Abstract
Burn injury disrupts the mechanical and biological barrier that the skin presents against infection by symbionts like the Pseudomonas aeruginosa, a Gram-negative bacteria. A combination of local factors, antimicrobial peptides, and resident effector cells form the initial response to mechanical injury of the skin. This activity is followed by an inflammatory response that includes influx of phagocytes and serum factors, such as complement and mannose-binding lectin (MBL), which is a broad-spectrum pattern recognition molecule that plays a key role in innate immunity. A growing consensus from studies in humans and mice suggests that lack of MBL together with other comorbid factors predisposes the host to infection. In this study we examined whether MBL deficiency increases the risk of P. aeruginosa infection in a burned host. We found that both wild-type and MBL null mice were resistant to a 5% total body surface area burn alone or s.c. infection with P. aeruginosa alone. However, when mice were burned then inoculated s.c. with P. aeruginosa at the burn site, all MBL null mice died by 42 h from septicemia, whereas only one-third of wild-type mice succumbed (p = 0.0005). This result indicates that MBL plays a key role in containing and preventing a systemic spread of P. aeruginosa infection following burn injury and suggests that MBL deficiency in humans maybe a premorbid variable in the predisposition to infection in burn victims.
The successful restriction of early infection is achieved by a well-orchestrated cooperative action of a network that intertwines overlapping and circulating molecules and cell-associated receptors. These include TLRs, lectin-like receptors, serum antimicrobial molecules, natural Abs, complement proteins, and collectins that include mannose-binding lectin (MBL),5 also known as mannose-binding protein or mannan-binding lectin (1–3). MBL is a pattern recognition molecule that recognizes certain carbohydrate moieties common to certain Gram-positive and Gram-negative bacteria, fungi, viruses, protozoa, and apoptotic cells (4–9). MBL, upon recognition of infectious agents, activates complement via the Ab independent lectin pathway where the MBL-associated serine protease-2 cleaves C4 and C2 (10, 11). This action generates the C3 convertase, C4bC2a, which is a critical step in the production of the downstream complement effector molecules. The skin is the largest and important organ of the innate immune system and provides a physical barrier consisting of epidermis and dermis. The former is packed with keratinocytes that make up a mechanical barrier whereas the latter contains capillaries and a variety of immune cells including Langerhans cells, dendritic cells, and macrophages. When skin is damaged by burn injuries the mechanical barrier is ruptured resulting in a complex interplay between pro- and anti-inflammatory responses that create an ideal milieu for bacterial invasion (12–17). The local inflammatory responses include a dynamic response by epithelial cells and keratinocytes, which modulates antimicrobial peptide gene expression (18) and orchestrate the diapedesis of neutrophils. These reactions appear to play a critical role in the local production of a variety of biologically active molecules, including proteases and metalloproteases that each play important roles in wound healing (19, 20). Other local inflammatory responses also increase endothelial cell permeability and leakage of plasma into tissue to form edema and blisters (21, 22). The edema provides a nutrient-rich environment for local bacterial growth and increases risk of bacterial escape into the blood stream leading to systemic infection (12, 14–17). We postulated that MBL, a serum protein, might play a key role as an innate immune molecule able to limit the systemic spread of locally infecting pathogens in burned tissue. This idea was informed by the knowledge that MBL is deployed quickly to sites of infection as has been shown in the lung (23) in addition to our previous work demonstrating that MBL null mice are highly susceptible to infection with Staphylococcus aureus (24) and HSV-2 (25).
In this study we investigated the roles of MBL against bacterial infection following a dry burn involving 5% of the total body surface area (TBSA). We chose P. aeruginosa because infection with this bacterium is a major cause of life-threatening complications in burn patients (16, 17, 26, 27). This opportunistic pathogen rarely infects immunocompetent hosts but exploits a weakened immune system caused by burns, cancer, AIDS, or other immunocompromising conditions (28, 29). We demonstrate that mice lacking both MBL-A and MBL-C (MBL null mice) are highly susceptible to postburn infection with P. aeruginosa. MBL has not previously been studied in association with thermal injuries, and our data raise the possibility that MBL deficiency may be a risk factor in burn patients.
Materials and Methods
Mice
MBL null mice were generated by crossing MBL-A null and MBL-C null mice as previously described (24). The MBL null and wild-type (WT) mice were maintained on a mixed background of 129Sv × C57BL/6J. Age- and gender-matched mice (8–10 wk of age) were used in each experiment. All animal experiments were performed under a protocol approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital (Boston, MA).
Burn model
One day before the burn, mice were anesthetized by i.p. injection of avertin (30 mg/kg; Sigma-Aldrich) to remove fur from the dorsal side with a hair removal lotion (Marzena). The following day the mice were anesthetized as described, and the hairless skinfold of the dorsal side was elevated. Two brass bars (1 cm2 in cross-section) were heated in boiling water and applied to compress the skinfold for 10 s, thus providing a dry, nonlethal full-thickness contact burn injury corresponding to ~5% of TBSA. Fluid replacement therapy was administrated immediately after the burn by i.p. injection of 1 ml of saline. The mice were placed on a heating pad (38°C) and kept under observation during the recovery period from anesthesia.
P. aeruginosa infection model
Bioluminescent P. aeruginosa, a modified strain 180 (no. 19660; American Type Culture Collection) (30), were cultured at 37°C in brain heart infusion media (Microbiology System; BD Diagnostic Systems) overnight, inoculated into fresh brain heart infusion media the following morning, and cultured for 3 h at 37°C to mid-log phase conditions. The bacteria were washed in saline and resuspended in saline to 2 × 105/ml (OD650 = 0.6, which corresponds to 1 × 108 CFU/ml in mid-log phase). The actual inoculation dose was determined by plating serial dilutions of the inoculum on tryptic soy agar plates (Sigma-Aldrich). Mice were anesthetized as described and inoculated s.c. with 2 × 104 P. aeruginosa in 100 μl per mouse at the middle of the burned skin. Survival was recorded subsequently.
Imaging
To record progression of the burn wound, digital photographs were taken immediately after the burn injury and serially every day. P. aeruginosa infection was recorded by the low light imaging system (Hamamatsu Photonics) that has been described elsewhere in detail (30). Briefly, it consists of an intensified charge-coupled device camera mounted in a light-tight specimen chamber, fitted with a light-emitting diode allowing a background grayscale image of the entire mouse to be captured. In the photon-counting mode, an image of the emitted light was captured at a maximum setting on the image-intensifier control module. Using ARGUS software the luminescence image was presented as a false-color image superimposed on top of the grayscale reference image. The image-processing component of the software gave mean pixel values from the luminescence images on defined areas within each burn on a 256-shade grayscale.
Reconstitution of MBL null mice with recombinant human MBL (rhMBL)
The rhMBL approved for human trials (as produced in a human cell line, HEK293 cells (31), and provided by NatImmune) was used to reconstitute MBL null mice. MBL null mice were injected i.p. with 75 μg of rhMBL in 0.2 ml of saline per mouse at 12 and 2 h before and 24 h after the burn injury.
Bacterial load in blood and organs
Mice were euthanized at 20 h after inoculation and burn because MBL null mice with postburn infection started to die ~23 h after bacterial inoculation. Subsequent cytokine analysis was also conducted at this time point for the same reason. Blood was collected by cardiac puncture and immediately mixed with heparin. Organs (liver, lung, kidney, and skin (burned skin and the surrounding edges of normal skin, 3 × 15 mm), were weighed and then homogenized in saline (0.5 ml for skin, lung, and kidney and 1 ml for liver) for bacterial counts, and supernatants were kept at −80°C for cytokine analysis. Serial dilutions of the blood and homogenates were plated on tryptic soy agar plates and colonies were counted after overnight incubation at 37°C. The results were calculated as CFU per milliliter for blood and CFU per gram of wet weight for organs (24).
Assay for bacterial growth in whole blood and plasma
The assay was performed as previously described (24). Briefly, blood was collected from MBL null and WT mice by cardiac puncture and mixed with hirudin. Human peripheral blood from an MBL-deficient healthy volunteer was also collected and mixed with hirudin. Whole blood or plasma (60 μl) of mice or 10% human blood diluted in saline was mixed with 40 μl of P. aeruginosa (1 × 103 CFU/ml) in saline. Samples were incubated at 37°C for 2 h then 10 μl from each tube was removed, serially diluted in saline containing 0.1% Triton X-100, and plated on tryptic soy agar plates. Colonies were counted after overnight incubation at 37°C. For a reconstitution experiment, MBL null mice were injected i.p. with 75 μg of rhMBL 2 h before collecting the blood. For the reconstitution of MBL-deficient human blood, rhMBL was used at 5 μg/ml.
MBL binding assays
P. aeruginosa (1 × 108 CFU) were incubated with 3 μl of WT mouse serum or MBL null mouse serum mixed with 30 ng of rhMBL in TBS (10 mM Tris-HCl (pH 7.4), 140 mM NaCl), 0.05% (v/v) Tween 20, 0.1% (w/v) NaN3, 5 mM Ca2+ with or without 100 mM mannose in a total volume of 300 μl. The samples were incubated at 4°C for 1 h followed by centrifugation at 7000 × g for 10 min to collect the supernatant. MBL-A and MBL-C in the WT mouse serum and the supernatant were quantified to determine total MBL-A and MBL-C and unbound MBL-A and MBL-C using assays previously described (32). rhMBL in the supernatant was determined to quantify unbound rhMBL as previously described (33). Bacterial binding was expressed as the percentage of bound MBL from the formula: ((recovered MBL in the supernatant)/(total MBL)) × 100. For ELISA type binding assay, microtiter wells were coated with P. aeruginosa (1 × 104 per well), incubated overnight at 4°C, blocked with 0.1% BSA in TBS for 1 h and subsequently incubated with 0.1 μg rhMBL/well in TBS, 0.05% Tween 20, and 5 mM CaCl2 with or without 100 mM mannose or 10 mM EDTA for 1 h at room temperature. Bound MBL was detected by 0.05 μg of mouse anti-human MBL Ab (clone 131–1) per well, followed by incubation with alkaline phosphatase-labeled anti-mouse Ab.
For FACS analysis, 1 × 106 P. aeruginosa (S. aureus as a control) were washed in HBSS (Invitrogen Life Technologies) and incubated with 20 μg of Cy3-labeled MBL for 10 min at room temperature (8). Binding of MBL was determined by FACS analysis (BD Biosciences).
Cytokine assays
Heparinized plasma and supernatants of organ homogenates were analyzed for TNF-α and IL-6 using commercially available ELISA kits (R&D Systems) according to the manufacturer’s instructions. For measuring cytokine responses after burn alone, blood and skin were collected at 20 h after burn for the reason described earlier.
Statistical analysis
Differences between groups were analyzed by the nonparametric Wilcoxon test (using the program JMP; SAS Institute). All experiments were repeated at least twice. Numbers of mice used in each experiment are indicated with figures.
Results
MBL null mice are highly susceptible to P. aeruginosa infection postburn
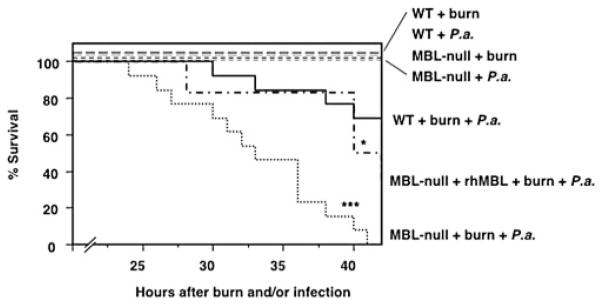
We examined the susceptibility of MBL null mice in an animal model of P. aeruginosa postburn infection. All WT and MBL null mice survived from burn injury alone (observed up to 3 wk) without showing any sign of illness, such as weight loss and ruffled fur. Likewise all unburned WT and MBL null mice survived from P. aeruginosa infection alone (s.c. inoculation of 2 × 104 CFU per mouse) with no sign of illness. However, when the mice were inoculated s.c. with P. aeruginosa (2 × 104 CFU per mouse) at the site of burn injury, all MBL null mice died within 42 h, compared with only 30% of the WT mice (p = 0.0005) (Fig. 1). Reconstitution of MBL null mice with rhMBL reversed survival to 50%, which was significantly improved (p = 0.03) compared with that of MBL null mice and was not different from that of WT mice (p = 0.24) (Fig. 1). This result indicated that the high mortality in MBL null mice was indeed due to the lack of MBL.
FIGURE 1.
Increased mortality in MBL null mice following P. aeruginosa (P.a.) infection after burn injury. WT, MBL null mice, and MBL null mice that were reconstituted with rhMBL were subjected to burn alone, infection alone, or infection postburn and survival was followed as described in Materials and Methods. WT (n = 13) mice, MBL null (n = 13) mice, and reconstituted MBL null (n = 6) mice for the infection postburn, WT and MBL null (n = 8) mice for burn alone, and WT and MBL null (n = 4) mice for infection alone were used. Two experiments were combined. ***, p < 0.001; *, p < 0.05.
MBL null mice have higher bacterial loads in blood and organs
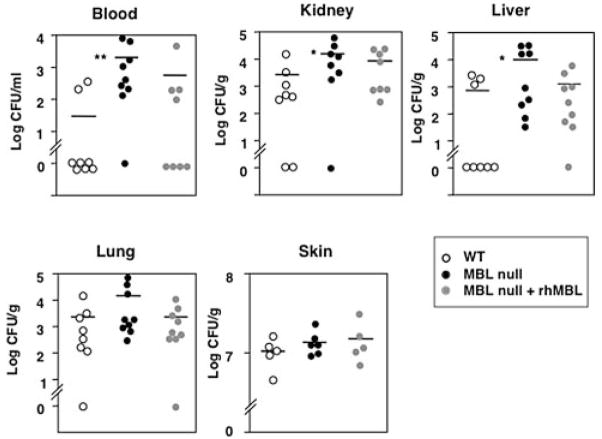
To investigate whether the high mortality was caused by increased infection we examined the state of infection by determining bacterial loads both locally and systemically. Bioluminescent P. aeruginosa were inoculated s.c. at the burn site and a low light imaging system recorded local infection. Images of infected skin lesions did not show differences in intensity and size between WT and MBL null mice, suggesting that there was no difference in local colonization (data not shown).
Bacterial load was determined in blood, liver, kidney, lung, and skin at 20 h after the postburn inoculation. Importantly, we found that the bacterial titer in the blood of MBL null mice was significantly higher (p = 0.004) than that of WT mice (Fig. 2). Like-wise, significantly elevated bacterial titers were also observed in kidney and liver (p = 0.045 and 0.033, respectively) of MBL null mice compared with that of WT mice (Fig. 2). The bacterial loads in lungs of MBL null mice were also elevated compared with that of WT mice, though this difference was not statistically significant. The elevated bacterial titers were significantly reduced in blood and kidneys (p = 0.03 for both) when MBL null mice were reconstituted with rhMBL and were not different in blood and kidneys (p = 0.33 and p = 0.1, respectively) from that of WT mice (Fig. 2). In contrast to the systemic infection, two independent methods, the observation by bioluminescent imaging of labeled bacteria (data not shown) and the colony counts (Fig. 2), indicated that there was no difference in local bacterial proliferation in the skin between MBL null and WT mice. Taken together these data suggested that MBL plays a role in inhibiting systemic spread of infection and subsequent seeding of distal organs rather than limiting the local infection at the site of inoculation.
FIGURE 2.
Increased bacterial load in MBL null mice at 20 h after burn injury and s.c. infection. Bacterial load was determined in blood and organ homogenates as described in Materials and Methods. Symbols represent individual mice for WT (n = 8), MBL null (n = 9), and reconstituted MBL null (n = 9) mice. For skin homogenate, WT (n = 5), MBL null (n = 6), and reconstituted MBL null (n = 5) mice were used. Bars indicate mean. **, p < 0.01; *, p < 0.05.
Whole blood of MBL null mice failed to restrict growth of P. aeruginosa in vitro
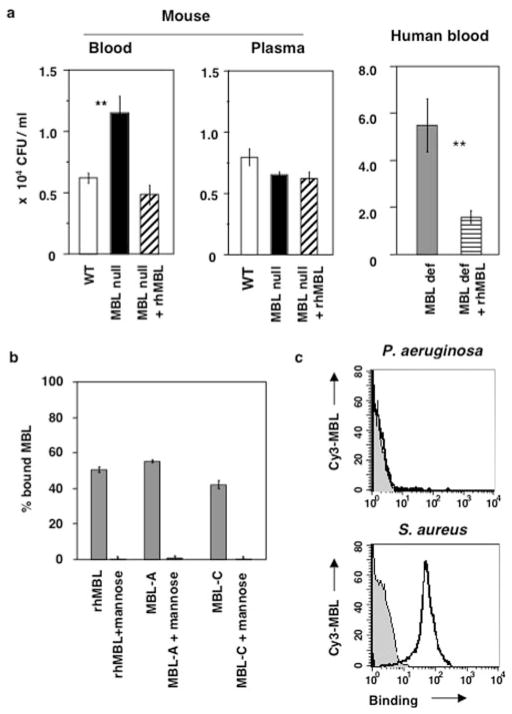
P. aeruginosa was incubated with whole blood or plasma from WT, MBL null mice, and MBL null mice that were reconstituted with rhMBL. Growth of P. aeruginosa was 2-fold higher in whole blood of MBL null mice compared with the growth in blood from WT mice after 2 h incubation (p = 0.005) (Fig. 3a). The low growth inhibition in whole blood of MBL null mice was restored when the mice were injected with rhMBL before blood collection (p = 0.005 between MBL null and MBL null with rhMBL) (Fig. 3a). We also tested this phenotype in whole blood from an MBL-deficient human volunteer. The growth of P. aeruginosa in MBL-deficient blood was 3-fold reduced upon reconstitution with rhMBL (p = 0.006) (Fig. 3a). There was no difference in growth of P. aeruginosa when bacteria were incubated with plasma from WT, MBL null, or the reconstituted MBL null mice (Fig. 3a). Likewise there was no difference in the bacterial growth when human MBL-deficient plasma was incubated with or without rhMBL (data not shown). These results indicate that soluble factors alone were ineffective in curtailing the infection, and that both MBL and phagocytes are required for the killing of P. aeruginosa.
FIGURE 3.
MBL-deficient blood fails to restrict growth of P. aeruginosa. a, Growth of P. aeruginosa was analyzed in ex vivo killing assay. Hirudin-treated whole blood or plasma from mice or whole blood from human donors was incubated with P. aeruginosa, and CFU per milliliter was determined at 120 min after the incubation at 37°C. Bars indicate mean ± SD of six results (three experiments in duplicates). **, p < 0.01. One representative result of two repeated experiments is shown. b, P. aeruginosa express ligands for MBL binding. Binding of P. aeruginosa to human MBL and mouse MBL-A and MBL-C was determined using suspension phase binding assay as described in Materials and Methods. Result is presented as a percentage of bound MBL. Bars indicate mean ± SD of four binding experiments. c, Binding of Cy3-MBL to P. aeruginosa was examined by FACS. Shaded area and solid line indicate bacteria alone and binding of Cy3-MBL to bacteria, respectively. S. aureus was used as a positive control.
As there are conflicting reports whether MBL binds this bacterium (34, 35), we re-examined the binding of MBL to P. aeruginosa by quantitative methods. Under our assay conditions, ~50% of the MBL in WT mouse serum as well as rhMBL that was mixed into MBL null mouse serum bound to P. aeruginosa (Fig. 3b). This result corresponded to 4 ng of MBL-A, 33 ng of MBL-C, and 15 ng of rhMBL that bound to 1 × 108 bacteria, which represent ~200 molecules of MBL bound per bacterium. A maximum of 2000 molecules of MBL could bind when an increased amount of MBL was used in the reaction mixture. The MBL was quantitatively recovered in the supernatant when mannose was added to the incubation with bacteria (Fig. 3b). Binding of MBL to P. aeruginosa could also be observed when the bacteria were coated on microtiter wells and the binding was inhibited by mannose or EDTA (OD415 rhMBL = 0.596, with mannose = 0.082, and with EDTA = 0.131, which was background level). Thus we show that MBLs do bind to P. aeruginosa in a mannose-specific and calcium-dependent manner. When MBL binding to P. aeruginosa was analyzed by FACS, no binding was detected in agreement with a previously published report (34), whereas binding to S. aureus was obviously detected under the same condition (Fig. 3c). These results suggest that FACS analysis is not sensitive to detect MBL binding to P. aeruginosa.
IL-6 and TNF-α levels in blood and organs during postburn infection
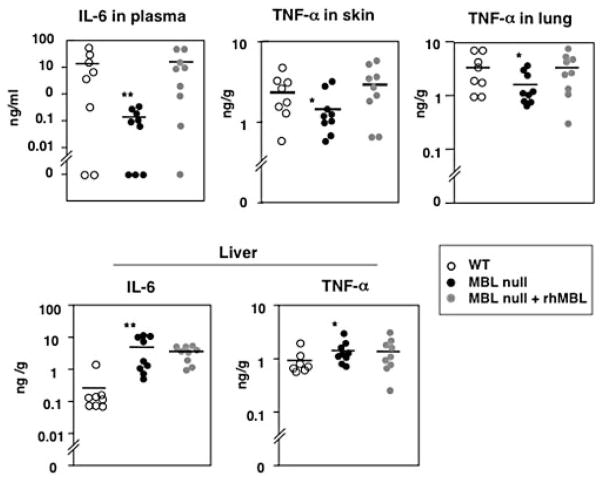
We then investigated inflammatory responses, as an overwhelming production of TNF-α and IL-6 has been associated with high mortality (36–38). We determined levels of TNF-α and IL-6 in plasma and in homogenates of skin, liver, kidney, and lung at 20 h after burn and bacterial inoculation. IL-6 levels in plasma were significantly lower in MBL null mice (p = 0.004) compared with that of WT mice (Fig. 4). Similarly TNF-α was lower in lung (p = 0.05) and in skin (p = 0.05) of MBL null mice compared with that of WT mice (Fig. 4). The low TNF-α in lung and IL-6 in plasma were significantly restored when the mice were reconstituted with rhMBL (p = 0.08 and 0.02, respectively) (Fig. 4), indicating that the differences can be ascribed to the presence or absence of MBL. In the liver the response was opposite as both IL-6 and TNF-α levels increased (p = 0.002 and p = 0.04, respectively) in MBL null mice compared with that of WT mice and the response was not reversed by reconstitution of mice with rhMBL (Fig. 4).
FIGURE 4.
IL-6 and TNF-α levels following infection after burn. Levels of IL-6 and TNF-α were determined in plasma and in homogenates of skin, lung, and liver as described in Materials and Methods. Symbols represent individual mice. Two experiments were combined, and mice used were WT (n = 8), MBL null (n = 9), and reconstituted MBL null (n = 9) mice. Bars indicate mean. *, p < 0.05; **, p < 0.01.
Reduced inflammatory response in MBL null mice following burn alone
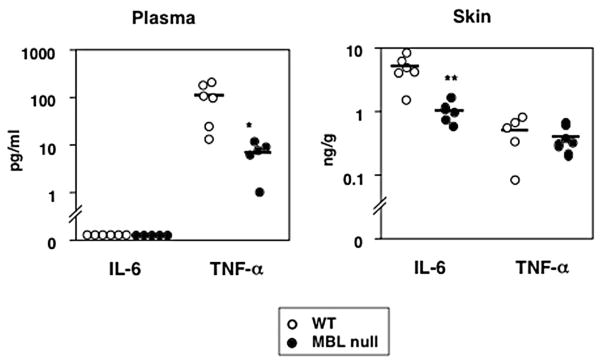
We also examined the effect of burn alone on inflammatory responses locally and systemically in MBL null and WT mice. TNF-α and IL-6 were measured in skin homogenate and plasma from MBL null and WT mice following burn injury. No IL-6 was detected in plasma from either MBL null or WT mice but TNF-α in plasma was significantly lower (p = 0.01) in MBL null mice than that in WT mice at 20 h (Fig. 5). IL-6 could be detected in skin and was lower in MBL null mice compared with that of WT mice (p = 0.007), whereas there was no difference in TNF-α levels in skin between WT and MBL null mice (Fig. 5).
FIGURE 5.
Decreased cytokine levels in MBL null mice following burn injury. Levels of TNF-α and IL-6 were determined in plasma and skin homogenates at 20 h after burn as described in Materials and Methods. Symbols represent individual mice. Two experiments were combined, and WT (n = 6) and MBL null (n = 5) mice were used. Bars indicate mean. *, p < 0.05; **, p < 0.01.
Discussion
This study provides for the first time in vivo evidence that MBL null mice are highly susceptible to P. aeruginosa infection following burn injury. The biological consequences of burn injuries vary as a result of combinations of heat source, length of input, and percentages of TBSA exposed. In this study we used a 5% TBSA, nonlethal dry burn. Burn alone had no obvious general health effect on either WT or MBL null mice. In striking contrast, when burn was followed by infection, all MBL null mice died within 42 h as a result of increased bacteremia and invasive infection in organs, which was demonstrated by significantly higher bacterial loads in MBL null mice compared with WT mice (Figs. 1 and 2). This bacterial spread from the skin triggered by burn injury is analogous to bacterial translocation from the gut following thermal injury (39, 40). The susceptibility phenotype was reversed by administration of rhMBL. In contrast there was no difference in bacterial proliferation at the local site between WT and MBL null mice. Furthermore, in the absence of concomitant burn injury MBL null mice contained P. aeruginosa and did not develop systemic infection. These results are consistent with the role of MBL as a circulating pattern recognition molecule that is rapidly deployed to local sites of infection with the precise purpose to limit and prevent systemic spread of the infection.
The skin is the largest innate immune organ as well as a physical barrier against noxious stimuli (41). Burn injury disrupts normal homeostasis and this results in predisposition to infection with a variety of potential pathogens, such as S. aureus and P. aeruginosa. In recognition of the high probability of local and systemic infection following burn injury, current standards of care involve the use of systemic and topical antimicrobial agents (42). There is an increasing awareness that the heterogeneous responses to infectious challenge may reflect genetic heterogeneity in the innate or the adaptive immune genotype of the individual. Although MBL has not been studied in relation to burn injuries or infections post-burn, the results of our in vivo study indicate that MBL plays an important role in the first line host defense against P. aeruginosa infection, the most common pathogen affecting burn patients. Mc-Manus and colleagues (43) found that 10% of all burn patients admitted to burn units developed P. aeruginosa bacteremia and 80% of those died. The MBL gene is known to have polymorphisms in the promoter region and in exon 1 that determine serum MBL levels, which vary from a few nanograms per milliliter to several micrograms per milliliter (44, 45). Since the first clinical study that correlated an MBL-dependent opsonic defect in serum with a phenotype of recurrent infections (46), such patients were found to have one of three amino acid substitutions due to single nucleotide polymorphisms in exon 1 of the MBL gene that disrupt the collagen helix (44). More detailed analysis of the MBL gene revealed seven distinct MBL haplotypes in humans, four of which (LYPB, LYQC, HYPD, and LXPA) dictate low serum levels (45, 47). MBL deficiency, defined as <100 ng of MBL per millileter in plasma, is very common in some ethnicities from 15% of the Caucasian population to as high as 30% in certain African populations (45, 47–50). Compelling clinical studies have suggested that MBL deficiency was associated with severe infections following chemotherapy, postoperative, and after stem cell transplantations (51–55). Other patient populations did not show such a relationship (56–58) and thus it appears that the effect of MBL deficiency is revealed only under certain clinical conditions. In our studies aimed at clarifying the role of MBL, we have found that MBL null mice are susceptible to infection with S. aureus (24) and HSV-2 (25). The present study adds P. aeruginosa to the list of pathogens that show increased infectivity in MBL-deficient animals.
MBL therefore appears to have a key role in the context of the acute inflammatory response, which includes the extravasation of serum and effector cells like neutrophils and monocytes. This idea is supported by our findings that MBL-dependent killing required serum factors as well as phagocytes. In this regard we did not observe a difference in the killing when bacteria were incubated in plasma alone from WT, MBL null mice, or MBL-deficient human plasma that was mixed with rhMBL (Fig. 3a), suggesting that MBL-mediated complement activation alone is not sufficient to restrict the growth of P. aeruginosa. By contrast, whole blood that contained effector cells of WT mice inhibited bacterial growth as compared with blood that contained MBL null mice effector cells (Fig. 3a). The inhibition of bacterial growth observed ex vivo in whole blood of MBL null mice was rescued by reconstitution with rhMBL (Fig. 3a). Likewise, MBL-deficient human blood efficiently inhibited bacterial growth when rhMBL was added (Fig. 3a). These results are consistent with previous work that demonstrated the importance of opsonophagocytosis in the effector arm of MBL against S. aureus (24, 59), and show that this mechanism is also operative against P. aeruginosa.
These results led us to examine whether MBL indeed bound to these bacteria because there had been no consensus as to whether MBL binds to P. aeruginosa. Neth et al. (34) failed to detect MBL binding to P. aeruginosa by FACS analysis. In contrast, Kuipers et al. (35) demonstrated MBL-dependent complement activation by P. aeruginosa in their assay using innocent bystander lysis of chicken erythrocytes. In this study, we reconcile these differences by reporting results that MBL binding to P. aeruginosa was demonstrated via both the suspension phase binding assay and the solid phase ELISA type assay (Fig. 3b), although it was not the case by FACS analysis (Fig. 3c). Importantly, the binding was via the carbohydrate recognition domain of MBL as it was inhibitable by excess mannose and required calcium as was not observed in the presence of EDTA (60, 61).
Innate immune surveillance mechanisms in the skin involve keratinocytes and Langerhans cells in the epidermis as well as dermal fibroblasts, mast cells, dendritic cells, and macrophages that are repositories for a vast array of stored and inducible molecules that include antimicrobial peptides, chemokines, cytokines, and cytokine antagonists (62). Recognizing the complexity of the innate immune response to burn injury and subsequent infection, we decided to measure the local and systemic production of two key cytokines IL-6 and TNF-α as the levels of these two cytokines correlated with outcome in murine burn models and clinical studies (36–38). Indeed, both cytokines were detected in the skin following burn alone, although we did not identify the cellular source. IL-6 was significantly lower in MBL null mice compared with WT mice, whereas there was no difference in TNF-α (Fig. 5). In contrast, systemic TNF-α was significantly lower in MBL null mice whereas IL-6 could not be detected systemically in either WT or MBL null mice (Fig. 5). The failure of MBL null mice to produce systemic TNF-α by burn injury alone is of considerable interest as it suggests that MBL is a modulator of inflammation. In this regard it will be interesting to define whether MBL interacts with TLRs that play a key role in NF-κB-dependent secretion of TNF-α. It is clear from clinical studies with TNF-α antagonists and in TNF null mice that TNF-α plays a key role in host defense, thus the sluggish TNF-α response in MBL null mice is likely to contribute toward the susceptibility of these mice to infection (24, 63, 64).
Unlike burn injury alone the combination of burn injury and infection induced significantly higher levels of plasma IL-6 in WT mice compared with MBL null mice (Fig. 4). Similarly TNF-α was lower in the lung and the skin of MBL null mice following burn and infection (Fig. 4). In contrast, both IL-6 and TNF-α were higher in the liver of MBL null mice than the liver in WT mice suggesting that cytokine responses differ depending on the organ. Of note, the reduced cytokine responses following the postburn infection could be reversed to WT levels by the reconstitution of MBL null mice with rhMBL, however, elevated cytokine responses in liver was not reversed (Fig. 4). Taken together, these results suggest that MBL modulates inflammatory responses not only to infectious challenge but also to sterile burn injury and that organ dependent mechanisms may be involved with the responses. MBL has a proinflammatory response when it discerns the fine molecular and macro molecular pattern of carbohydrates decorating the surface of pathogens (2). However MBL also recognizes altered-self in the context of necrotic and apoptotic cells (6–8). In this context MBL is reported to induce an anti-inflammatory response (6). MBL is a member of a group of molecules called collectins that include the lung collectins, surfactant protein-A and surfactant protein-D. Surfactant protein-A and -D have been described to have an anti-inflammatory role in the lung. However, under certain circumstances when the lung is infected, they may induce proinflammatory responses (65). The downstream signaling required to regulate these opposing responses is not well understood but is a focus of intense investigation.
In this study, we provide in vivo evidence that MBL is important in curtailing the systemic expansion of P. aeruginosa infection following burn injury. As the human MBL gene is polymorphic and certain haplotypes have been associated with increased risk to infection, we postulate that MBL deficiency may be an important risk factor among burn patients in susceptibility to systemic infection. Clinical studies in burn patients would be a natural extension of this work. Furthermore, the high incidence of carbapenem-resistant P. aeruginosa in burn patients (66) suggests that MBL therapy could be an important adjuvant in the antimicrobial armamentarium for this patient population.
Acknowledgments
We thank members of the Laboratory of Developmental Immunology, Massachusetts General Hospital, in particular Drs. L. M. Stuart and Iain P. Fraser for helpful discussion and critical reading of this manuscript. We also thank Dr. E. A. Carter, Pediatrics, Gastro Infectious Laboratory, Massachusetts General Hospital and Dr. R. L. Sheridan, Surgery, Massachusetts General Hospital, Shriner’s Burn Institute for helpful discussions. We thank NatImmune A/S for providing rhMBL.
Footnotes
This work is supported by National Institutes of Health Grant RO1AI42788.
Abbreviations used in this paper: MBL, mannose-binding lectin; rhMBL, recombinant human MBL; TBSA, total body surface area; WT, wild type.
Disclosures
R. A. B. Ezekowitz, J. Chr. Jensenius, and S. Thiel have a financial interest in NatImmune A/S, Copenhagen, the company providing the rhMBL. All other authors have no financial conflict of interest.
References
- 1.Krutzik SR, Sieling PA, Modlin RL. The role of Toll-like receptors in host defense against microbial infection. Curr Opin Immunol. 2001;13:104–108. doi: 10.1016/s0952-7915(00)00189-8. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 3.Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 4.Fraser IP, Koziel H, Ezekowitz RA. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin Immunol. 1998;10:363–372. doi: 10.1006/smim.1998.0141. [DOI] [PubMed] [Google Scholar]
- 5.Epstein J, Eichbaum Q, Sheriff S, Ezekowitz RA. The collectins in innate immunity. Curr Opin Immunol. 1996;8:29–35. doi: 10.1016/s0952-7915(96)80101-4. [DOI] [PubMed] [Google Scholar]
- 6.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nauta AJ, Raaschou-Jensen N, Roos A, Daha MR, Madsen HO, Borrias-Essers MC, Ryder LP, Koch C, Garred P. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853–2863. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- 8.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–3226. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 9.Saevarsdottir S, Vikingsdottir T, Valdimarsson H. The potential role of mannan-binding lectin in the clearance of self-components including immune complexes. Scand J Immunol. 2004;60:23–29. doi: 10.1111/j.0300-9475.2004.01437.x. [DOI] [PubMed] [Google Scholar]
- 10.Thiel S, Vorup-Jensen T, Stover CM, Schwaeble W, Laursen SB, Poulsen K, Willis AC, Eggleton P, Hansen S, Holmskov U, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–1502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparkes BG. Immunological responses to thermal injury. Burns. 1997;23:106–113. doi: 10.1016/s0305-4179(96)00089-7. [DOI] [PubMed] [Google Scholar]
- 13.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29:1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 14.Allgöwer M, Schoenenberger GA, Sparkes BG. Burning the largest immune organ. Burns. 1995;21(Suppl 1):S7–S47. doi: 10.1016/0305-4179(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 15.Pruitt BA, Jr, McManus AT, Kim SH, Goodwin CW. Burn wound infections: current status. World J Surg. 1998;22:135–145. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 16.Revathi G, Puri J, Jain BK. Bacteriology of burns. Burns. 1998;24:347–349. doi: 10.1016/s0305-4179(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 17.Song W, Lee KM, Kang HJ, Shin DH, Kim DK. Microbiologic aspects of predominant bacteria isolated from the burn patients in Korea. Burns. 2001;27:136–139. doi: 10.1016/s0305-4179(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 18.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong DG, Jude EB. The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc. 2002;92:12–18. doi: 10.7547/87507315-92-1-12. [DOI] [PubMed] [Google Scholar]
- 20.Borregaard N, Theilgaard-Mönch K, Cowland JB, Ståhle M, Sørensen OE. Neutrophils and keratinocytes in innate immunity: cooperative actions to provide antimicrobial defense at the right time and place. J Leukocyte Biol. 2005;77:439–443. doi: 10.1189/jlb.0704381. [DOI] [PubMed] [Google Scholar]
- 21.Arturson G. Microvascular permeability to macromolecules in thermal injury. Acta Physiol Scand Suppl. 1979;463:111–122. [PubMed] [Google Scholar]
- 22.Ward PA, Till GO. Pathophysiologic events related to thermal injury of skin. J Trauma. 1990;30:S75–S79. doi: 10.1097/00005373-199012001-00018. [DOI] [PubMed] [Google Scholar]
- 23.Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi L, Takahashi K, Dundee J, Shahroor-Karni S, Thiel S, Jensenius JC, Gad F, Hamblin MR, Sastry KN, Ezekowitz RA. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199:1379–1390. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadjeva M, Paludan SR, Thiel S, Slavov V, Ruseva M, Eriksson K, Lowhagen GB, Shi L, Takahashi K, Ezekowitz A, Jensenius JC. Mannan-binding lectin modulates the response to HSV-2 infection. Clin Exp Immunol. 2004;138:304–311. doi: 10.1111/j.1365-2249.2004.02616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankowsky HA, Callioux LS, Tredget EE. North American survey of hydrotherapy in modern burn care. J Burn Care Rehabil. 1994;15:143–146. doi: 10.1097/00004630-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Tredget EE, Shankowsky HA, Rennie R, Burrell RE, Logsetty S. Pseudomonas infections in the thermally injured patient. Burns. 2004;30:3–26. doi: 10.1016/j.burns.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Martin MA. Nosocomial infections in intensive care units: an overview of their epidemiology, outcome, and prevention. New Horiz. 1993;1:162–171. [PubMed] [Google Scholar]
- 29.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis. 2003;187:1717–1725. doi: 10.1086/375244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vorup-Jensen T, Jensen UB, Liu H, Kawasaki T, Uemura K, Thiel S, Dagnaes-Hansen F, Jensen TG. Tail-vein injection of mannan-binding lectin DNA leads to high expression levels of multimeric protein in liver. Mol Ther. 2001;3:867–874. doi: 10.1006/mthe.2001.0335. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Jensen L, Hansen S, Petersen SV, Takahashi K, Ezekowitz AB, Hansen FD, Jensenius JC, Thiel S. Characterization and quantification of mouse mannan-binding lectins (MBL-A and MBL-C) and study of acute phase responses. Scand J Immunol. 2001;53:489–497. doi: 10.1046/j.1365-3083.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 33.Thiel S, Møller-Kristensen M, Jensen L, Jensenius JC. Assays for the functional activity of the mannan-binding lectin pathway of complement activation. Immunobiology. 2002;205:446–454. doi: 10.1078/0171-2985-00145. [DOI] [PubMed] [Google Scholar]
- 34.Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose-binding lectin binds to a range of clinically relevant microorgan-isms and promotes complement deposition. Infect Immun. 2000;68:688–693. doi: 10.1128/iai.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuipers S, Aerts PC, van Dijk H. Differential microorganism-induced mannose-binding lectin activation. FEMS Immunol Med Microbiol. 2003;36:33–39. doi: 10.1016/S0928-8244(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 36.Neely AN, Hoover DL, Holder IA, Cross AS. Circulating levels of tumour necrosis factor, interleukin 6 and proteolytic activity in a murine model of burn and infection. Burns. 1996;22:524–530. doi: 10.1016/0305-4179(96)00029-0. [DOI] [PubMed] [Google Scholar]
- 37.Yamada Y, Endo S, Inada K. Plasma cytokine levels in patients with severe burn injury–with reference to the relationship between infection and prognosis. Burns. 1996;22:587–593. doi: 10.1016/s0305-4179(96)00052-6. [DOI] [PubMed] [Google Scholar]
- 38.Rumbaugh KP, Hamood AN, Griswold JA. Cytokine induction by the P. aeruginosa quorum sensing system during thermal injury. J Surg Res. 2004;116:137–144. doi: 10.1016/j.jss.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Alexander JW, Gianotti L, Pyles T, Carey MA, Babcock GF. Distribution and survival of Escherichia coli translocating from the intestine after thermal injury. Ann Surg. 1991;213:558–566. doi: 10.1097/00000658-199106000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eaves-Pyles T, Alexander JW. Comparison of translocation of different types of microorganisms from the intestinal tract of burned mice. Shock. 2001;16:148–152. doi: 10.1097/00024382-200116020-00011. [DOI] [PubMed] [Google Scholar]
- 41.Steinstraesser L, Oezdogan Y, Wang SC, Steinau HU. Host defense peptides in burns. Burns. 2004;30:619–627. doi: 10.1016/j.burns.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Klasen HJ. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns. 2000;26:117–130. doi: 10.1016/s0305-4179(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 43.McManus AT, Mason AD, Jr, McManus WF, Pruitt BA., Jr Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur J Clin Microbiol. 1985;4:219–223. doi: 10.1007/BF02013601. [DOI] [PubMed] [Google Scholar]
- 44.Sumiya M, Super M, Tabona P, Levinsky RJ, Arai T, Turner MW, Summerfield JA. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–1570. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 45.Steffensen R, Thiel S, Varming K, Jersild C, Jensenius JC. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J Immunol Methods. 2000;241:33–42. doi: 10.1016/s0022-1759(00)00198-8. [DOI] [PubMed] [Google Scholar]
- 46.Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;2:1236–1239. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 47.Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol. 1998;161:3169–3175. [PubMed] [Google Scholar]
- 48.Sumiya M, Summerfield JA. Mannose-binding protein, genetic variants and the risk of infection. QJM. 1996;89:723–726. doi: 10.1093/qjmed/89.10.723. [DOI] [PubMed] [Google Scholar]
- 49.Super M, Gillies SD, Foley S, Sastry K, Schweinle JE, Silverman VJ, Ezekowitz RA. Distinct and overlapping functions of allelic forms of human mannose binding protein. Nat Genet. 1992;2:50–55. doi: 10.1038/ng0992-50. [DOI] [PubMed] [Google Scholar]
- 50.Christiansen OB, Kilpatrick DC, Souter V, Varming K, Thiel S, Jensenius JC. Mannan-binding lectin deficiency is associated with unexplained recurrent miscarriage. Scand J Immunol. 1999;49:193–196. doi: 10.1046/j.1365-3083.1999.00473.x. [DOI] [PubMed] [Google Scholar]
- 51.Peterslund NA, Koch C, Jensenius JC, Thiel S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–638. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 52.Neth O, Hann I, Turner MW, Klein NJ. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet. 2001;358:614–618. doi: 10.1016/S0140-6736(01)05776-2. [DOI] [PubMed] [Google Scholar]
- 53.Siassi M, Hohenberger W, Riese J. Mannan-binding lectin (MBL) serum levels and post-operative infections. Biochem Soc Trans. 2003;31:774–775. doi: 10.1042/bst0310774. [DOI] [PubMed] [Google Scholar]
- 54.Ytting H, Jensenius JC, Christensen IJ, Thiel S, Nielsen HJ. Increased activity of the mannan-binding lectin complement activation pathway in patients with colorectal cancer. Scand J Gastroenterol. 2004;39:674–679. doi: 10.1080/00365520410005603. [DOI] [PubMed] [Google Scholar]
- 55.Mullighan CG, Heatley S, Doherty K, Szabo F, Grigg A, Hughes TP, Schwarer AP, Szer J, Tait BD, Bik To L, Bardy PG. Mannose-binding lectin gene polymorphisms are associated with major infection following allogeneic hemopoietic stem cell transplantation. Blood. 2002;99:3524–3529. doi: 10.1182/blood.v99.10.3524. [DOI] [PubMed] [Google Scholar]
- 56.Kilpatrick DC, Delahooke TE, Koch C, Turner ML, Hayes PC. Mannan-binding lectin and hepatitis C infection. Clin Exp Immunol. 2003;132:92–95. doi: 10.1046/j.1365-2249.2003.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergmann OJ, Christiansen M, Laursen I, Bang P, Hansen NE, Ellegaard J, Koch C, Andersen V. Low levels of mannose-binding lectin do not affect occurrence of severe infections or duration of fever in acute myeloid leukaemia during remission induction therapy. Eur J Haematol. 2003;70:91–97. doi: 10.1034/j.1600-0609.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 58.Carlsson M, Sjoholm AG, Eriksson L, Thiel S, Jensenius JC, Segelmark M, Truedsson L. Deficiency of the mannan-binding lectin pathway of complement and poor outcome in cystic fibrosis: bacterial colonization may be decisive for a relationship. Clin Exp Immunol. 2005;139:306–313. doi: 10.1111/j.1365-2249.2004.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neth O, Jack DL, Johnson M, Klein NJ, Turner MW. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J Immunol. 2002;169:4430–4436. doi: 10.4049/jimmunol.169.8.4430. [DOI] [PubMed] [Google Scholar]
- 60.Childs RA, Feizi T, Yuen CT, Drickamer K, Quesenberry MS. Differential recognition of core and terminal portions of oligosaccharide ligands by carbohydrate-recognition domains of two mannose-binding proteins. J Biol Chem. 1990;265:20770–20777. [PubMed] [Google Scholar]
- 61.Lee RT, Ichikawa Y, Fay M, Drickamer K, Shao MC, Lee YC. Ligand-binding characteristics of rat serum-type mannose-binding protein (MBP-A): homology of binding site architecture with mammalian and chicken hepatic lectins. J Biol Chem. 1991;266:4810–4815. [PubMed] [Google Scholar]
- 62.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wellmer A, Gerber J, Ragheb J, Zysk G, Kunst T, Smirnov A, Bruck W, Nau R. Effect of deficiency of tumor necrosis factor α or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infect Immun. 2001;69:6881–6886. doi: 10.1128/IAI.69.11.6881-6886.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Netea MG, van Tits LJ, Curfs JH, Amiot F, Meis JF, van der Meer JW, Kullberg BJ. Increased susceptibility of TNF-α lymphotoxin-α double knockout mice to systemic candidiasis through impaired recruitment of neutrophils and phagocytosis of Candida albicans. J Immunol. 1999;163:1498–1505. [PubMed] [Google Scholar]
- 65.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 66.National Nosocomial Infections Surveillance (NNIS) System Report, Data Summary from January 1992 through June 2004. Am J Infect Control. 2004 October;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]