Abstract
Background and Objectives
Laser-activated photodynamic biologic tissue glues may be useful for closing incisions in ophthalmology. We report on the use of two such preparations to close perforating corneal incisions in living rats.
Study Design/Materials and Methods
A previously described preparation containing a covalent albumin-chlorin e6 (ce6) conjugate (bovine serum albumin (BSA)–ce6), and a novel mixture of albumin and Janus Green (BSA/JG), both activated with a 665-nm diode laser were used to glue mouse skin ex vivo. The optimized glues were then used to seal incisions in rat corneas and results were compared to control incisions. Rats were sacrificed at day 1, 7, and 14 and eyes tested for leaking pressure and examined histopathologically.
Results
One day after treatment eyes closed with BSA–ce6 had a leaking pressure (in mmHg) of 357 compared to 193 for control incisions (P<0.01); closure with BSA/JG gave a leaking pressure of 430 (P<0.05 compared to BSA–ce6, and P<0.001 compared to control). Histological examination showed eyes sealed with BSA/JG have less inflammation present than untreated eyes at 7 days.
Conclusions
These data demonstrate that photodynamic laser activated tissue glues can be used to effectively seal corneal incisions in living animals without thermal damage or undue inflammation.
Keywords: Photodynamic therapy, tissue glue, laser activation, rat corneal incision, Janus Green
INTRODUCTION
Closing, sealing, and bonding wounds and defects in tissues remain problem areas in medicine. Alternative methods to the traditional mechanical means designed to close incisions, wounds, and anastomoses have received attention in the last several years. While sutures and surgical staples remain the gold standard of closure techniques, there are many situations where they have major disadvantages. Sutures are labor intensive (especially laparoscopically and in microsurgery), and can lead to infection, and scarring. Particularly for closure of delicate tissues, such as ophthalmic wounds, primary closure with sutures can lead to unavoidable issues with tissue warping that can have deleterious effects on vision. Staples are much less labor-intensive than sutures but can lead to scarring and uneven healing. Neither of these techniques can easily close a defect in which the edges cannot be evenly opposed or where the tissues are extremely sensitive such as the eyes. Therefore much work has gone into the discovery of novel tissue sealants, adhesives, and glues [1]. These alternatives can be divided into four classes.
Firstly, cyanoacrylate glues have had widespread use among the surgical specialties [2]. Although surgeons have used cyanoacrylates for over 20 years, these adhesives continue to cause concerns over tissue necrosis and cytotoxicity [3]. Secondly, fibrin sealants have been developed as tissue adhesives that “mimic” the body’s own natural clotting mechanisms [4]. Because fibrin sealant is derived from blood products, it may be associated with viral-disease transmission [5]. Thirdly, is the group of products such as gelatin–resorcinol–formol glue. This adhesive is formed from gelatin, resorcinol, and distilled water in the presence of formaldehyde, glutaraldehyde, and heat [6]. Toxicity issues and the fact that the glue cannot be used in a wet environment have dampened enthusiasm for the product [7]. Another glue in this class is albumin cross-linked with glutaraldehyde, known as Bio-Glue that has been used to seal dissections in vessels such as the aorta [8]. This has reported problems with vascular stenosis [9] and inflammation [10]. Fourthly, is a miscellaneous group of light-activated tissue adhesives and bonding technologies. Hydrogels are a class of synthetic tissue adhesives that, in general, are applied to the site as liquids and then polymerize into solids in the presence of light [11]. In preclinical use, this type of sealant has also been shown to be effective as a hemostatic adjunct to prevent anastomotic bleeding and to seal other types of closure such as the dura, pancreatic stump, or open wounds. Various dye-activated protein solders have been investigated as well. These rely on the addition of dyes to proteins (usually albumin) to absorb light and produce local heating. Dyes that have been used include indocyanine green [12], methylene blue [13], and fluorescein [14]. There is a technique, that does neither need a solder nor a glue, known as photochemical tissue bonding that uses the dye Rose Bengal without exogenous proteins to form direct covalent bonds between collagen surfaces [15,16].
The ideal light-activated tissue glue for use in the eye will form a strong bond without producing heat, as delicate tissues such as the cornea do not have a great tolerance for thermal damage. We previously showed that photodynamically active dyes could be used to initiate the bonding process and proposed that the reactive oxygen species and radicals produced by the interaction between the excited dye molecules and oxygen could lead to the formation of covalent protein cross-links [17]. We reported on chlorin e6 (ce6) as a dye capable of producing light-activated strong bonds in cadaver scleral wounds when formulated with albumin as a covalent conjugate [18]. In the present report, we investigate and compare the in vivo characteristics of photodynamic light-activated tissue glues (PLATG) formulated with two different dyes; ce6 and Janus Green (JG), another dye capable of photodynamic reactions. The dye–protein mixture in the glue may also be varied, as reported in a previous article [18]. Although it is relatively easy to demonstrate tissue adhesion ex vivo, it is important to study the technology in living animals. Here, we are concerned not only with effective strength of the glue, but also to verify that the process does not injure the tissues or cause undue inflammation. We chose to test the glues by sealing freshly made penetrating corneal incisions in living rats and assessing leaking pressure at sacrifice at various times after wounding and by examining eyes for inflammation by routine histology.
MATERIALS AND METHODS
Preparation of Glues
The preparation of BSA–ce6 was essentially as described [18]. Briefly the N-hydroxy succinimide (NHS) ester of ce6 (Porphyrin Products, Logan, UT) was prepared by reacting 1.5 equivalents of dicyclohexylcarbodiimide and 1.5 equivalents of NHS with 1 equivalent of ce6 in dry DMSO for 24 hours). Bovine serum albumin (BSA, Sigma Chemical, St. Louis, MO) was dissolved in 0.1 M NaHCO3 buffer pH =9.3 at a concentration of 500 mg/ml (7.4 mM). Equivalents (1.2) of ce6–NHS ester in DMSO were added to the protein solution and the mixture allowed to stand overnight. The mixture was then dialyzed twice against 5 L of PBS to remove unconjugated ce6 and DMSO and provided a conjugate with a 1:1 substitution ratio. The conjugate then had one, two, or three times the amount of unconjugated BSA added and additional PBS to give an overall 50% w/w protein solution. JG (Aldrich Chemical, Milwaukee, WI) was dissolved in water to give a 2% w/w solution (25 mM accounting for 65% dye content). The albumin solution was prepared at 50% w/w in distilled water (7.4 mM) and sufficient JG solution was added to give concentrations of 7.4, 3.7, 2.5, and 1.9 mM (ratios of protein to dye of 1:1, 1:0.5, 1:0.33, 1:0.25). Glues were characterized by absorption spectroscopy after suitable dilution in 0.1 M NaOH/1% SDS [18].
Preliminary Testing of Glues
We first conducted ex vivo studies in order to test the appropriate consistency and viscosity of the glues as well as to compare the adhesive strength of the bonds produced by illuminating BSA–ce6 conjugate and BSA+JG mixture at different molar ratio of protein to dye. C57/BL6 mice were purchased from Charles River Laboratories (Wilmington, MA) and were used in a protocol approved by the Subcommittee on Research Animal Care of Massachusetts General Hospital. The animals were later euthanized by carbon dioxide inhalation in accordance with the recommendations of 2000 Report of the AVMA Panel on euthanasia and then the skin was dissected with a no. 10 blade and instantly placed in buffered 10% formaldehyde to avoid desiccation. First, skin was cut into thin strips (3 cm long×0.5 cm wide) and two pieces were held end-to-end by clips so that the gap where the glue would be applied did not touch any surface. The two pieces of skin were in gentle contact and the gap between the two pieces varied from 0 to 0.3 mm depending on the minor irregularities of the skin edge. We applied the glue(approximately50–100μl)between the two opposed ends of the strips using a 1 ml syringe and a 18-gauge needle. Then the area was illuminated with the appropriate wavelength laser (665 nm 1 W-diode laser (High Power Devices, Inc., North Brunswick, NJ) at different fluences(109;87.3;65.5; and32.5J/cm2)with an irradiance of 181 mW/cm2. Once the two ends had stuck together, the strength of this union was measured by placing the samples in the tensiometer (Chatillon TCD 200, Commercial Scale Co., Inc., Agawam, MA). This machine holds the ends of the tissue in clamps and exerts an increasing force until the tissue separates and reads the force exerted in Newtons. We repeated the experiments with different ratios of the different glue preparations.
Rat Model of Penetrating Corneal Wound
The protocol was reviewed and approved by the Schepens Eye Research Institute Animal Care and Use Committee and was in compliance with NIH and Association for Research in Vision and Ophthalmology guidelines. Only one eye of each animal was operated upon. Sprague Dawley (Charles River) rats weighing approximately 350 g were obtained. The rats were anesthetized using ketamine (80 mg/kg) and xylazine (12/mg/kg) by injection in the peritoneal cavity. Immediately prior to surgery, mydriasis was achieved in the experimental eye by placing two drops of cyclopentolate 1% and phenylephrine 10%. In order to reduce the flow of aqueous across the wound, timolol 0.5% was also placed on the eye. Immediately prior to surgery, the topical local anesthetic proparacaine was placed on the eye. Under a surgical microscope, traction sutures using 6-0 silk were placed through conjunctiva/Tenon’s capsule at 3:00 and 9:00 o’clock and used to prolapse and secure the globe. The corneal epithelium was removed using a Grieshaber scarifier blade. A diamond knife was set to 300 μM and used to make a 6-mm incision in the center of the cornea. The full-thickness of the incision was ensured by placement of one blade of a Vannas scissors through the wound to ensure complete perforation for the extent of the wound. For the control group, the procedure ended here by removing the traction sutures, injecting a sub-Tenon’s depot of the anti-inflammatory steroid, dexamethasone (0.3 ml), and irrigating the wound.
For the experimental groups, one of the two formulations of PLATG (BSA+BSA–ce6, and BSA+JG both at 1:0.33 protein to dye ratio) was applied to the wound using a 1-ml syringe and a 18-gauge needle. Using the tip of the needle, approximately 50–100 μl of PLATG was placed between the wound edges, but not injected into the anterior chamber. A 665 nm laser (BW-Tek Laser Systems, Baltimore, MD) operating at 300 mW and giving a 4-mm diameter spot size, was applied to the wound in a continuous back and forth motionforapproximately2minutes. Allowing for the average dwell time of the light to be approximately 25%on any area of the glue, the total fluence delivered was 71.5 J/cm2 at an irradianceof600mW/cm2. Any area of the wound found to be leaking aqueous underwent further glue and laser application as outlined above. The silk sutures were removed, a sub-Tenon’s depot of the anti-inflammatory steroid, dexamethasone (0.3 ml) placed, and the wound irrigated.
The eyes of the rats were photographed using a slit-lamp biomicroscope (Model 900, Haag-Streit AG, Koeniz, Switzerland) and sacrificed at 1, 7, and 14 days following the treatment. The treated eyes were enucleated with forceps and scissors (to cut muscles and optic nerve) and randomly divided into two groups for measuring of leaking pressure and histopathological examination.
Leaking Pressure
In order to measure leaking pressures, an 18-gauge butterfly needle was connected via plastic tubing to a water bottle. The inner pressure in the bottle was controlled by a hand-pumped sphygmomanometer. The butterfly needle was inserted in the vitreous cavity through equatorial sclera, and the sphygmomanometer was increasingly pressurized in ~10-mmHg increments. Leaking pressure was recorded the moment the wound leaked air or fluid.
Histopathology
A 6-0 silk suture was placed at the limbus of each enucleated eye to mark the end of the incision. The eyes were fixed in formalin, cross-sectioned perpendicular to the wound, and stained using hematoxylin and eosin.
RESULTS
Glue Formulation
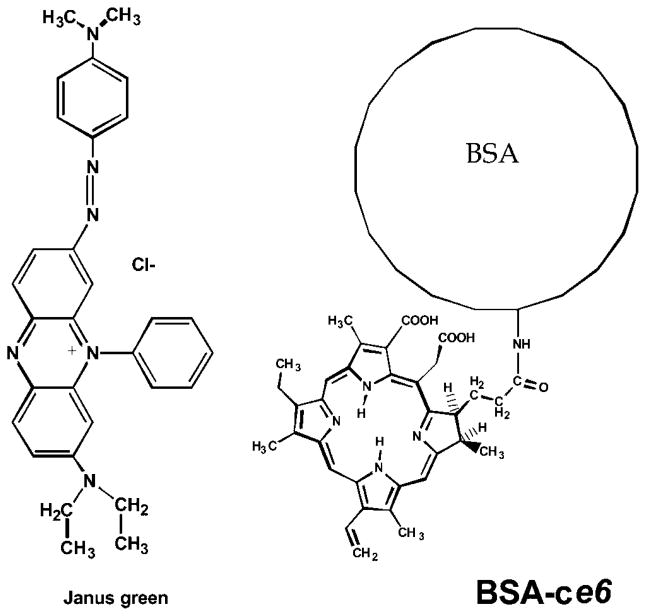
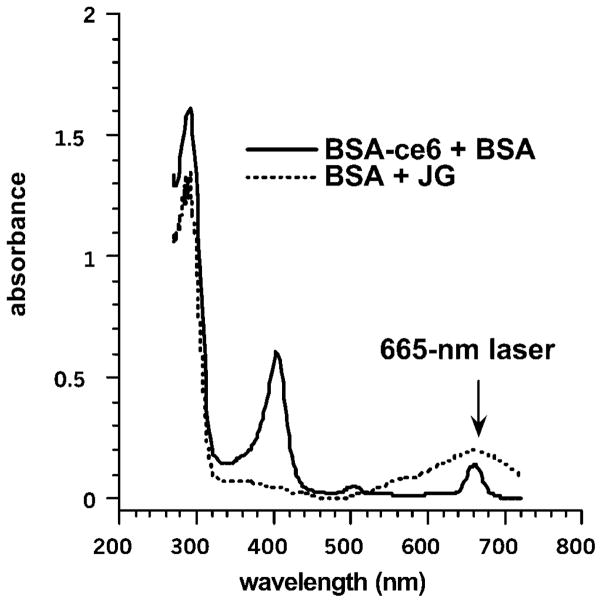
The chemical structures of BSA–ce6 and JG are shown in Figure 1. The spectra of the glues prepared at a protein:dye ratio of 1:0.33 are shown in Figure 2 illustrating the wavelength of the red laser used to activate the dyes.
Fig. 1.
Chemical structures of the photodynamic light-activated tissue glues (PLATG) preparations: Janus Green (JG) dye and bovine serum albumin (BSA)–chlorin e6 (ce6) conjugate.
Fig. 2.
Absorption spectra of the PLATG preparations. Mixtures with protein:dye ratios of 1:0.33 were dissolved in 0.1 M NaOH at a concentration of 5 μM equivalent dye.
Experiments on Glue Formulation
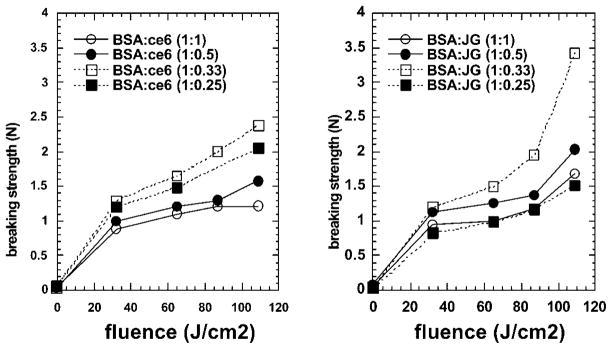
The effect that the protein:dye ratio had on the strength of the bonds when BSA–ce6 was mixed with BSA was observed previously [18]. In those experiments the conjugate (BSA:ce6 =1:1) with added unconjugated BSA (to make BSA:ce6 =4:1) was shown to give significantly higher breaking strength than conjugate or mixtures with lower BSA:ce6 ratios. In the present work, we undertook a preliminary series of experiments to determine the optimum ratio of BSA to JG and sought to confirm our previous finding with BSA–ce6+BSA. In contrast to the previous study [18] that used cadaver eyes with scleral incisions sealed using Argon laser light (488 and 514-nm), in the present case we used strips of mouse skin and 665-nm diode laser light and tested the breaking strength with a tensiometer. The results are shown in Figure 3. The strips were bonded edge to edge and the surface area of each edge was approximately 4 mm2 (5-mm long by 0.8-mm thick). The breaking strength measured of 2.5 N was therefore equal to 62.5 N/cm2.
Fig. 3.
Ex vivo strength skin of glued skin strips. Strips of mouse skin were glued with the designated ratio of protein to dye with the designated fluence and immediately the breaking strength was measured by tensiometer.
The BSA–ce6 based glue was most effective at a ratio of 1 protein to 0.33 dye, followed by the 1:0.25 ratio and the least strong bond (but nevertheless effective) was produced by the 1:1 and 1:0.5 ratios (Fig. 3a). The JG based glue gave overall stronger bonds than the corresponding ce6 based glues. Again the 1:0.33 ratio gave the strongest bonds followed by the 1:0.5 with the other ratios slightly less strong (Fig. 3b). In all cases the strength of the bonds was directly proportional to the delivered fluence as might be expected for a photochemical process.
Clinical Outcome
The rats showed no acute intraoperative or postoperative complications such as collapse of the anterior chamber, bleeding/hyphema, etc. After the first day, there were no eyelid adhesions and all lid movements were normal. There was no excessive tearing. On many eyes, the glue was still present by the 1 day (Fig. 4A) and traces were occasionally observed at the 7-day examinations (Fig. 4B), but was always gone by the 14-day exam (Fig. 4C). There was no conjunctival inflammation or hyperemia by the 7-day point. Figure 4 shows examples of the slit-lamp photographs of the glued eyes at the 1, 7, and 14 day time points. There was no significant differences observed between eyes glued with BSA+JG and with BSA+BSA–ce6.
Fig. 4.
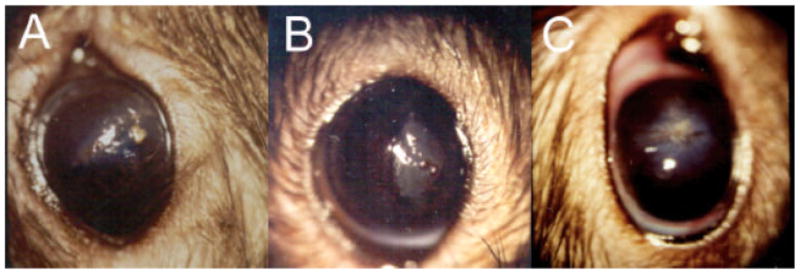
Slit lamp photographs of rat eyes glued with JG formulation at (A) 1-day; (B) 7 days; and (C) 14 days post wounding. [Figure can be viewed in color online via www.interscience.wiley.com.]
Leaking Pressure
Information on the relative strengths of the wounds sealed with the PLATG or left untreated was obtained by using a pressurized system to inflate the enucleated eyes with water until the wound either burst or leaked. The results are shown in Table 1. In the eyes which were taken from rats sacrificed 24 hours after wounding we see that those eyes that had wounds closed with both BSA–ce6 and JG PLATG had leaking pressure significantly higher than control eyes, and the eyes sealed with JG had significantly higher leaking pressures than those sealed with BSA–ce6. At 1 week after wounding, the leaking pressures of the PLATG sealed eyes had fallen to match the pressures of control eyes as the protein glues biodegraded. At 2 weeks after wounding, the incisions had healed and the leaking pressures of all the eyes were similar and higher than the capacity of the system to measure.
TABLE 1.
Leaking Pressure of Eyes (in mmHg) Removed at Designated Times After Treatment
| Control | Chlorin e6 (ce6) | Janus Green (JG) | |
|---|---|---|---|
| 1 day | 193 ± 6 (n =4) | 357 ± 25* (n =4) | 430 ± 36**,† (n =4) |
| 1 week | 232 ± 47 (n =3) | 200 ± 26 (n =3) | 220 ± 30 (n =3) |
| 2 weeks | >500 (n =3) | >500 (n =3) | >500 (n =3) |
P <0.01 versus control.
P <0.001 versus control.
P <0.05 versus ce6.
Histopathology
Enucleated eyes were fixed in formalin and processed for standard hematoxylin and eosin histology. Figure 5 illustrates the typical findings. In panel (i) the untreated wound at 1-day post wounding shows an immature gaping wound with significant peri-inciscional inflammation, more than 50% neutrophils. There are also some inflammatory cells including neutrophils present in the anterior chamber. In contrast very few neutrophils are found in the subepithelial area away from the site of the incision. Panel (iv) shows a JG glue treated wound at 1 day post-treatment. There is a moderate inflammatory infiltrate present in the subepithelial area of the cornea with a significantly diminished degree of infiltration in the mid and deep layers. The inflammation is divided equally between neutrophils and mononuclear cells. The wound with overlying hardened glue could easily be observed. Other than this anterior synechiae, there was a mild degree of exudate and very few inflammatory cells in the anterior chamber. Panel (vii) shows the ce6 glue at 1 day after treatment. Moderate amounts of glue were present above the corneal epithelium and in the anterior chamber against the lens capsule. There was recruitment of neutrophils in the subepithelial area throughout the cornea and intense stroma edema at the sides of the wound. Overall, there were significantly more neutrophils in the peri-insicional areas and in the stroma as compared to JG glue. No inflammatory cells were seen in the anterior chamber.
Fig. 5.
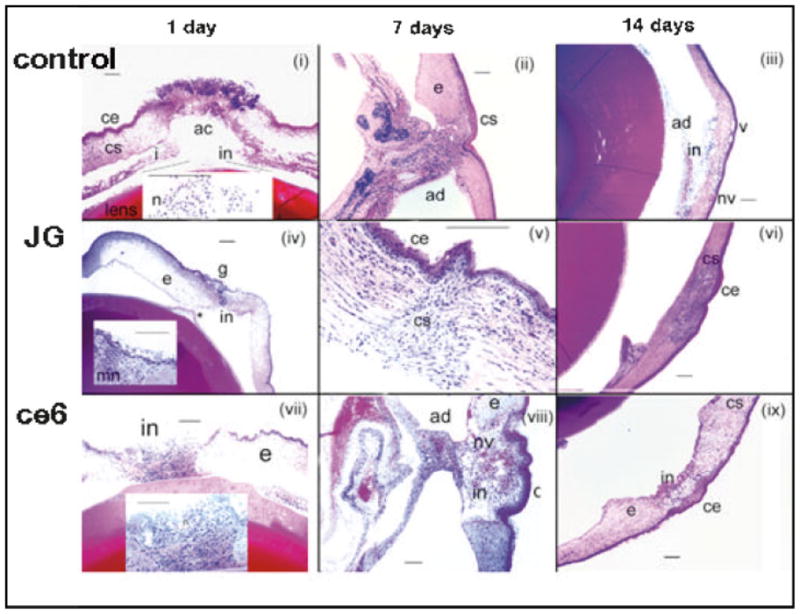
Histopathology of eyes. Panels (i–iii) are of control unglued eyes, panels (iv–vi) are of JG glued eyes, and panels (vii–ix) are of ce6 glued eyes. Panels (i), (iv), and (vii) are at 1 day after wounding; panels (ii), (v), and (vii) are at 7 days after wounding. Panels (iii), (vi), and (ix) are at 14 days after wounding. In all cases the scale bar is 200 μm. Abbreviations are: ac, anterior chamber; ad, adhesion; ce, corneal epithelium; cs, corneal stroma; e, edema; g, glue; i, iris; in, inflammation; mn, mononuclear cells; n, neutrophils; nv, neovascularization; v, vacuoles. [Figure can be viewed in color online via www.interscience.wiley.com.]
At 1 week after wounding the control wound in panel (ii) shows stromal edema with a moderate mixed cell infiltrate around the site of incision. There is epithelial bridging and a moderate amount of inflammation, mostly neutrophils, in the anterior chamber and the stroma is also heavily infiltrated at the site of the bridging. The epithelium is normal, and in fact absent in some areas. The posterior surface is covered with macrophages and neutrophils with a lot of disorganization in the stromal lamellae and a break in Descemet’s membrane. The 1-week JG glued eye (detail in panel v) shows epithelial healing over the area of the penetrating wound. A few inflammatory cells, mostly neutrophils with a few macrophages, were present around the area of the wound. The wound shows significant maturation, especially anteriorly. There is no ongoing inflammatory stimulus at the wound for inflammatory cell recruitment and the anterior chamber is devoid of inflammatory cells. The 1-week ce6 glued wound (panel viii) showed more stromal edema than at 1 day. Epithelial and anterior stroma bridging were found. Inflammation and neutrophils were seen in the stroma, mostly in the anterior and mid stroma and a significant neovascularization had taken place in the corneal healing.
The control wound at 2 weeks (panel iii) shows a mature wound with very significant corneal neovascularization. Although neovascularization is necessary for wound healing, an excessive amount can diminish corneal function. There is iris adherent to the posterior aspect of the wound, a large area without Descemet’s, a few inflammatory cells in the cornea, and none in the anterior chamber. There is some disorganization and scarring in the cornea. The JG glued wound at 2 weeks showed a mature wound with fibrous scar tissue deposition found at the wound site and hyperplasia of the overlying epithelial cells. There were very few inflammatory cells in the stroma with primarily keratocytes and a few mononuclear inflammatory cells being found. No neutrophils were observed and the anterior chamber was quiet. The ce6 wound at 2 weeks (panel ix) showed very few inflammatory cells, the wound was completely closed, normal on the surface, with excellent epithelial, stromal, and even endothelial cell layers. The anterior chamber is perfectly quiet. Scar tissue filled in the majority of the depth of the incision.
The adhesions seen in panels (ii), (iii), and (viii) can be a sign that the wound was “gaping” at a critical stage during the healing process. This has the effect of forcing the cornea back against the iris and with the inflammatory process involved in healing allows adhesions to form.
DISCUSSION
This study has shown that PLATG is able to effectively and safely seal corneal incisions in living rats. The data from the leaking pressure measurements and histopathology studies respectively support the effective strength and the non-toxic nature of the glues. Although varying amounts of inflammation were present, this was not excessive in the glued wounds and in some cases was less than in the unglued control wounds. It should be noted that inflammation is a normal consequence of the healing process. The measurements of bond strength clearly showed that the corneal wounds sealed with the JG formulation had a greater adhesive strength than those wounds sealed with ce6 formulation, and both glues were significantly better than control (untreated) wounds. Furthermore, the glues were biodegraded by week 1 as expected, and corneal healing was equal in all eyes after 2 weeks. The leaking pressure measured in control wounds at day 1 was 193 mmHg showing that to some extent the wounds were self-sealing. The fact that the leaking pressures in the PLATG treated eyes dropped to match control eyes at the 1 week time point showed that the beneficial effects of the increased strength of the seal was only temporary. Further work could involve looking at time points between 1 day and 1 week to determine the time course of the loss of strength in the glued wounds. However, the lower incidence of adhesions and neovascular scar formation in the glued eyes did suggest that the glue might reduce the “gaping” effect of an unsealed wound. It is possible that a wound could be both “gaping” and self-sealing at the same time. This could happen if the rear aspect of the wound sealed and this forced the front edges apart to produce a gaping effect. In addition the PLATG might provide an addition clinical benefit in that the seal would exclude bacteria and particulate matter from the open wound. It remains to be seen what effect this procedure would have on vision.
JG, a novel dye not hitherto used for tissue welding applications, was shown to give superior performance compared to a covalent conjugate of ce6. Despite JG having shown promising results in our experiments, we conclude that it still needs further study as a potential PLATG component. This cationic phenazine azo-dye has been used as a photosensitizer in a series of experiments on micro-irradiation of vitally stained single cells [19,20]. More recently it has been used in ophthalmology as a vital stain to quantify toxicity to the corneal endothelium [21]. In addition, due to its mitochondrial localization it has been proposed as an anti-malarial compound [22]. Nevertheless, it has not to our knowledge been used in any chromophore-assisted tissue glue or welding application. Studies will need to be conducted to measure to what extent the dye is absorbed by cells and tissue when topically applied and to verify its lack of long-term toxicity or ill effects.
The results of the leaking pressure measurements are very similar to those found by us in previous work [18] where BSA–ce6 based glue was used to seal corneal wounds in cadaveric human eyes (375-mmHg immediately afterwards), compared to the present study using wounds in living rat eyes (350-mmHg 1 day after). Our group has previously shown that a light-activated glue composed of riboflavin-6-phosphate and fibrinogen gave a somewhat lower strength seal in cadaver eyes (260 mmHg) [17] and this preparation was used to seal incisions in eyes of living rabbits with satisfactory bonding and non-inflammatory outcome [23,24]. Fibrinogen, as the exogenous protein, however, is problematical in that it needs to be isolated from human plasma with the accompanying risk of virus transmission. Photochemical keratodesmos (PKD) is a technique that employs the dye Rose Bengal to directly bond collagen surfaces and has also shown good results in terms of bonding strength (254-mmHg), but once again, those studies were performed in enucleated eyes [16]. Moreover, in contrast to PLATG, which can also be used to fill irregular wound gaps, close contact of the wound edges is imperative for PKD to be successful. The high irradiances used with the argon laser and Rose Bengal [16] increased the likelihood of thermal damage to the cornea with values of 2.55 and 3.82 W/cm2 being reported as problematical.
The present results suggest that good seals can be obtained using PLATG without the requirement of high irradiance (only 600 mW/cm2), which reduces considerably the risk of thermal damage to the tissue (no evidence of thermal damage in any of our histopathology studies). In general photochemical processes depend on fluence and not on irradiance, while photothermal processes depend on irradiance rather than fluence. The irradiance of 600 mW/cm2 is exactly the same as the value used for delivering 690-nm light during photodynamic therapy (PDT) mediated by Visudyne for choroidal neovascularization caused by wet macular [25]. However, in the case of PDT the light is directly focused onto the retina through the pupil, while in the present case of PLATG on the corneal surface only a small portion of light directed to the glue would be expected to reach the retinal surface and thus the risk of retinal damage caused by this procedure should be small.
Besides the strong bonds that can be obtained with PLATG, there are other relevant advantages of this technique such as its easy biodegradability (not found in alternative products such as cyanoacrylates) and its use of a readily available non-infective protein substrate (not found with Fibrin sealants). Moreover, its non-inflammatory nature and its apparent lack of cell toxicity suggest that PLATG could also be preferable to cyanocrylates and gelatin–resorcinol–formol glues.
In a similar fashion to findings in our previous report [18], we found that we needed to increase the ratio of protein to dye in our ex vivo skin gluing experiments in order to obtain maximum bond strength. While the explanation for this finding is by no means obvious, we suggest that effectively decreasing the dye concentration in the mixture while maintaining the protein concentration at levels necessary to keep its viscosity high, had the effect of allowing the light to penetrate deeper into what was otherwise an optically dense material. This deeper penetration of light would make the glue set all the way through and maximize the bond strength. Still many questions remain to be addressed in this photodynamic tissue glue procedure. For example, can the oxygen-dependent photochemical processes that generate covalent cross-links between albumin and collagen be demonstrated? Can PLATG form tissue seals without harming the surrounding living cells?
Wound sealing using photodynamic processes is an attractive concept as an alternative to traditional closure techniques such as sutures, staples, and clips especially in those defects in which the edges cannot be apposed or where the tissues are sensitive to even slight mechanical deformation as can be induced by sutures, such as ocular tissues. This innovative technique has been shown to be efficacious, non-thermal, non-toxic, and non-inflammatory; all highly advantageous features. However, much more work needs to be carried out before any clinical application could be considered. This should include testing the glues in a non self-sealing wound model in vitro or in vivo. This may determine how important a strong but relatively short-lasting bond is in wound closure. It is possible that in the cornea a less strong bond such as that provided by fibrin glue might perform as well as PLATG. The fact, however, that such strong bonds can be obtained in the eye encourages further studies on the application of this technology to other disciplines that encounter delicate tissues or involve microsurgery such as orthopedic or dermatologic applications.
Acknowledgments
We thank Tayyaba Hasan for support for this project.
Contract grant sponsor: NIH; Contract grant number: R01-CA/AI838801.
Footnotes
JK and MRH have disclosed potential conflicts of interests with this study.
References
- 1.Charters A. Wound glue: A comparative study of tissue adhesives. Accid Emerg Nurs. 2000;8:223–227. doi: 10.1054/aaen.2000.0168. [DOI] [PubMed] [Google Scholar]
- 2.Tseng YC, Hyon SH, Ikada Y, Shimizu Y, Tamura K, Hitomi S. In vivo evaluation of 2-cyanoacrylates as surgical adhesives. J Appl Biomater. 1990;1:111–119. doi: 10.1002/jab.770010203. [DOI] [PubMed] [Google Scholar]
- 3.Toriumi DM, Raslan WF, Friedman M, Tardy ME. Histotoxicity of cyanoacrylate tissue adhesives. A comparative study. Arch Otolaryngol Head Neck Surg. 1990;116:546–550. doi: 10.1001/archotol.1990.01870050046004. [DOI] [PubMed] [Google Scholar]
- 4.Sierra DH. Fibrin sealant adhesive systems: A review of their chemistry, material properties, and clinical applications. J Biomater Appl. 1993;7:309–352. doi: 10.1177/088532829300700402. [DOI] [PubMed] [Google Scholar]
- 5.Clark RA. Fibrin glue for wound repair: Facts and fancy. Thromb Haemost. 2003;90:1003–1006. doi: 10.1160/TH03-08-0526. [DOI] [PubMed] [Google Scholar]
- 6.Albes JM, Krettek C, Hausen B, Rohde R, Haverich A, Borst HG. Biophysical properties of the gelatin–resorcin–formaldehyde/glutaraldehyde adhesive. Ann Thorac Surg. 1993;56:910–915. doi: 10.1016/0003-4975(93)90354-k. [DOI] [PubMed] [Google Scholar]
- 7.Nomori H, Horio H, Suemasu K. The efficacy and side effects of gelatin–resorcinol formaldehyde–glutaraldehyde (GRFG) glue for preventing and sealing pulmonary air leakage. Surg Today. 2000;30:244–248. doi: 10.1007/s005950050053. [DOI] [PubMed] [Google Scholar]
- 8.Passage J, Jalali H, Tam RK, Harrocks S, O’Brien MF. BioGlue surgical adhesive—An appraisal of its indications in cardiac surgery. Ann Thorac Surg. 2002;74:432–437. doi: 10.1016/s0003-4975(02)03689-5. [DOI] [PubMed] [Google Scholar]
- 9.Economopoulos GC, Dimitrakakis GK, Brountzos E, Kelekis DA. Superior vena cava stenosis: A delayed BioGlue complication. J Thorac Cardiovasc Surg. 2004;127:1819–1821. doi: 10.1016/j.jtcvs.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 10.Erasmi AW, Sievers HH, Wolschlager C. Inflammatory response after BioGlue application. Ann Thorac Surg. 2002;73:1025–1026. doi: 10.1016/s0003-4975(01)03524-x. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney T, Rayan S, Warren H, Rattner D. Intestinal anastomoses detected with a photopolymerized hydrogel. Surgery. 2002;131:185–189. doi: 10.1067/msy.2002.119492. [DOI] [PubMed] [Google Scholar]
- 12.Zuger BJ, Ott B, Mainil-Varlet P, Schaffner T, Clemence JF, Weber HP, Frenz M. Laser solder welding of articular cartilage: Tensile strength and chondrocyte viability. Lasers Surg Med. 2001;28:427–434. doi: 10.1002/lsm.1070. [DOI] [PubMed] [Google Scholar]
- 13.Birch JF, Mandley DJ, Williams SL, Worrall DR, Trotter PJ, Wilkinson F, Bell PR. Methylene blue based protein solder for vascular anastomoses: An in vitro burst pressure study. Lasers Surg Med. 2000;26:323–329. doi: 10.1002/(sici)1096-9101(2000)26:3<323::aid-lsm11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Poppas DP, Mininberg DT, Hyacinthe L, Spencer JR, Schlossberg SM. Patch graft urethroplasty using dye enhanced laser tissue welding with a human protein solder: A preclinical canine model. J Urol. 1993;150:648–650. doi: 10.1016/s0022-5347(17)35573-8. [DOI] [PubMed] [Google Scholar]
- 15.Chan BP, Kochevar IE, Redmond RW. Enhancement of porcine skin graft adherence using a light-activated process. J Surg Res. 2002;108:77–84. doi: 10.1006/jsre.2002.6516. [DOI] [PubMed] [Google Scholar]
- 16.Mulroy L, Kim J, Wu I, Scharper P, Melki SA, Azar DT, Redmond RW, Kochevar IE. Photochemical keratodesmos for repair of lamellar corneal incisions. Invest Ophthalmol Vis Sci. 2000;41:3335–3340. [PubMed] [Google Scholar]
- 17.Khadem J, Truong T, Ernest JT. Photodynamic biologic tissue glue. Cornea. 1994;13:406–410. doi: 10.1097/00003226-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Khadem J, Veloso AA, Jr, Tolentino F, Hasan T, Hamblin MR. Photodynamic tissue adhesion with chlorin(e6) protein-conjugates. Invest Ophthalmol Vis Sci. 1999;40:3132–137. [PubMed] [Google Scholar]
- 19.Baba K. Selective injury of mitochondria with Janus Green B and ruby laser light—Enzyme morphological and ultrastructural study. Acta Pathol Jpn. 1970;20:59–78. doi: 10.1111/j.1440-1827.1970.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 20.van Duijn C., Jr Photodynamic effects of vital staining with diazine green (Janus Green) on living bull spermatozoa. Exp Cell Res. 1961;25:120–130. doi: 10.1016/0014-4827(61)90313-5. [DOI] [PubMed] [Google Scholar]
- 21.Dua HS, Benedetto DA, Azuara-Blanco A. Protection of corneal endothelium from irrigation damage: A comparison of sodium hyaluronate and hydroxypropylmethylcellulose. Eye. 2000;14(Pt 1):88–92. doi: 10.1038/eye.2000.19. [DOI] [PubMed] [Google Scholar]
- 22.Vennerstrom JL, Makler MT, Angerhofer CK, Williams JA. Antimalarial dyes revisited: Xanthenes, azines, oxazines, and thiazines. Antimicrob Agents Chemother. 1995;39:2671–2677. doi: 10.1128/aac.39.12.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goins KM, Khadem J, Majmudar PA, Ernest JT. Photodynamic biologic tissue glue to enhance corneal wound healing after radial keratotomy. J Cataract Refract Surg. 1997;23:1331–1338. doi: 10.1016/s0886-3350(97)80111-3. [DOI] [PubMed] [Google Scholar]
- 24.Goins KM, Khadem J, Majmudar PA. Relative strength of photodynamic biologic tissue glue in penetrating keratoplasty in cadaver eyes. J Cataract Refract Surg. 1998;24:1566–1570. doi: 10.1016/s0886-3350(98)80343-x. [DOI] [PubMed] [Google Scholar]
- 25.Michels S, Schmidt-Erfurth U. Sequence of early vascular events after photodynamic therapy. Invest Ophthalmol Vis Sci. 2003;44:2147–2154. doi: 10.1167/iovs.02-0604. [DOI] [PubMed] [Google Scholar]