTiter 96 AQueous Assay as per the manufacturer's protocol (Promega).
Assessment of CD4+ T cell proliferation
In separate experiments, PBMCs were stained for 7 min with CFSE (Invitrogen) before dye inactivation with human Ab serum (Mediatech), after which PBMCs were treated with PHA and IL-2, incubated for 3 d with 0.5% SP or medium, and the percentage of CD4+ T cells proliferating was assessed via the decrement in CFSE staining by multiparameter flow cytometry.
Effect of SP on culture media pH
We measured the pH of RPMI plus 10% FBS containing SP or an equivalent volume of medium at room temperature.
Measurement of cytokine levels in SP
We measured the titers of 27 proinflammatory and anti-inflammatory cytokines in SP samples from the same 20 subjects in whom the impact of SP on CD4+ target cell infection was assessed using multiplex cytokine bead technology per the manufacturer's protocol (Luminex; Bio-Rad, Hercules, CA). Briefly, after 30 min of incubation with a cytokine standard, sample, spikes, or blank, multiplex beads were washed and stained with a mixture of biotinylated Abs for 30 min and then washed and stained with streptavidin-PE for 10 min before resuspension and measurement of fluorescence intensity using the Bio-Plex array reader (BioRad). Data validity was verified using calibration curves from recombinant cytokine standards as well as high and low spikes from the supernatant of stimulated human dendritic cells, with standards and spikes measured in triplicate, samples measured once, and blank values subtracted from all readings. Cytokine assays were carried out by the Immune Monitoring Laboratory.
Statistical analyses
We used Mann-Whitney U tests for univariate comparisons and Spearman correlation coefficients for correlations between the magnitude of T cell expression of receptors, coreceptors, activation markers, and the magnitude of CD4+ T cell proliferation as well as SP cytokine levels. The percentage TZM-bl HIV infection, CD4, CXCR4, and CCR5 expression was derived via the ratio of the individual readouts over the average readout of the non-SP positive control conditions. Percentage reduction in T cell CD4 mean fluorescence intensity (MFI), CD38 MFI, proliferation, and HIV infection attributable to SP were derived via the ratio of the average readout of the non-SP positive control conditions minus the individual readouts in the SP conditions over the average readout of the non-SP positive control conditions. Similarly, we derived the percentage reduction in TZM-bl cell infection attributable to SP via the ratio of TZM-bl luminescence in the HIV condition minus TZM-bl luminescence in the SP plus HIV condition over TZM-bl luminescence in the HIV condition. Two-sided p values <0.05 were considered statistically significant. Data from flow cytometry-based experiments were analyzed with FlowJo 8.8.6 (Tree Star, Ashland, OR), and we conducted statistical analyses on Prism 4 (GraphPad Software, La Jolla, CA). All experiments were repeated at least three times, with the exception of the multiplex cytokine assay, which we replicated once.
Results
SP reduces HIV infection of CD4+ target cells
We used two standard experimental models to characterize the impact of SP on CD4+ target cell susceptibility to HIV infection: PHA-stimulated PBMCs and TZM-bl cells. With both models, we found that SP potently protects CD4+ target cells from infection with X4 tropic HIV IIIB and HC4 and R5 tropic HIV BaL and SF162 (Fig. 1).
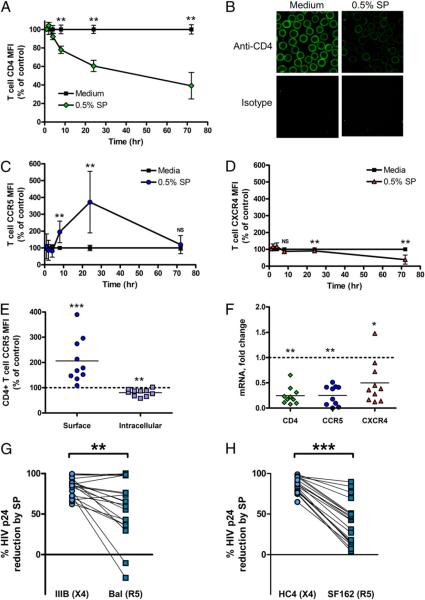
FIGURE 1.
SP protects CD4+ target cells from infection by R5 tropic and X4 tropic HIV. CD4+ T cells incubated with SP were protected from infection by X4 tropic HIV IIIB (A, C, G) and HC4 (E, n = 20) and R5 tropic HIV BaL (B, D, H) and SF162 (F, n = 20) at an MOI of 0.1 as assessed by intracellular HIV p24 staining. We observed a dose-response relationship, with incubation with increasing concentrations of SP associated with increasing protection of CD4+ TZM-bl cells from infection with HIV IIIB and HIV BaL (G, H, n = 20). **p < 0.01; ***p < 0.001. RLU, relative light units.
SP reduces CD4+ T cell expression of the HIVR CD4
We examined the impact of SP on T cell expression of the HIVR CD4. In a time-dependent fashion, incubation with SP reduced CD4+ T cell surface expression of the HIVR CD4 (Fig. 2A). We observed a similar reduction in CD4+ T cell expression of CD4 by confocal microscopy (Fig. 2B). Notably, CD4+ T cell expression of the HIVR CD4 was not significantly affected by 1-h incubation with SP, suggesting a time-dependent process that did not involve nonspecific interference with CD4 staining. Furthermore, we found that CD71-normalized CD4 mRNA expression in bead-purified CD4+ T cells incubated for 24 h with 0.5% by volume SP was reduced 0.24-fold in comparison with that of medium-treated CD4+ T cells (p = 0.0070; Fig. 2F).
FIGURE 2.
SP modulates the expression of the HIVR CD4 and the HIV coreceptors CCR5 and CXCR4. Incubation with SP reduced CD4+ T cell expression of the HIVR CD4 in a time-dependent fashion compared with medium controls (A, n = 6). CD4+ T cell expression of the HIVR CD4 by confocal microscopy was reduced after 24-h incubation with SP compared with medium control (B). There was a rapid and robust increase in surface expression of CCR5 in CD4+ T cells incubated with 0.5% SP (C, n = 6), along with a late and limited repression of surface CXCR4 expression (D, n = 6). We found that simultaneous with the increase in surface expression of CCR5, SP reduces intracellular CCR5 staining (E, n = 10). By real-time PCR, there was a clear SP-mediated reduction in CD4+ T cell transcription of CD4, CXCR4, and CCR5 (F, n = 10). Fold change was calculated via the 2−ΔΔCT method using the difference in CD4, CXCR4, and CCR5 mRNA Ct values between the SP-treated and media controls after normalization of CD4, CXCR4, and CCR5 mRNA expression using CD71 housekeeping gene mRNA levels. SP protects CD4+ T cells from X4 tropic HIV IIIB infection more potently than it protects CD4+ T cells from R5 tropic HIV BaL (G, n = 20) and X4 tropic HIV HC4 more potently than R5 tropic HIV SF162 (H, n = 20). Error bars depict SD. *p < 0.05; **p < 0.01; ***p < 0.001.
SP modulates CD4+ T cell expression of the HIV coreceptors CCR5 and CXCR4
SP triggered a rapid and profound increase in surface expression on CD4+ T cells of the HIV coreceptor CCR5 (Fig. 2C), as well as a late and modest fall in surface expression of the HIV coreceptor CXCR4 (Fig. 2D). Comparing surface expression of CCR5 with the intracellular expression of CCR5 in separate permeabilized CD4+ T cells from identical donors, we found that SP significantly but subtly reduced intracellular expression of CCR5 at the same time it enhanced surface expression of CCR5 (Fig. 2E). Concordant with this suggestion that SP promotes trafficking of intracellular CCR5 to the cell surface while inhibiting CCR5 transcription, we found that CD71-normalized expression of mRNA for the HIV coreceptors CCR5 and CXCR4 was cut by incubation with SP (Fig. 2F).
SP protection of CD4+ T cells from HIV infection is more potent against X4 tropic HIV IIIB and HC4
Because SP enhanced CD4+ target cell surface expression of the HIV coreceptor CCR5 but not CXCR4, we examined whether SP preferentially inhibits the transmission of X4 tropic HIV by challenging identical PBMCs with MOI of 0.1 of either X4 or R5 tropic HIV and then compared the relative percentage reduction in target cell infection. SP protects CD4+ target cells from X4 tropic HIV IIIB and HC4 infection more potently than it protects CD4+ target cells from R5 tropic HIV BaL and SF162 infection (Fig. 2G,2H).
SP reduces CD4+ T cell activation and proliferation
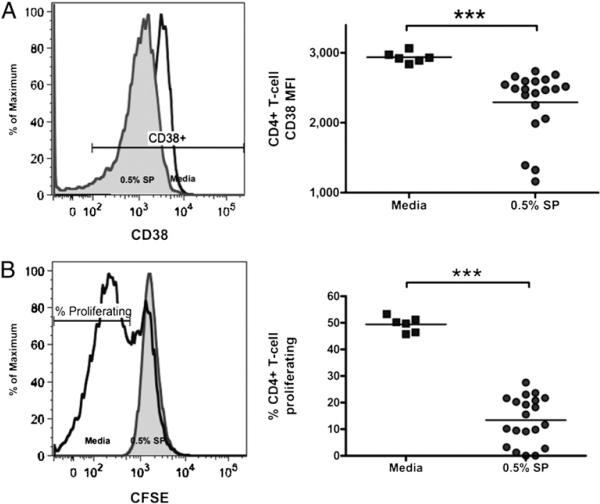
Activation and proliferation influence target cell susceptibility to HIV infection (22–24) and are potentially affected by immunomodulatory factors in semen (25–27). We found that SP significantly reduced CD4+ T cell expression of the activation marker CD38 after 24-h incubation (Fig. 3A), an effect that was maintained after 72 h (CD38 MFI 1914 versus 700; p = 0.0003, n = 20). Incubation with SP also diminished PHA-stimulated CD4+ T cell proliferation at 72 h (Fig. 3B).
FIGURE 3.
SP reduces T cell activation and proliferation. CD4+ T cell incubation with SP reduced CD4+ T cell expression of the activation marker CD38 (A, n = 20) and PHA- and IL-2–stimulated CD4+ T cell proliferation as measured by CFSE dye dilution (B, n = 20). ***p < 0.001.
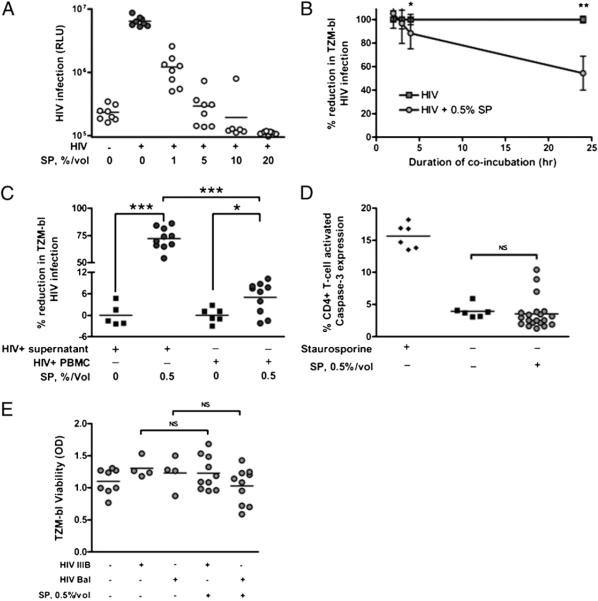
The magnitude of protection of CD4+ target cells from HIV infection is influenced by SP concentration, coincubation time, and cell contact but not target cell apoptosis or pH effects
We observed increasing inhibition of CD4+ target cell infection with HIV with increasing concentrations of SP (Fig. 4A). Similarly, the magnitude of SP-mediated reduction in CD4+ target cell infection with HIV was higher with incubation times greater than 2 h (Fig. 4B). SP inhibited cell-free and cell-associated HIV infection of CD4+ target cells, although the magnitude of protection was greater for cell-free infection (Fig. 4C). Because high concentrations of SP cause immune cell apoptosis (28), we assessed the impact of SP on CD4+ target cell viability using two standard assays. By intracellular caspase-3 staining, there was no alteration in CD4+ T cell viability after 24 h (Fig. 4D). Caspase-3 expression was not significantly enhanced after 72 h of incubation with 0.5% SP (2.20 versus 3.66%; p = 0.1519, n = 20). Further, when we confined our analyses of CD4+ T cell infection with HIV to cells not expressing activated caspase-3, incubation with SP still reduced HIV infection of CD4+ T cells (27.03 versus 22.15% for HIV IIIB, p = 0.0310; 11.93 versus 3.79% for HIV BaL, p = 0.0005; n = 20). Similarly, 24-h incubation with 0.5% SP had no effect on TZM-bl cell viability using MTT staining (Fig. 4E). At SP concentrations above 1%, we observed induction in target cell apoptosis by both assays (data not shown). Importantly, the pH of 0.5% SP conditions was not different from medium control (8.047 versus 8.051; p = 0.6923; n = 10), thus excluding the possibility that SP at this concentration produced the observed effects via pH alterations (29, 30).
FIGURE 4.
Magnitude of protection of CD4+ target cells from HIV infection by SP is influenced by SP concentration, incubation duration, and cell contact, but not by target cell apoptosis. Incubation with higher concentrations of SP conferred greater CD4+ TZM-bl cell protection from HIV IIIB infection at an MOI of 0.2 (A, n = 8). Incubation with SP for greater than 2 h was associated with greater protection of CD4+ TZM-bl cells from infection with HIV BaL (B, n = 8). SP inhibits both cell-associated and cell-free HIV IIIB infection of TZM-bl cells, but the magnitude of SP inhibition of cell-associated HIV infection of TZM-bl cells was smaller than the magnitude of SP inhibition of cell-free HIV infection of TZM-bl cells (C, n = 10). Incubation with 0.5% SP for 24 h did not induce CD4+ T cell apoptosis as measured by intracellular expression of activated caspase-3 (D, n = 20) or by MTT viability assay in TZM-bl cells (E, n = 10). Staurosporine 0.25 μM was used as a positive control for intracellular activated caspase-3 expression. Error bars depict SD. *p < 0.05; **p < 0.01; ***p < 0.001. RLU, relative light units.
Magnitude of SP-mediated reductions in CD4 expression, activation, and proliferation correlate with the reduction in T cell HIV infection
We correlated reductions in markers of target cell susceptibility to HIV infection with the magnitude of SP-mediated reductions in CD4+ T cell HIV infection. The reduction in CD4+ T cell infection with both X4 tropic HIV IIIB and R5 tropic HIV BaL correlated closely and significantly with the reduction in T cell CD4 MFI, the reduction in T cell expression of activation marker CD38, and the reduction in T cell proliferation (Fig. 5A–F).
FIGURE 5.
SP-mediated protection from HIV infection correlates closely with SP-mediated reductions in CD4+ T cell expression of CD4 and CD38 as well as CD4+ T cell proliferation. The magnitude of the reduction in CD4+ T cell infection by HIV IIIB correlated positively with the magnitude of the SP-mediated reduction in CD4+ T cell MFI of the HIVR CD4 (A), the MFI of the activation marker CD38 (B), and with the reduction in CD4+ T cell proliferation (C) by CFSE staining. Likewise, the magnitude of the reduction in CD4+ T cell infection by HIV BaL correlated positively with the magnitude of the SP-mediated reduction in CD4+ T cell CD4 MFI (D), CD4+ T cell CD38 MFI (E), and CD4+ T cell proliferation (F). SP contains multiple cytokines and chemokines (G). n = 20 for A–G.
No correlation between cytokine and chemokine concentrations in SP with SP-mediated protection of CD4+ target cells from HIV infection
Multiple cytokines and chemokines are enriched in human SP (Fig. 5G), but cytokine and chemokine concentrations in SP did not correlate with the magnitude of SP-mediated modulation of CD4+ T cell infection or with CD4+ T cell expression of markers of susceptibility to HIV infection (data not shown).
Discussion
Human semen protects CD4+ target cells from infection by X4 and R5 tropic HIV. This protective effect was intimately associated with semen-mediated reduction of CD4+ T cell expression of major determinants of target cell susceptibility to HIV infection, including the expression of the HIVR CD4, HIV coreceptor CXCR4, as well as CD4+ T cell activation and proliferation. Conversely, semen induced CD4+ T cell expression of the HIV coreceptor CCR5 and promoted preferential protection of CD4+ target cells from R5 tropic HIV compared with X4 tropic HIV. These data suggest that semen contributes to both the relative inefficiency of sexual transmission of HIV (2) and to the preferential transmission of R5 tropic HIV at mucosal surfaces (31–34).
CD4 is the chief HIVR on target cells (35, 36). CD4 blockade protects T cells from HIV infection (37), and CD4 downregulation is known to occur in vivo as a host (38) or pathogen-mediated process (39). African green monkeys have ~40% less CD4 T cell expression compared with that of rhesus macaques, and this is thought to contribute to protection against SIV-mediated T cell depletion and disease progression in these hosts (38). We have shown that 24-h incubation with SP reduces surface expression of the HIVR CD4 on target CD4+ T cells by more than 39% and that this effect correlated closely with the magnitude of SP-mediated reduction in CD4+ T cell infection by HIV IIIB and BaL.
R5 tropic HIV predominates during early HIV infection, whereas X4 tropic HIV typically emerges late in the course of HIV disease (31–34). The reason for this selectivity has been elusive, although explanatory hypotheses include selective trapping and transcytosis of R5 viruses, selective infection of mucosal dendritic cells, macrophages, and CD4+ T cells by R5 viruses, and more effective replication of R5 viruses in regional lymph nodes relative to X4 tropic viruses (40). Our data suggest that whereas semen protects CD4+ T cells from HIV infection, the few CD4+ target cells still susceptible to infection by HIV have preferentially augmented vulnerability to infection by R5 virus. Given that sexual transmission of HIV likely stems from the establishment of infection by one or at most a few HIV virions (41), we hypothesize that semen-mediated quadrupling of CD4+ target cell expression of CCR5 expression likely exerts a profound influence on the tropism of the transmitted/founder virus.
HIV preferentially infects activated and proliferating target cells (22, 23, 42), and activated CD4+ T cells are thought to be critical early targets of HIV infection at mucosal surfaces especially during coincident sexually transmitted infection (8, 10, 43). We have shown that SP also reduces CD4+ T cell expression of the activation marker CD38 as well as CD4+ T cell proliferation. The magnitude of these effects correlated strongly with the magnitude of SP-mediated reduction in CD4+ T cell infection by HIV, consistent with our hypothesis that the anti-inflammatory impact of SP inhibits HIV transmission by reducing target cell availability at mucosal surfaces (24), perhaps via the same anti-inflammatory mediators in SP that promote immune tolerance in the female reproductive tract (25–27). Of the 27 cytokines and chemokines measured in SP samples, none showed consistent correlation with the observed SP-mediated reduction in CD4+ T cell infection with HIV. We hypothesize that either the specific soluble factor(s) in SP responsible for the observed protection of CD4+ target cells from HIV infection were not captured by the multiplex assay or that we measured the effector cytokine but that single cytokine/chemokine concentrations did not correlate with protection because the prevention of target cell infection occurs over a threshold level for each cytokine/chemokine or, alternatively, because more than one cytokine and/or chemokine act in combination to produce the observed effects.
Mucin-6 in SP reduces HIV attachment to dendritic cells and subsequent transfer to CD4+ target cells (13, 14). The current study, however, is to our knowledge the first to demonstrate that SP directly protects CD4+ T cells from HIV infection. This is important because HIV infection of CD4+ T cells clearly occurs without such cellular intermediaries (8–11, 44). Although the relative contribution of cell-free versus cell-associated HIV transmission at mucosal surfaces remains unresolved (4, 10, 44, 45), cell-associated transmission of HIV is well documented (44–49), and our data suggest that this mode of HIV transmission may be inhibited less by SP.
In direct contrast with our results and those of other authors (13–15), SEVI, a highly purified component of agitated SP, has been shown to enhance HIV infection of multiple target cell types including CD4+ T cells (16, 50). The likely source for the divergence of results is that the complicated immunomodulatory content of semen both inhibits and facilitates HIV infection of target cells, and different experimental approaches likely accentuate these opposing effects. For instance, all experiments in our studies were done using unfractionated SP, whereas most of the SEVI findings detailed the influence on target cell infection of a highly purified semen fraction. In addition, our SP samples were not agitated prior to coincubation with target cells. Most importantly, in contrast with the 2-h coincubation times used in the SEVI papers, we used longer coincubation times consistent with the forensic observation that semen is typically detectable in the vagina days after deposition (51). Our time-course experiments suggest that the shorter coincubation times described by Münch et al. (16, 50) would hide the seminal protection of CD4+ T cells. Reconciling these results, we believe the data showing that SEVI enhances HIV infection of target cells is convincing, but our data and data from multiple other studies (13–15) suggest that the chief impact of unfractionated SP incubated with target cells during most physiological time frames is protection of CD4+ T cells from HIV infection.
At higher SP concentrations, we observed both greater CD4+ target cell protection and increasing CD4+ T cell apoptosis (28). However, the induction of CD4+ target cell apoptosis is not the only contributor to SP-mediated protection of CD4+ target cells from HIV infection. We found powerful SP-mediated protection of CD4+ T cells from HIV infection at concentrations that did not elicit target cell apoptosis and when proapoptotic cells were excluded from our analyses. Importantly, the use of low SP concentrations likely mimics the low physiological concentrations of semen seen in the female reproductive tract, as semen persists for days after vaginal deposition (51) and is thus likely susceptible to local diffusion and dilution by genital secretions (52).
We acknowledge important limitations of this work. First, we used SP from HIV-negative donors, thereby allowing assessment of the impact of SP on CD4+ target cell infection without the confounding presence of donor HIV (3, 4) or HIV-specific Abs (53). It is possible, however, that HIV infection reduces the capacity of SP to protect CD4+ T cells from HIV infection. We also showed that the observed protection of target cells occurs independent of pH, thereby excluding that SP impact on pH is responsible for the observed effects. However, the buffered medium used to predilute semen samples could have excluded a pH-dependent effect of semen that is independent of our observations. Next, although we excluded samples with active signs of genital tract inflammation using standard World Health Organization semen leukocyte thresholds and testing for gonorrhea and Chlamydia, it is conceivable that a small minority of subjects in this or any semen collection protocol had undetected genital tract inflammation at the time of sample donation. Whereas such genital inflammation conceivably could contribute to the anti-HIV or other properties of semen in that small minority of samples, the consistent effects observed in our studies suggest the anti-HIV activity and impact on markers of susceptibility to HIV infection is attributable not to rare undetected genital tract inflammation but to an intrinsic property of semen. Further, the standard ex vivo models of CD4+ target cell HIV infection used in this study cannot capture the potential contributions to mucosal HIV transmission of mucus and epithelial barriers, intricate cell-to-cell interactions, other innate factors, and the complex extracellular microenvironment (11, 54, 55). Nonetheless, the available evidence suggests that these models capture a physiological interaction of semen with CD4+ T cells at mucosal sites. First, ex vivo tissue explant studies showed that HIV reaches the rich population of subepithelial CD4+ T cells independent of epithelial cell interactions with HIV and in the presence of an intact epithelial cell lining (9, 56, 57). It seems plausible that soluble factors in semen can reach CD4+ target cells in a similar way. That soluble factors in semen likely impact CD4+ target cells in the subepithelial layer is supported by the fact that semen alters T cell phenotype in vivo in animal models (26). In addition, like HIV itself, soluble factors in semen likely access CD4+ target cells more easily in the presence of mucosal microtrauma after intercourse (58) or during mucosal inflammation caused by sexually transmitted infections (59, 60).
SP reduces CD4+ T cell expression of the HIVR CD4, as well as CD4+ T cell activation and proliferation. The downmodulation of these major determinants of CD4+ T cell susceptibility to HIV infection correlated strongly and positively with protection of CD4+ T cells against HIV infection, partly explaining the relative inefficiency of HIV transmission across mucosal surfaces (2). SP-mediated induction of CD4+ T cell surface expression of CCR5 mitigated the protective effects of SP against infection with R5 viruses, likely contributing to the selective transmission of R5 HIV across mucosal surfaces (31–34). Understanding the mechanisms through which SP modulates CD4+ T cell susceptibility to HIV infection could open up novel means of preventing mucosal transmission of HIV.
Acknowledgments
We thank the study subjects. We also thank Charles Wira, Ruth Connor, Kathleen Martin, Paul Palumbo, John Fahey, Stephanie Dorosko, Mimi Ghosh, Molly Housman, Deepti Saxena, and Kenneth Orndorff for thoughtful discussions and technical support. We also thank Judy Stern, Paul Manganiello, Richard Reindollar, Misty Blanchette Porter, Jacqueline Smith, Kathy Smith, Kate Ruoff, and Joseph Schwartzman for assistance with specimen collection and assay conduct and for helpful discussions.
This work was supported by Mentored Clinical Scientist Development Award 1K08AI069915-05 from the National Institutes of Health, Research Grants from the Hitchcock Foundation and the Campbell Foundation (to T.L.), and AIDS International Training and Research Program Fellowship D43-TW006807 from the Fogarty International Center (to E.B.). The Immune Monitoring Laboratory is supported in part by Core Grant CA-23108 of the Norris Cotton Cancer Center (Lebanon, NH) and COBRE Award P20 RR16437.
Abbreviations used in this paper
- Ct
threshold cycle
- MFI
mean fluorescence intensity
- MOI
multiplicity of infection
- NIH
National Institutes of Health
- RLU
relative light units
- SEVI
semen-derived enhancer of viral infection
- SP
seminal plasma
Footnotes
Portions of this work were presented at the XVIII International AIDS Conference, July 18–23, 2010, Vienna, Austria, and at the 4th International Workshop on HIV Transmission, Principles of Intervention, July 17–18, 2009, Cape Town, South Africa.
Disclosures The authors have no financial conflicts of interest.
References
- 1.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 2.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat. Rev. Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 3.Ball JK, Curran R, Irving WL, Dearden AA. HIV-1 in semen: determination of proviral and viral titres compared to blood, and quantification of semen leukocyte populations. J. Med. Virol. 1999;59:356–363. doi: 10.1002/(sici)1096-9071(199911)59:3<356::aid-jmv16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Butler DM, Delport W, Kosakovsky Pond SL, Lakdawala MK, Cheng PM, Little SJ, Richman DD, Smith DM. The origins of sexually transmitted HIV among men who have sex with men. Sci. Transl. Med. 2010;2:18re1. doi: 10.1126/scitranslmed.3000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcelin AG, Tubiana R, Lambert-Niclot S, Lefebvre G, Dominguez S, Bonmarchand M, Vauthier-Brouzes D, Marguet F, Mousset-Simeon N, Peytavin G, Poirot C. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma. AIDS. 2008;22:1677–1679. doi: 10.1097/QAD.0b013e32830abdc8. [DOI] [PubMed] [Google Scholar]
- 6.Pilcher CD, Joaki G, Hoffman IF, Martinson FE, Mapanje C, Stewart PW, Powers KA, Galvin S, Chilongozi D, Gama S, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21:1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheth PM, Kovacs C, Kemal KS, Jones RB, Raboud JM, Pilon R, la Porte C, Ostrowski M, Loutfy M, Burger H, et al. Toronto Mucosal Immunology Group Persistent HIV RNA shedding in semen despite effective anti-retroviral therapy. AIDS. 2009;23:2050–2054. doi: 10.1097/QAD.0b013e3283303e04. [DOI] [PubMed] [Google Scholar]
- 8.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 9.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat. Med. 2003;9:847–852. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- 11.Shacklett BL. Mucosal immunity to HIV: a review of recent literature. Curr. Opin. HIV AIDS. 2008;3:541–547. doi: 10.1097/COH.0b013e32830ab9ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabatté J, Ceballos A, Raiden S, Vermeulen M, Nahmod K, Maggini J, Salamone G, Salomón H, Amigorena S, Geffner J. Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN. J. Virol. 2007;81:13723–13734. doi: 10.1128/JVI.01079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stax MJ, van Montfort T, Sprenger RR, Melchers M, Sanders RW, van Leeuwen E, Repping S, Pollakis G, Speijer D, Paxton WA. Mucin 6 in seminal plasma binds DC-SIGN and potently blocks dendritic cell mediated transfer of HIV-1 to CD4(+) T-lymphocytes. Virology. 2009;391:203–211. doi: 10.1016/j.virol.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Martellini JA, Cole AL, Venkataraman N, Quinn GA, Svoboda P, Gangrade BK, Pohl J, Sørensen OE, Cole AM. Cationic polypeptides contribute to the anti-HIV-1 activity of human seminal plasma. FASEB J. 2009;23:3609–3618. doi: 10.1096/fj.09-131961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Münch J, Rücker E, Ständker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Ceballos A, Remes Lenicov F, Sabatté J, Rodríguez Rodrígues C, Cabrini M, Jancic C, Raiden S, Donaldson M, Agustín Pasqualini R, Jr., Marin-Briggiler C, et al. Spermatozoa capture HIV-1 through heparan sulfate and efficiently transmit the virus to dendritic cells. J. Exp. Med. 2009;206:2717–2733. doi: 10.1084/jem.20091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff H. Methods for the detection of male genital tract inflammation. Andrologia. 1998;30(Suppl 1):35–39. doi: 10.1111/j.1439-0272.1998.tb02824.x. [DOI] [PubMed] [Google Scholar]
- 19.Brown WF. Variance estimation in the Reed-Muench fifty per cent endpoint determination. Am. J. Hyg. 1964;79:37–46. doi: 10.1093/oxfordjournals.aje.a120362. [DOI] [PubMed] [Google Scholar]
- 20.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol. Biol. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Cullen BR, Greene WC. Regulatory pathways governing HIV-1 replication. Cell. 1989;58:423–426. doi: 10.1016/0092-8674(89)90420-0. [DOI] [PubMed] [Google Scholar]
- 23.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZQ, Wietgrefe SW, Li Q, Shore MD, Duan L, Reilly C, Lifson JD, Haase AT. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc. Natl. Acad. Sci. USA. 2004;101:5640–5645. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322:43–52. doi: 10.1007/s00441-005-1127-3. [DOI] [PubMed] [Google Scholar]
- 26.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlström AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol. Reprod. 2009;80:1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson SA, Sharkey DJ. The role of semen in induction of maternal immune tolerance to pregnancy. Semin. Immunol. 2001;13:243–254. doi: 10.1006/smim.2000.0320. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto M, Byrn R, Eyre RC, Mullen T, Church P, Kiessling AA. Seminal plasma induces programmed cell death in cultured peripheral blood mononuclear cells. AIDS Res. Hum. Retroviruses. 2002;18:797–803. doi: 10.1089/08892220260139549. [DOI] [PubMed] [Google Scholar]
- 29.Bouvet JP, Grésenguet G, Bélec L. Vaginal pH neutralization by semen as a cofactor of HIV transmission. Clin. Microbiol. Infect. 1997;3:19–23. doi: 10.1111/j.1469-0691.1997.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 30.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J. Virol. 2009;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van't Wout AB, Kootstra NA, Mulder-Kampinga GA, Albrecht-van Lent N, Scherpbier HJ, Veenstra J, Boer K, Coutinho RA, Miedema F, Schuitemaker H. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J. Clin. Invest. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolinsky SM, Wike CM, Korber BT, Hutto C, Parks WP, Rosenblum LL, Kunstman KJ, Furtado MR, Muñoz JL. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992;255:1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto T, Tsunetsugu-Yokota Y, Mitsuki YY, Mizukoshi F, Tsuchiya T, Terahara K, Inagaki Y, Yamamoto N, Kobayashi K, Inoue J. Selective transmission of R5 HIV-1 over X4 HIV-1 at the dendritic cell-T cell infectious synapse is determined by the T cell activation state. PLoS Pathog. 2009;5:e1000279. doi: 10.1371/journal.ppat.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu T, Mo H, Wang N, Nam DS, Cao Y, Koup RA, Ho DD. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 35.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 36.Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 37.Ji C, Kopetzki E, Jekle A, Stubenrauch KG, Liu X, Zhang J, Rao E, Schlothauer T, Fischer S, Cammack N, et al. CD4-anchoring HIV-1 fusion inhibitor with enhanced potency and in vivo stability. J. Biol. Chem. 2009;284:5175–5185. doi: 10.1074/jbc.M808745200. [DOI] [PubMed] [Google Scholar]
- 38.Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, Brenchley JM. CD4 down-regulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat. Med. 2009;15:879–885. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang J, McLinden JH, Rydze RA, Chang Q, Kaufman TM, Klinzman D, Stapleton JT. Viruses within the Flaviviridae decrease CD4 expression and inhibit HIV replication in human CD4+ cells. J. Immunol. 2009;183:7860–7869. doi: 10.4049/jimmunol.0902276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margolis L, Shattock R. Selective transmission of CCR5-utilizing HIV-1: the `gatekeeper' problem resolved? Nat. Rev. Microbiol. 2006;4:312–317. doi: 10.1038/nrmicro1387. [DOI] [PubMed] [Google Scholar]
- 41.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camara M, Dieye TN, Seydi M, Diallo AA, Fall M, Diaw PA, Sow PS, Mboup S, Kestens L, Jennes W. Low-level CD4+ T cell activation in HIV-exposed seronegative subjects: influence of gender and condom use. J. Infect. Dis. 2010;201:835–842. doi: 10.1086/651000. [DOI] [PubMed] [Google Scholar]
- 43.Cohen CR, Moscicki AB, Scott ME, Ma Y, Shiboski S, Bukusi E, Daud I, Rebbapragada A, Brown J, Kaul R. Increased levels of immune activation in the genital tract of healthy young women from sub-Saharan Africa. AIDS. 2010;24:2069–2074. doi: 10.1097/QAD.0b013e32833c323b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 45.Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J. Virol. 2007;81:1000–1012. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehman DA, Chung MH, John-Stewart GC, Richardson BA, Kiarie J, Kinuthia J, Overbaugh J. HIV-1 persists in breast milk cells despite antiretroviral treatment to prevent mother-to-child transmission. AIDS. 2008;22:1475–1485. doi: 10.1097/QAD.0b013e328302cc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sallé B, Brochard P, Bourry O, Mannioui A, Andrieu T, Prevot S, Dejucq-Rainsford N, Dereuddre-Bosquet N, Le Grand R. Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J. Infect. Dis. 2010;202:337–344. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
- 48.Sato H, Orenstein J, Dimitrov D, Martin M. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology. 1992;186:712–724. doi: 10.1016/0042-6822(92)90038-q. [DOI] [PubMed] [Google Scholar]
- 49.Vendrame D, Sourisseau M, Perrin V, Schwartz O, Mammano F. Partial inhibition of human immunodeficiency virus replication by type I inter-ferons: impact of cell-to-cell viral transfer. J. Virol. 2009;83:10527–10537. doi: 10.1128/JVI.01235-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim KA, Yolamanova M, Zirafi O, Roan NR, Staendker L, Forssmann WG, Burgener A, Dejucq-Rainsford N, Hahn BH, Shaw GM, et al. Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI. Retrovirology. 2010;7:55. doi: 10.1186/1742-4690-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willott GM, Allard JE. Spermatozoa—their persistence after sexual intercourse. Forensic Sci. Int. 1982;19:135–154. doi: 10.1016/0379-0738(82)90040-8. [DOI] [PubMed] [Google Scholar]
- 52.Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 2009;61:86–100. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haimovici F, Mayer KH, Anderson DJ. Quantitation of HIV-1-specific IgG, IgA, and IgM antibodies in human genital tract secretions. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997;15:185–191. doi: 10.1097/00042560-199707010-00001. [DOI] [PubMed] [Google Scholar]
- 54.Mselle TF, Howell AL, Ghosh M, Wira CR, Sentman CL. Human uterine natural killer cells but not blood natural killer cells inhibit human immunodeficiency virus type 1 infection by secretion of CXCL12. J. Virol. 2009;83:11188–11195. doi: 10.1128/JVI.00562-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. Trappin-2/Elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology. 2010;129:207–219. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat. Rev. Immunol. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, Corey L. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat. Med. 2009;15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norvell MK, Benrubi GI, Thompson RJ. Investigation of microtrauma after sexual intercourse. J. Reprod. Med. 1984;29:269–271. [PubMed] [Google Scholar]
- 59.de Sousa JD, Müller V, Lemey P, Vandamme AM. High GUD incidence in the early 20 century created a particularly permissive time window for the origin and initial spread of epidemic HIV strains. PLoS ONE. 2010;5:e9936. doi: 10.1371/journal.pone.0009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mauck CK, Ballagh SA, Creinin MD, Weiner DH, Doncel GF, Fichorova RN, Schwartz JL, Chandra N, Callahan MM. Six-day randomized safety trial of intravaginal lime juice. J. Acquir. Immune Defic. Syndr. 2008;49:243–250. doi: 10.1097/QAI.0b013e318186eae7. [DOI] [PubMed] [Google Scholar]