Abstract
Research on the prevention of human immunodeficiency virus (HIV)–1 infection is at a critical juncture. Major methodological challenges to performing prevention trials have emerged, and one after another promising biomedical interventions have failed to reduce the incidence of HIV-1 infection. Nevertheless, there is growing optimism that progress can be achieved in the near term. Mathematical modeling indicates that 2 new strategies, “test and treat” and preexposure prophylaxis, could have a major impact on the incidence of HIV-1 infection. Will our hopes be justified? We review the potential strengths and limitations of these antiretroviral “treatment as prevention” strategies and outline other new options for reducing the incidence of HIV-1 infection in the near term. By maximizing the potential of existing interventions, developing other effective strategies, and combining them in an optimal manner, we have the opportunity to bring the HIV-1 epidemic under control.
The human immunodeficiency virus (HIV)–1 epidemic remains out of control despite the intense efforts of clinicians, scientists, public health specialists, activists, and others for nearly 3 decades. There have been dramatic gains, particularly in treatment, but for every 2 persons who start antiretroviral therapy (ART), 5 become newly infected [1]. UNAIDS estimates that there were 2.7 million new HIV-1 infections and 2.0 million deaths due to AIDS in 2008. Decreases in some regions were offset by increases in others [2]. In North America, western Europe, and Australia, there is evidence that the epidemic is expanding in the population most affected, men who have sex with men [3-6].
Research on prevention of HIV-1 infection is at a critical juncture. Major methodological challenges to performing prevention trials have emerged [7]. A recent review found that just 5 of 37 randomized trials of promising interventions for sexual transmission reported a reduction in HIV-1 acquisition [8]. Aside from male circumcision, only 2 biomedical interventions had been shown to be effective: the ALVAC/AIDSVAX (RV144) vaccine, which had a modest, transient benefit and was not recommended for implementation, and treatment of curable sexually transmitted infections, which was not replicated in 5 other trials.
Nevertheless, there is growing optimism that progress can be achieved in the near term. Mathematical modeling indicates that 2 new strategies, “test and treat” and preexposure prophylaxis (PrEP), could have a major impact on the incidence of HIV-1 infection [9-13]. CAPRISA 004, the first PrEP trial to report efficacy results, found that 1% tenofovir gel applied intravaginally before and after coitus reduced HIV acquisition by 39% overall [14]. Will our expectations for these 2 strategies be met? Are there other options that should also be considered?
TREATMENT AS PREVENTION
Treatment as prevention is not a new concept. “Treatment is Prevention” was a popular slogan for tuberculosis >20 years ago, and models were constructed to use this strategy to improve tuberculosis control in Africa and elsewhere [15-18]. Multiple studies have shown that treatment can reduce the incidence of sexually transmitted infections other than HIV-1 infection [19].
The earliest evidence that ART reduces HIV-1 transmission came from ecological studies. The introduction of 3-drug ART in the mid-1990s was associated with a decrease in the prevalence of HIV-1 infection in a number of countries and settings [20, 21]. In Vancouver, British Columbia, a decrease in the median plasma HIV-1 RNA concentration was correlated with a similar decrease in the incidence of HIV-1 infection among injection drug users (IDUs; Spearman correlation coefficient, 0.48; P = .024) [22]. Despite the limitations of ecological associations, it is plausible that a decreased community viral load due to increased ART coverage contributed to the observed decrease in incidence.
Observational studies have supported these findings. In a recent meta-analysis of 11 cohort studies, HIV-1 transmission was 92% lower among couples in whom the index partner was taking ART (0.46 vs 5.64 cases per 100 person-years) [23]. Other evidence includes the success of maternal ART for prevention of mother-to-child transmission [24]. The single randomized phase III clinical trial that is examining the hypothesis in discordant couples, HPTN 052, is still ongoing at sites in Africa, India, Brazil, and Thailand [25].
A number of groups have modeled the available data and reported that a greatly expanded testing and treatment strategy could have a major impact on the incidence of HIV-1 infection [9-11, 26]. One early model suggested that ART could potentially “eradicate” the HIV-1 epidemic if applied broadly for a long enough period of time [9]. The authors also found, however, that the outcome was sensitive to the level of HIV-1 risk behavior. If treatment led to so-called “disinhibition” and risk behaviors increased by 50%, a significant population-level benefit was unlikely.
The most optimistic model was developed by Granich et al [11]. Using data from South Africa, they reported that if all persons are tested for HIV-1 once yearly and all persons with positive results immediately start and maintain use of effective ART for life, the incidence could be reduced to <1 new infection per 1000 persons within 10 years, and the prevalence would decrease to <1% within 50 years. Major assumptions of this model include that all transmission is via heterosexual sex, that uptake and adherence rates are extremely high, that ART reduces transmission 100-fold, that treatment is coupled with ≥1 prevention intervention that collectively reduces incidence by 40%, that significant drug resistance does not emerge, and that treatment failure rates are low (3% per year).
Unfortunately, many of these assumptions are currently unattainable in real-world settings. For example, on the basis of actual use, our best current estimate of the effectiveness of ART is a 92% reduction in transmission, not 99% [23]. Another key assumption of the Granich et al [11] model, that acute infections account for only 9% of all new transmissions, may not apply in many settings. Hollingsworth et al [27] showed that 9% is a good estimate for populations in which serial monogamy is the rule, but if there is substantial partner mixing, ≥31% transmissions may be caused by recent infections. Because most recent infections would not be detected by annual testing, many transmissions would be undeterred. Dodd et al [28] showed that partner mixing and other epidemiological parameters could have a major influence on the overall impact of test and treat programs.
Perhaps the biggest question we must ask regarding the Granich et al [11] “test and treat” strategy, however, is “Can we implement it?” Currently, no country comes close to testing everyone for HIV-1 infection every year. This is crucial, because it is clear that most new transmissions can be attributed to persons who are unaware of their HIV-1 status [29]. In the United States, where opt-out testing is now practiced in many states, the Centers for Disease Control and Prevention estimates that 21% of all HIV-1–positive persons—including 48% of those aged 13–24 years—are unaware of their infection status [30, 31]. In parts of sub-Saharan Africa, ≥80% of infected persons are unaware [32].
Linkage to care and initiation of treatment are also major challenges. Some persons are reluctant to accept their diagnosis and receive care. Others are not treated, because the provider does not believe that the patient will adhere to therapy. Among those offered ART, some do not accept because of concerns about drug toxicities, cost, or potential stigma if their employer, family, or friends learn that they are taking AIDS medications.
Given these challenges, far less than 100% of HIV-1–positive persons opt to take ART. In the United States, it is estimated that the proportion of HIV-1–positive persons who are in care and who have a viral load <500 copies/mL is as low as 26% (Figure 1). Of those who are aware of their status, only ~60% were successfully linked to care (ie, they were seen at least once in the past 6 months), and of those in care, only 55% had viral loads <500 copies/mL [29, 33, 34]. The proportion of persons in care with undetectable viral loads may have increased in recent years in response to the improved tolerability and durability of newer ART regimens, but it is unclear whether there has been a significant increase in the overall uptake of treatment [35, 36]. It is also too soon to know the extent to which these rates will be affected by the recent changes in the US Department of Health and Human Services guidelines, which encourage earlier initiation of ART [37].
Figure. 1.
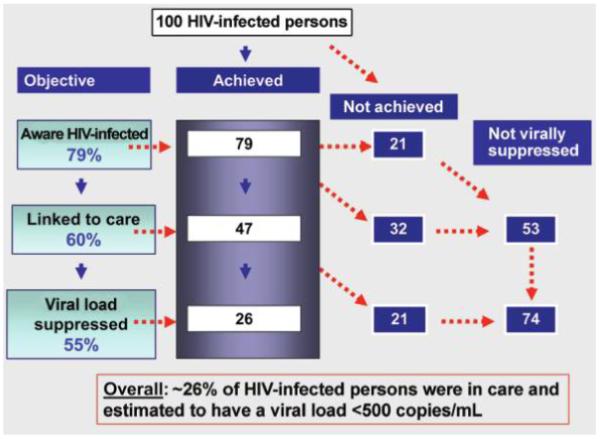
The test, link, and treat cascade. Gaps limiting the impact of human immunodeficiency virus (HIV)–1 testing and treatment on achievement of viral suppression. Data from [29, 30, 33, 34].
Thus, there is considerable room for improvement in our current testing and treatment efforts, and with it, a major opportunity to reduce ongoing transmission of HIV-1. One study being launched in the United States, “Test, Link, and Care Plus” (HPTN 065), will examine these gaps and assess the feasibility of an enhanced testing, linkage to care, and treatment strategy [38]. Similar studies are also being planned for other populations and settings [39].
In summary, substantial data suggest that the incidence of HIV-1 infection can be reduced by implementation of a test, link, and treat strategy. It is unlikely, however, that we will be able to eliminate the epidemic by treatment alone. We must also protect high-risk uninfected persons from HIV-1 acquisition. This means broader implementation of existing interventions, such as male circumcision, blood and injection safety, condom use, evidence-based behavioral strategies, and structural interventions. In addition, another “treatment for prevention” strategy, PrEP, has considerable promise.
PREP
Five ongoing clinical trials are examining the impact of oral and/or vaginal tenofovir or emtricitabine-tenofovir (Truvada; Gilead) on the acquisition of HIV-1 infection in various high-risk populations (Table 1). The results of a recently completed efficacy trial, CAPRISA 004, have now been reported [14]. Most of these trials are evaluating daily dosing, but other ongoing or planned studies are also assessing the feasibility of various intermittent dosing strategies [40-42].
Table 1.
Ongoing Phase II and III Human Immunodeficiency Virus (HIV)–1 Preexposure Prophylaxis Efficacy trials. Modified from [40]
| Trial | Sponsor(s) | Site(s) | Population | Intervention | Status | Date results expected |
|---|---|---|---|---|---|---|
| Bangkok Tenofovir Study | CDC | Thailand | 2400 Injection drug users | Daily oral TDF | Follow-up continuing | 2010 or 2011 |
| CAPRISA 004 | USAID, DST | South Africa | 889 Heterosexual women |
Topical TDF gel applied intravaginally ≤12 h before and as soon as possible after coitus (but within 12 h) |
Completed. TDF gel was associated with a 39% overall reduction in HIV acquisition (95% confidence interval, 6%–60%; P = .017) [14] |
2010 or 2011 |
| iPrEx | NIH, BMGF | Peru, Ecuador, Brazil, South Africa, Thai- land, United States |
2500 Men who have sex with men |
Daily oral TDF-FTC | Follow-up continuing | 2010 |
| Partners PrEP | BMGF | Kenya, Uganda | 3900 Serodiscordant het- erosexual couples |
Daily oral TDF or TDF-FTC | Enrolling | 2012 |
| FEM-PrEP | USAIDS, BMGF | Kenya, Malawi, South Africa, Tanzania, Zambia |
3900 Heterosexual women |
Daily oral TDF-FTC | Enrolling | 2012 |
| VOICE (MTN 003) | NIH | South Africa, Uganda, Malawi, Zimbabwe | 5000 Heterosexual women |
Daily oral TDF or TDF- FTC or daily topical TDF gel (or daily oral placebo or topical placebo gel) |
Enrolling | 2013 |
NOTE. BMGF, Bill & Melinda Gates Foundation; CAPRISA, Centre for the AIDS Programme of Research in South Africa; CDC, Centers for Disease Control and Prevention; DST, Department of Science and Technology, Republic of South Africa; FTC, emtricitabine; MTN, Microbicide Trials Network; NIH, US National Institutes of Health; TDF tenofovir disoproxil fumarate; USAID, United States Agency for International Development.
Initial enthusiasm for PrEP was based on positive efficacy results in nonhuman primates [43] and pharmacokinetic studies showing that emtricitabine and tenofovir are concentrated in human genital secretions [44, 45]. Mathematical modeling found that up to 3.2 million new HIV-1 infections could be averted in sub-Saharan Africa over 10 years if PrEP is 90% effective and targeted at those at highest risk [12]. Although the efficacy of topical PrEP in CAPRISA 004 was well below this level [14], it has established “proof of concept” for the approach.
The effectiveness of PrEP will be limited by acceptability, adherence, and other factors. In addition, identifying every person at high risk of acquiring HIV-1 infection and offering them PrEP will be even greater challenges than implementing a test, link, and treat program. Moreover, as with other prevention interventions, risk compensation (disinhibition) could substantially reduce its benefit in some persons [12].
We must also recognize that, even if PrEP is highly effective, it cannot be passed out like condoms. Regardless of whether drug toxicity proves to be a serious concern, the potential for drug resistance among those who do become infected while taking PrEP will necessitate close monitoring. Furthermore, the cost-effectiveness of PrEP will decrease rapidly with the level of risk [13]. Given these factors, if a favorable risk-benefit ratio is found for PrEP, its implementation is likely to be limited, at least initially, to highly motivated, high-risk persons who have access to monitoring services.
Strategies for countering these expected limitations are being evaluated. A pipeline approach is being established to identify and develop new PrEP agents that have a prolonged half-life, achieve high concentrations in target tissues, have a high threshold for resistance, and have other ideal properties. An extremely long-acting (eg, for ≥4 weeks), safe, and highly effective PrEP agent would begin to resemble an ultimate prevention research goal: a safe and effective HIV-1 vaccine.
For the foreseeable future, however, PrEP, like test, link, and treat strategies, is unlikely to serve as a stand-alone prevention strategy. We therefore clearly need ongoing research programs to identify the most effective combination of interventions for each population and setting. For example, in selected settings in sub-Saharan Africa, this might include an enhanced testing strategy, male circumcision, treatment of HIV-1–infected persons, and if found to be safe and effective, administration of topical or oral PrEP for the uninfected partner in serodiscordant couples. Structural and behavioral interventions are also essential, particularly strategies to optimize adherence and to reduce stigma and discrimination.
COMBINATION PREVENTION
In 2002, UNAIDS published a call for an expanded HIV response and listed 21 actions that should be taken, 12 of which were prevention interventions [46]. Evidence for how to best combine these interventions for a given population and setting was extremely limited, however. To be successful, it is now widely recognized that HIV-1 prevention programs must incorporate biomedical, behavioral, and structural strategies [47].
But how do we combine different prevention interventions in a manner that has maximal impact? Under one current initiative (Methods for Prevention Packages Program), multi-disciplinary teams composed of epidemiologists, mathematical modelers, behavioral and biomedical scientists, and experts in HIV-1 clinical research design and implementation are developing prevention packages for specific populations and settings. The 6 current projects are targeting serodiscordant couples, households, persons with acute HIV-1 infection, and an entire village, all in sub-Saharan Africa; IDUs in Eastern Europe; and men who have sex with men in North and South America. The HIV Prevention Trials Network is likewise developing combination prevention interventions for various populations and settings [41].
The prevention package approach combines individual interventions with known efficacy into a combination strategy that is predicted by mathematical modeling to have increased effectiveness. Modeling is used because the main alternative, a stepwise factorial design, would require two or more trials and take far more time and resources. If new interventions became available in the meantime, further testing might be necessary and delay clinical trials interminably. Pilot studies of the prevention packages are required to demonstrate acceptability and adherence before full-scale testing is performed.
At the same time, implementation science can be used to gain insights from current HIV-1 prevention programs that are incorporating multiple interventions [48]. Major projects funded by the Global Fund and PEPFAR offer particularly good opportunities for this approach. We must make every effort to use this information to increase the effectiveness of both new and ongoing prevention programs.
Clinical trials of new HIV-1 prevention interventions must also continue. In addition to vaccines, vaginal microbicides, and oral PrEP, researchers are examining rectal microbicides, slow-release vaginal rings, medication-assisted therapy for IDUs, and other products and strategies. Progress has been slowed, however, by an array of methodological challenges to HIV-1 prevention trials. These challenges have themselves become the focus of ongoing research.
CHALLENGES TO HIV-1 PREVENTION RESEARCH
A recent Institute of Medicine report enumerated the methodological challenges facing biomedical HIV-1 prevention trials [8]. Included were the accurate determination of the incidence of HIV-1 infection in the study population, adherence to treatment, risk-taking behavior, and retention of participants in the trials. The report also cited the need to identify surrogate markers for infection and product activity and to develop alternative trial designs that can be used to more efficiently compare 2 active interventions.
An accurate HIV-1 incidence assay is needed to determine whether a prevention trial is feasible in a particular population (ie, whether the baseline incidence is sufficient to obtain statistically meaningful results), to assess incidence outcomes in community (cluster)–randomized prevention trials, and to evaluate the overall effectiveness of a combination prevention program at the population level. The assay must discriminate recent cases (that occurred within a known window period—ideally the previous 6–12 months) from other prevalent cases. Current assays are of uncertain value because they have generally been tested on relatively small numbers of specimens from a narrow range of sources (chiefly clade B) [49]. When applied in clinical settings, they have been found to overestimate the number of recent infections [50-52]. This can be countered by using a second incidence assay and excluding long-standing cases and elite suppressors by clinical history, HIV-1 RNA testing, and/or CD4+ cell count [51, 52], but this approach quickly becomes quite costly. Development of simpler, more cost-effective incidence assays with greater specificity is therefore an urgent priority.
Adherence is also a major challenge. In one microbicide trial that failed to demonstrate efficacy, an objective measure of adherence found that the product was used, on average, 42% of the time—less than one-half of the self-reported rate of 96% [53]. In another trial examining whether suppressive therapy for herpes simplex virus infection could reduce the incidence of HIV-1 infection, the median rate of adherence was >90% as measured by tablet count, but only 55% of urine samples from women randomized to the active agent, acyclovir, had detectable levels of the drug [54].
Incomplete adherence tends to bias efficacy assessments toward the null, making it more difficult to detect a prevention effect if, in fact, one is present. The same is true for a number of the other methodological challenges to HIV-1 prevention research. Masse et al [55] cited 4 major reasons for “efficacy dilution” in randomized, placebo-controlled trials of vaginal microbicides: incomplete adherence, time “off” the study product (eg, for pregnancy), anal intercourse, and placebo gel efficacy. As would be expected, in the CAPRISA 004 trial of 1% tenofovir gel used intravaginally before and after coitus, more highly adherent women had higher rates of protection than less adherent women [14].
Risk compensation can also be a significant challenge [56, 57]. We can predict that each time some participants complete their assigned prevention intervention(s) they will be reminded of their vulnerability and reduce their HIV-1 risk taking. Others, however, may conclude that they are protected and increase their risk, even though they have been carefully advised otherwise. Methods that allow us to predict how different individuals will react in these settings could help us to develop and target more effective behavioral interventions. In addition, as further research enables us to more accurately measure adherence, risk-taking, and related variables, we can begin to adjust for these factors in the design and analysis of our prevention trials.
Research at the implementation stage will also be critical for optimizing our prevention strategies for each population and setting. Success is clearly the responsibility of both providers and clients. In everyday practice, each can fall alarmingly short. For example, a recent study indicates that even when patients are at risk of life-threatening complications such as emboli, only approximately one-half may be prescribed anticoagulants [58]. Underuse of ART for HIV-1–positive persons has also been reported, particularly for IDUs [59]. Rates of patient adherence to long-term regimens have likewise been strikingly low in certain settings—often in the range of 50% [60]. Thus, a prevention intervention with 60% efficacy in a clinical trial might only reduce HIV-1 incidence by 15% in practice (60% × 0.5 [coverage] × 0.5 [adherence]) unless additional strategies to increase coverage and enhance adherence are implemented.
CONCLUSIONS
In the end, prevention is a commitment that individuals make to themselves and others. To enable success, we must provide the most effective combination of biomedical and behavioral interventions possible for each population and setting. We must also promote complementary structural changes, particularly those that increase access to services, decrease costs, and reduce stigma and discrimination [61, 62]. The focus must be on the health and well-being of individuals, not merely public health goals. It is when all of these interventions are in place that we can expect to see a sustained, progressive decrease in the incidence of HIV-1 infection.
We must recognize, however, that funding for HIV-1 prevention will rarely be sufficient to fully implement an ideal strategy. Cost-effectiveness must also be considered in identifying the combination of interventions that will have the greatest impact. Hecht et al [63] have called this the “hard choices for prevention” scenario. This requires careful planning and integration of our research efforts.
In conclusion, a more comprehensive and coordinated prevention research strategy that includes basic research, clinical trials, and implementation science is urgently needed. By maximizing the potential of test, link, and treat strategies; developing other effective interventions and strategies; and combining them in an optimal manner with proven interventions, such as prevention of mother-to-child transmission and male circumcision, we have the opportunity to bring the HIV-1 epidemic under control.
Acknowledgments
Financial support. National Institutes of Health (U01AI068619 to S.H.V.).
Footnotes
Potential conflicts of interest. S.H.V. has been a consultant for Pfizer and Mead-Johnson within the past year. D.N.B. and C.W.D.: no conflicts.
The opinions and statements in this article are those of the authors and do not represent the official policy, endorsement, or views of their institutions or agencies. of HIV-1 infection in a number of countries and settings
References
- 1.UNAIDS . Report on the global HIV/AIDS epidemic 2008. UNAIDS; Geneva: [Accessed 5 February 2010]. 2008. http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp. [Google Scholar]
- 2.UNAIDS . AIDS epidemic update: December 2009. UNAIDS; Geneva: [Accessed 5 February 2010]. 2009. http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive/2009/default.asp. [Google Scholar]
- 3.Sullivan PS, Hamouda O, Delpech V, et al. Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996–2005. Ann Epidemiol. 2009;19:423–431. doi: 10.1016/j.annepidem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Marcus U, Voss L, Kollan C, Hamouda O. HIV incidence increasing in MSM in Germany: factors influencing infection dynamics. Euro Surveill. 2006;11:157–160. [PubMed] [Google Scholar]
- 5.Bezemer D, deWolf F, Boerlijst MC, et al. A resurgent HIV-1 epidemic among men who have sex with men in the era of potent antiretroviral therapy. AIDS. 2008;22:1071–1077. doi: 10.1097/QAD.0b013e3282fd167c. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Trends in HIV/AIDS diagnoses among men who have sex with men. MMWR Morb Mortal Wkly Rep. 2008;57:681–686. [PubMed] [Google Scholar]
- 7.Lagokos SW, Gable AR, editors. Methodological challenges in biomedical HIV prevention trials. National Academies Press; Washington, DC: 2008. [Google Scholar]
- 8.Padian NS, McCoy SI, Balkus JE, Wasserheit JN. Weighing the gold in the gold standard: challenges in HIV prevention research. AIDS. 2010;24:621–635. doi: 10.1097/QAD.0b013e328337798a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velasco-Hernandez JX, Gershengorn HB, Blower SM. Could wide-spread use of combination antiretroviral therapy eradicate HIV epidemics? Lancet Infect Dis. 2002;2:487–493. doi: 10.1016/s1473-3099(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 10.Lima VD, Johnston K, Hogg RS, et al. Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic. J Infect Dis. 2008;198:59–67. doi: 10.1086/588673. [DOI] [PubMed] [Google Scholar]
- 11.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 12.Abbas UL, Anderson RM, Mellors JW. [Accessed 5 February 2010];Potential impact of antiretroviral chemoprophylaxis on HIV-1 transmission in resource-limited settings. PLoS ONE. 2007 9:e875. doi: 10.1371/journal.pone.0000875. http://www.plosone.org/article/info:doi/10.1371/journal.pone.0000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paltiel AD, Freedberg KA, Scott CA, et al. HIV pre-exposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis. 2009;48:806–815. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karim QA, Karim SS, Frohlich JA, et al. [Accessed 24 July 2010];Effectiveness and safety of tenofovir gel, an antiretroviral microbe, for the prevention of HIV infection in women. Science. 2010 doi: 10.1126/science.1193748. http://www.sciencemag.org/cgi/content/abstract/science.1193748v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heymann SJ. Modelling the efficacy of prophylactic and curative therapies for preventing the spread of tuberculosis in Africa. Trans Royal Soc Trop Med Hygiene. 1993;87:406–411. doi: 10.1016/0035-9203(93)90014-h. [DOI] [PubMed] [Google Scholar]
- 16.Blower SM, Small PM, Hopewell PC. Control strategies for tuberculosis epidemics: new models for old problems. Science. 1996;273:497–500. doi: 10.1126/science.273.5274.497. [DOI] [PubMed] [Google Scholar]
- 17.Foster S, Godfrey-Faussett P, Porter J. Modelling the economic benefits of tuberculosis preventive therapy for people with HIV: the example of Zambia. AIDS. 1997;11:919–925. doi: 10.1097/00002030-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Dye C, Fengzeng Z, Scheele S, Williams B. Evaluating the impact of tuberculosis control: number of deaths prevented by short course chemotherapy in China. Int J Epidemiol. 2000;29:558–564. [PubMed] [Google Scholar]
- 19.Manhart LE, Holmes KK. Randomized controlled trials of individual-level, population-level, and multilevel interventions for preventing sexually transmitted infections: what has worked? J Infect Dis. 2005;191(suppl 1):S7–S24. doi: 10.1086/425275. [DOI] [PubMed] [Google Scholar]
- 20.Fang C-T, Hsu H-M, Twu S-J, et al. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. J Infect Dis. 2004;190:879–885. doi: 10.1086/422601. [DOI] [PubMed] [Google Scholar]
- 21.Castilla J, del Romero J, Hernando V, Marincovich B, García S, Rodríguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005;40:96–101. doi: 10.1097/01.qai.0000157389.78374.45. [DOI] [PubMed] [Google Scholar]
- 22.Wood E, Kerr T, Marshall BDL, et al. [Accessed 5 February 2010];Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009 338:b1649. doi: 10.1136/bmj.b1649. http://www.bmj.com/cgi/content/full/338/apr30_1/b1649?view=long&pmid=19406887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attia S, Egger M, Műller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 24.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 25.HIV Prevention Trials Network [Accessed 5 February 2010];HPTN 052: A randomized trial to evaluate the effectiveness of antiretroviral therapy plus HIV primary care versus HIV primary care alone to prevent the sexual transmission of HIV-1 in serodiscordant couples. http://www.hptn.org/research_studies/hptn052.asp.
- 26.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: antiretroviral therapy in San Francisco. Science. 2000;287:650–654. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 27.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission by stage of infection. J Infect Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 28.Dodd PJ, Garnett GP, Hallett TB. Examining the promise of HIV elimination by ‘test and treat’ in hyperendemic settings. AIDS. 2010;24:729–735. doi: 10.1097/QAD.0b013e32833433fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20:1447–1450. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention HIV prevalence estimates—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:1073–1076. [PubMed] [Google Scholar]
- 31.Campsmith ML, Rhodes PH, Hall HI, Green TA. Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006. J Acquir Immune Defic Syndr. 2010;53:619–624. doi: 10.1097/QAI.0b013e3181bf1c45. [DOI] [PubMed] [Google Scholar]
- 32.Anand A, Shiraishi RW, Bunnell RE, et al. Knowledge of HIV status, sexual risk behaviors and contraceptive need among people living with HIV in Kenya and Malawi. AIDS. 2009;23:1565–1573. doi: 10.1097/QAD.0b013e32832cb10c. [DOI] [PubMed] [Google Scholar]
- 33.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19:423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 34.Richardson JL. Dataset: Partnership for Health Study. University of Southern California; Los Angeles CA: 2001. [Google Scholar]
- 35.Willig JH, Abroms S, Westfall AO, et al. Increased regimen durability in the era of once-daily fixed-dose combination antiretroviral therapy. AIDS. 2008;22:1951–1960. doi: 10.1097/QAD.0b013e32830efd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hull MW, Lima VD, Hogg RS, Harrigan PR, Montaner JS. Epidemiology of treatment failure: a focus on recent trends. Curr Opin HIV AIDS. 2009;4:467–473. doi: 10.1097/COH.0b013e328331d353. [DOI] [PubMed] [Google Scholar]
- 37.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed 5 February 2010]. Dec 1, 2009. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 38.Vermund SH, Hodder SL, Justman JE, et al. Addressing research priorities for prevention of HIV infection in the United States. Clin Infect Dis. 2010;50(S3):S149–S155. doi: 10.1086/651485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization Modelling the impact of antiretroviral therapy on TB and HIV: informal working group meeting; Geneva, Switzerland. 4–6 November 2009; [Accessed 5 February 2010]. http://www.who.int/hiv/topics/artforprevention/modelling_meeting/en/index.html. [Google Scholar]
- 40.AVAC: Global Advocacy for HIV Prevention [Accessed 5 February 2010];Ongoing ARV-based prevention (oral PrEP and topical microbicide) trials. 2009 December; http://www.avac.org/ht/a/GetImageAction/i/4474.
- 41.HIV Prevention Trials Network [Accessed 5 February 2010];Ongoing studies and studies in development. http://hptn.org/research_studies.asp.
- 42.International AIDS Vaccine Initiative [Accessed 5 February 2010];Studies evaluating the safety and acceptability of intermittent pre-exposure prophylaxis (PrEP) regimen. http://www.iavi.org/research-development/trials/Pages/PrEPstudy.aspx.
- 43.Kwara A, Delong A, Rezk N, et al. Antiretroviral drug concentrations and HIV RNA in the genital tract of HIV-infected women receiving long-term highly active antiretroviral therapy. Clin Infect Dis. 2008;46:719–725. doi: 10.1086/527387. [DOI] [PubMed] [Google Scholar]
- 44.Vourvahis M, Tappouni HL, Patterson KB, et al. The pharmacokinetics and viral activity of tenofovir in the male genital tract. J Acquir Immune Defic Syndr. 2008;47:329–333. doi: 10.1097/QAI.0b013e3181632cc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García-Lerma JG, Otten RA, Qari SH, et al. [Accessed 5 February 2010];Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008 5:e28. doi: 10.1371/journal.pmed.0050028. http://www.plosmedicine.org/article/info:doi%2F10.1371%2Fjournal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stover J, Walker N, Garnett GP, et al. Can we reverse the HIV/AIDS pandemic with an expanded response? Lancet. 2002;360:73–77. doi: 10.1016/S0140-6736(02)09339-X. [DOI] [PubMed] [Google Scholar]
- 47.Horton R, Das P. Putting prevention at the forefront of HIV/AIDS. Lancet. 2008;372:421–422. doi: 10.1016/S0140-6736(08)60882-X. [DOI] [PubMed] [Google Scholar]
- 48.Madon T, Hofman KJ, Kupfer L, Glass RI. Implementation science. Science. 2007;318:1728–1729. doi: 10.1126/science.1150009. [DOI] [PubMed] [Google Scholar]
- 49.Guy R, Gold J, García Calleja JM, et al. Accuracy of serological assays for detection of recent infection with HIV and estimation of population incidence: a systematic review. Lancet Infect Dis. 2009;9:747–759. doi: 10.1016/S1473-3099(09)70300-7. [DOI] [PubMed] [Google Scholar]
- 50.Sakarovitch C, Rouet F, Murphy G, et al. Do tests devised to detect recent HIV-1 infection provide reliable estimates of incidence in Africa? J Acquir Immune Defic Syndr. 2007;45:115–122. doi: 10.1097/QAI.0b013e318050d277. [DOI] [PubMed] [Google Scholar]
- 51.Laeyendecker O, Rothman RE, Henson C, et al. The effect of viral suppression on cross-sectional incidence testing in the Johns Hopkins Hospital Emergency Department. J Acquir Immune Defic Syndr. 2008;48:211–215. doi: 10.1097/QAI.0b013e3181743980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marinda ET, Hargrove J, Preiser W, et al. Significantly diminished long-term specificity of the BED capture enzyme immunoassay among patients with HIV-1 with very low CD4 counts and those on antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;53:496–499. doi: 10.1097/qai.0b013e3181b61938. [DOI] [PubMed] [Google Scholar]
- 53.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of carraguard for prevention of HIV infection in women in South Africa: a randomized, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 54.Watson-Jones D, Baisley K, Rusizoka M, et al. Measurement and predictors of adherence in a trial of HSV suppressive therapy in Tanzania. Contemp Clin Trials. 2009;30:504–512. doi: 10.1016/j.cct.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Masse BR, Boily M-C, Dimitrov D, Desai K. [Accessed 5 February 2010];Efficacy dilution in randomized placebo-controlled vaginal microbicide trials. Emerg Themes Epidemiol. 2009 6:5. doi: 10.1186/1742-7622-6-5. http://www.ete-online.com/content/6/1/5/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cassell MM, Halperin DT, Shelton JD, Stanton D. Risk compensation: the Achilles’ heel of innovations in HIV prevention? BMJ. 2006;332:605–607. doi: 10.1136/bmj.332.7541.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eaton LA, Kalichman S. Risk compensation in HIV prevention: implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Curr HIV/AIDS Rep. 2007;4:165–172. doi: 10.1007/s11904-007-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niska R, Han B. Anticoagulation for patients with atrial fibrillation in ambulatory care settings. J Am Board Fam Med. 2009;22:299–306. doi: 10.3122/jabfm.2009.03.080218. [DOI] [PubMed] [Google Scholar]
- 59.Mathers BM, Degenhardt L, Ali H, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375:1014–1028. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- 60.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 61.Gupta GR, Parkhurst JO, Ogden JA, Aggleton P, Mahal A. Structural approaches to HIV prevention. Lancet. 2008;372:764–775. doi: 10.1016/S0140-6736(08)60887-9. [DOI] [PubMed] [Google Scholar]
- 62.Vermund SH, Karim QA. HIV prevention at a crossroads: advances in reducing sexual risk. Curr Opin HIV AIDS. 2009;4:266–273. doi: 10.1097/COH.0b013e32832c91dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hecht R, Bollinger L, Stover J, et al. Critical choices in financing the response to the global HIV/AIDS pandemic. Health Aff (Millwood) 2009;28:1591–1605. doi: 10.1377/hlthaff.28.6.1591. [DOI] [PubMed] [Google Scholar]


