Abstract
The worldwide rise in antibiotic resistance necessitates the development of novel antimicrobial strategies. Although many workers have used photodynamic therapy (PDT) to kill bacteria in vitro, the use of this approach has seldom been reported in vivo in animal models of infection. We have previously described the first use of PDT to treat excisional wound infections by Gram-(−) bacteria in living mice. However, these infected wound models involved a short timespan between infection (30 min) and treatment by PDT. We now report on the use of PDT to treat an established soft-tissue infection in mice. We used Staphylococcus aureus stably transformed with a Photorhabdus luminescens lux operon (luxABCDE ) that was genetically modified to be functional in Gram-(+) bacteria. These engineered bacteria emitted bioluminescence, allowing the progress of the infection to be monitored in both space and time with a low light imaging charge-coupled device (CCD) camera. One million cells were injected into one or both thigh muscles of mice that had previously been rendered neutropenic by cyclophosphamide administration. Twenty-four hours later, the bacteria had multiplied more than one hundredfold; poly-L-lysine chlorin e6 conjugate or free chlorin e6 was injected into one area of infected muscle and imaged with the CCD camera. Thirty minutes later, red light from a diode laser was delivered as a surface spot or by interstitial fiber into the infection. There was a light dose dependent loss of bioluminescence (to <5% of that seen in control infections) not seen in untreated infections or those treated with light alone, but in some cases, the infection recurred. Treatment with conjugate alone led to a lesser reduction in bioluminescence. Infections treated with free chlorin e6 responded less well and the infection subsequently increased over the succeeding days, probably due to PDT-mediated tissue damage. PDT-treated infected legs healed better than legs with untreated infections. This data shows that PDT may have applications in drug-resistant soft-tissue infections.
Introduction
Photodynamic therapy (PDT) uses the combination of a non-toxic dye, known as a photosensitizer (PS), visible light (usually red) and oxygen to produce reactive oxygen species that cause cell death and tissue destruction.1 It has gained clinical approval as a treatment for several forms of cancer, choroidal neovascularization in age-related macular degeneration and actinic keratoses.1 It has been known since the first days of PDT early in the last century that certain microorganisms can be killed by the combination of dyes and light in vitro.2 At the present time, the molecular characteristics of PS suitable for killing either Gram-(+) or Gram-(−) bacterial species (or both) are fairly well understood.3 Nevertheless, despite a century of using photodynamic inactivation (PDI) to kill bacteria in vitro, the use of PDT in vivo to treat infections has not been developed.
The rapidly increasing emergence of antibiotic resistance amongst pathogenic bacteria is becoming a serious public health problem.4 Bacteria replicate very rapidly and a mutation that helps a microbe survive in the presence of an antibiotic drug will quickly become predominant throughout the microbial population. The inappropriate prescription of antibiotics and the failure of some patients to complete their treatment regimen also exacerbate the problem. Antimicrobial resistance is becoming a factor in virtually all hospital-acquired (nosocomial) infections and physicians are concerned that several bacterial infections soon may be untreatable.5 These concerns have led to major research effort to discover alternative strategies that could be used to combat infections in patients, such as naturally occurring antimicrobial peptides, bacteriophages and PDT. All studies that have examined in vitro PDI of antibiotic-resistant bacteria have found them to be as equally susceptible as their naïve counterparts.6,7
In order to effectively treat infections with PDT in living animals, certain conditions must be met. It is necessary for the PS to be selective for bacteria compared to host cells and tissue, a suitable route of administration of the PS to the infected area must exist, the infected area must allow effective light delivery and an appropriate method of monitoring the result of treatment should be employed. Our approach to meeting these conditions is as follows. We use the covalent attachment of a PS, chlorin e6 (ce6), to a polycationic peptide, poly-L-lysine (pL), to form a molecular-targeting vehicle that can bind to and penetrate both Gram-(+) and Gram-(−) bacteria.8,9 Because the resulting conjugate is a macromolecule, it should only be taken up by mammalian cells through the time-dependent process of endocytosis, thus giving a temporal selectivity for bacteria. The conjugate is directly administered by topical application or by injection directly into the infected area, and after a suitable interval to allow the PS to bind and penetrate the bacteria, light is delivered by surface illumination (or possibly by interstitial fiber optic). We use genetically engineered bacteria that emit bioluminescence and can be detected in vivo using an intensified CCD camera.10 By quantifying the bioluminescence images, the extent of infection can be determined in real time in living animals, providing both temporal and spatial information about the labeled bacteria.11,12
We previously showed that both a non-pathogenic strain of Escherichia coli 10 and a highly pathogenic strain of Pseudomonas aeruginosa 13 could be effectively and rapidly killed in excisional wounds in living mice.14 In the latter case, PDT-treated mice were saved from death caused by the bacteria invading the bloodstream, which was the fate of mice whose wounds were untreated or received light or conjugate alone.13 However, these experiments were carried out on animals whose wounds were recently contaminated with relatively large numbers of colony-forming units (CFU). It is unlikely that patients would present for treatment under these circumstances. A more realistic and clinically relevant model would consist of inoculation of a smaller number of bacteria and then allowing the infection to grow and become established in tissue over time.
In this paper, we report on the establishment of a soft-tissue infection model using stably transformed bioluminescent Staphylococcus aureus in mice who have been rendered temporarily neutropenic by cyclophosphamide administration. These infections were treated by direct injection of polycationic PS conjugate into the infected area, followed by illumination with red laser light.
Materials and methods
Preparation of polylysine–ce6 conjugate
This was carried essentially as previously described.14 Briefly, pL–HBr (average molecular weight 22 000, degree of polymerization 110, Sigma Chemical Co., St Louis, MO, USA) was dissolved in dry dimethyl sulfoxide, to which was added ce6 (Porphyrin Products, Logan, UT, USA) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (Sigma). Triethylamine was then added and the mixture was stirred for 24 h, when methanol and water were added and the mixture was evaporated to dryness under vacuum. The residue was dissolved in sodium acetate buffer (10 mM, pH 5.5), applied to a column of Sephadex G25 (60 × 1 cm) and eluted with the same buffer. Product-containing fractions were collected and evaporated to give the product, pL–ce6, with a substitution ratio of 7.4 ce6 per pL chain, assuming the absorption coefficient of conjugated ce6 is the same as that of free ce6 (ε400 nm = 150 000 M−1 cm−1).
Bacteria
We used a strain of S. aureus 8325-4 that has been widely studied for pathogenicity and virulence factors.15 Bioluminescent S. aureus were generated by transforming the strain 8325-4 with a modified Photorhabdus luminescens lux operon 16 using the Gram-(+) lux transposon plasmid pAUL-ATn4001 luxABCDE KmR,17 which was introduced into the cells by electroporation as previously described.16 Transformants were grown overnight in tryptic soy broth (TSB) containing erythromycin (5 μg mL−1) and then plated onto TSB media containing kanamycin (200 μg mL−1) to select for those clones where the Tn4001 luxABCDE KmR cassette had transposed and inserted downstream of a promoter. Highly bioluminescent colonies were selected using an IVIS™ imaging system (Xenogen Corporation, Alameda, CA, USA). One clone, designated as S. aureus Xen 8.1, was selected and further characterized.
Mice
All animal experiments were approved by the Subcommittee on Research Animal Care of Massachusetts General Hospital and were in accordance with US National Institutes of Health guidelines. Male Balb/c mice weighing 20–25 g were shaved on one or both back legs and depilated with Nair (Carter-Wallace Inc., New York, NY, USA). Mice were anesthetized with an i.p. injection of ketamine–xylazine cocktail (90 mg kg−1 ketamine, 10 mg kg−1 xylazine) for infection, and for subsequent PDT and imaging.
Bioluminescence imaging
The low light imaging system (Hamamatsu Photonics KK, Bridgewater, NJ, USA) has been previously described in detail.14 It consists of an intensified CCD (ICCD) camera mounted in a light-tight specimen chamber fitted with a light-emitting diode, allowing a background grayscale image of the entire mouse to be captured. In the photon-counting mode, an image of the emitted light was captured using an integration time of 2 min at a maximum setting on the image intensifier control module. Using ARGUS software, the luminescence image was presented as a false-color image superimposed on top of the grayscale reference image. The image-processing component of the software gave total pixel values from the luminescence images on user-defined areas within each wound on a 256-grayscale. The analysis area was adjusted to cover the whole infection and the resulting total pixel count was a combined measure of both the extent and intensity of infection. For calculation of total luminescence signals from each image, the values were normalized to a bit range of 2–4. For presentation of composite series of luminescence images, the same bit range was used for each component image.
Infection model
Initial studies were carried out to compare the growth of soft-tissue infections in normal and cyclophosphamide-treated mice. Mice were pre-treated with two separate doses of cyclophosphamide in order to create a temporary state of neutropenia. 150 mg kg−1 of cyclophosphamide (Sigma) dissolved in sterile saline was injected i.p. (0.1 mL per mouse) on day 1, followed by a second dose of 100 mg kg−1 injected i.p. on day 4. One million mid-log phase bioluminescent S. aureus Xen 8.1 cells suspended in 50 μL phosphate buffered saline (PBS) were injected 2 mm beneath the surface of the thigh muscle in normal and neutropenic mice (5 per group). Mice were anesthetized and were imaged with the luminescence camera immediately after infection and then after 4 and 24 h, and daily thereafter. They were monitored closely for symptoms of disease (weight, ruffled fur and inactivity) and mice that become moribund were sacrificed. In some mice, we studied a model where both hind legs received an equal bacterial infection in each hind leg.
Photodynamic therapy
All experiments using mice that had been injected with PS were carried out under subdued room lighting or in the dark, except when illumination was taking place. Mice were injected with pL–ce6 at a dose of 50 μL of a 1 mM ce6 equivalent solution into the area of subcutaneous and/or intramuscular infection. The area of infection was imaged using the bacterial bioluminescence in the Hamamatsu camera before injection so the infected area was known for each mouse. Injection was carried out using a 50 μL Hamilton syringe fitted with a 28-gauge needle. We injected 10 μL in the middle of the infected area and four additional 10 μL aliquots of conjugate at the edges of the four quadrants of the infected area.
Thirty minutes after conjugate injection, illumination was carried out by one of two methods. Both used a 1 W, 665 nm diode laser (BWTek, Newark, DE, USA) and 200 μm optical fibers coupled to the laser via SMA connectors. Surface illumination was accomplished by a fiber with a plane-polished distal end that provided a spot on the mouse leg with a diameter of up to 1.5 cm. Interstitial light delivery was accomplished by inserting a fiber fitted with a spherical diffusing tip at the distal end into the infected area. The power was routinely measured using a laser power meter (model FM/GS, Coherent, Santa Clara, CA, USA); a power density of 100 mW cm−2 was employed for surface illumination and a power of 40 mW total out of the diffusing tip for interstitial light delivery
Mice were imaged in the luminescence camera immediately before PS injection, immediately after the injections and again after the 30 min incubation time. Mice were again imaged after each increment of light dose had been delivered (this was frequently after each 40 J cm−2). In one set of experiments, we treated mice with a single soft-tissue infection with PDT mediated by free (unconjugated) ce6. We injected 50 μL of a 1 mM solution of ce6 in PBS using the same methodology as for pL–ce6, i.e. 5 × 10 μL aliquots into the infection and carried out surface illumination 30 min later. Mice were imaged after each light dose and followed for healing as before.
Follow-up of mice
Each day mice were weighed and imaged in the luminescence camera after brief anesthesia. Their legs were observed for function and marked on the following scale from 0–4: 4 = perfectly normal leg in appearance and motion; 3 = slight limp, slight impairment in movement; 2 = significant impairment in movement, mouse cannot walk normally; 1 = leg is paralyzed and dragged behind mouse; 0 = legs suffers from frank necrosis or is absent. In addition, the size of the visible lesion on the leg was measured in two dimensions using vernier calipers.
Statistics
Differences between the means of lesion areas and leg-function scores were analyzed for statistical significance by the unpaired 2-tailed Student’s t test. P values of <0.05 were considered significant.
Results
Normal versus neutropenic mice
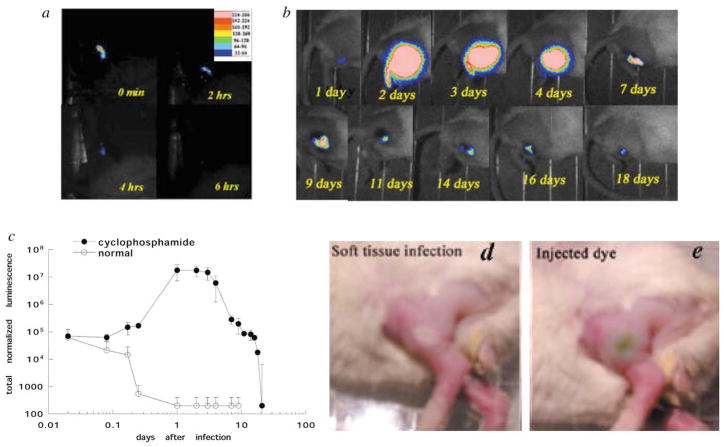
Since any clinical use of PDT for treatment of soft-tissue infections would be for patients that presented with an already established growth of bacteria in tissue, we wanted to test a model where the bacteria had been allowed to grow and multiply many-fold over time. We therefore compared the injection of 1 million log-phase S. aureus CFU into normal mice and those which had previously been rendered temporarily neutropenic by pre-treatment with cyclophosphamide. Typical time-course sets of bioluminescence images from infected mice are shown in Fig. 1(a) and (b) and the mean normalized bioluminescence values from 5 mice per group in Fig. 1(c). The normal mouse has lost all trace of bioluminescent bacteria 6 h after injection. In contrast, the neutropenic mouse shows a greater than one hundredfold increase in signal 24 h after infection and this remains at a very high level for a further 3 days. Mice frequently developed a whitish area on the skin at the location of the underlying infection after 24 h [Fig. 1(d)]. Significant amounts of bioluminescence were still present in the neutropenic mouse thighs for up to 18 days after infection. The cyclophosphamide-treated mice exhibited symptoms arising from their infection (see next section), while the normal mice did not display any adverse effects.
Fig. 1.
Series of bioluminescence images (captured at a bit range of 2–4) from a normal mouse (a) and a cyclophosphamide pre-treated mouse (b) injected in the thigh with 1 million bioluminescent S. aureus cells. (c) Mean values of total normalized bioluminescence from infected mouse (normal and neutropenic) thighs (5 per group). Error bars show the SEM. (d) Photograph of cyclophosphamide-treated mouse 24 h after infection. (e) Photograph of mouse with soft-tissue infection after local injection of pL–ce6.
PDT of a single-leg infection
When the pL–ce6 conjugate (50 μL) at a concentration of 1 mM ce6 equivalent was injected into the infected area, there was a visible green coloration noticeable beneath the skin [Fig. 1(e)]. This allowed a judgment to be made about the uniformity of the PS distribution within the infection. Although these mice were neutropenic and the infection did not accumulate the considerable quantities of pus expected from immunocompetent mice, there was still some matter present in the infection. This occasionally allowed the injected PS to flow within the tissue in unexpected ways so that an even distribution of green color was not seen. Another problem was that it was difficult to estimate the precise depth of infection from the luminescence image and sometimes it appeared the PS was injected above the majority of the bacteria if they happened to have penetrated some way into the muscle. We had previously suspected that the conjugate might diffuse relatively rapidly through the tissue; however, this did not prove to be the case. The green coloration remained in place for some time (several hours) especially in unilluminated mice.
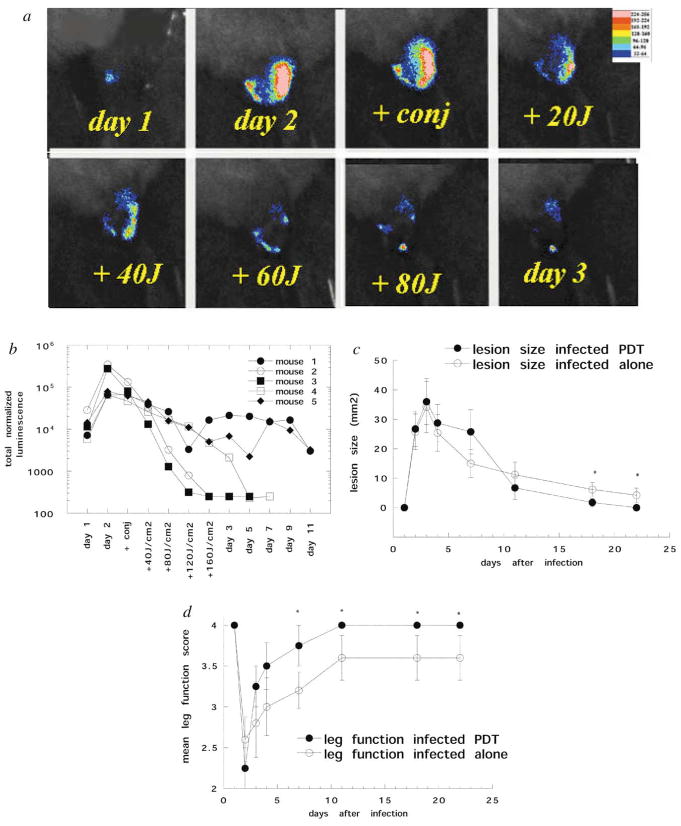
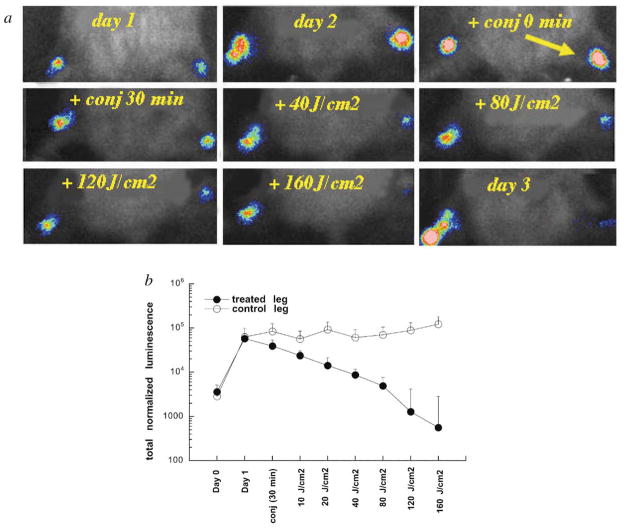
There was a slight reduction in bacterial bioluminescence observed immediately after the conjugate was injected into the infection. Luminescence was further reduced after the 30 min incubation period in the dark. When illumination was commenced, there was a light dose dependent decrease in luminescence after each 40 J cm−2 increment of red light [Fig. 2(a) and (b)]. In two of the mice (no. 2 and 3), at the completion of delivery of 160 J cm−2, the bioluminescence had declined to the limit of detection and there was no re-growth of the bacteria in these animals, as evidenced by imaging for the following days. In mouse 4, the response during light delivery was less impressive, but the infection continued to decline over the 5 days following PDT and became undetectable. The remaining mice (no. 1 and 5) also showed only a moderate response to light delivery, but these animals exhibited an increase in the bioluminescence (indicating bacterial re-growth) that remained at moderate intensity for 10 days.
Fig. 2.
(a) Series of bioluminescence images (captured at a bit range of 2–4) from a mouse infected on day 1 and treated on day 2 by injection of pL–ce6 into the infected area, followed after 30 min by illumination with 665 nm light at a fluence rate of 100 mW cm−2. (b) Individual normalized bioluminescence values from 5 infected mice treated with PDT as described above. (c) Mean lesion sizes of the 5 mice treated with PDT. (d) Mean leg-function scores (see Materials and methods for definition) of the 5 mice treated with PDT. Error bars show the SEM and asterisks signify 2-tailed unpaired Student’s t test P < 0.05.
As mentioned previously, infected mice showed symptoms arising from their infections 24 h after injection of bacteria. They developed a white area visible on the skin of the thigh corresponding in location to the bioluminescence image. The legs of the mice showed impaired function, ranging from a slight limp to complete paralysis. The mean lesion sizes and leg-function scores of control infected mice and infected mice that received PDT are shown in Fig. 2(c) and (d). The lesion size reached a peak on day 3 for both control and PDT-treated mice, and for the next 8 days it appeared that the PDT lesions were somewhat larger than the control lesions (although this difference was not statistically significant). However, on day 10, the PDT lesions had healed somewhat better than the control lesions and this difference became statistically significant (P < 0.05) from days 17 to 22. The leg-function score reached a minimum on the day of PDT (24 h after infection) for all mice and then the PDT-treated legs began to improve faster than the control legs; from day 7 until the end of the experiment the PDT-treated legs performed significantly better than the controls (P < 0.05). Four cyclophosphamide-treated mice were treated with PDT (injection of conjugate and 160 J cm−2 light) in the thigh muscle without injection of bacteria and therefore with no infection present. There was no visible lesion and no impairment of leg function seen at any time (data not shown).
Combined interstitial and surface illumination
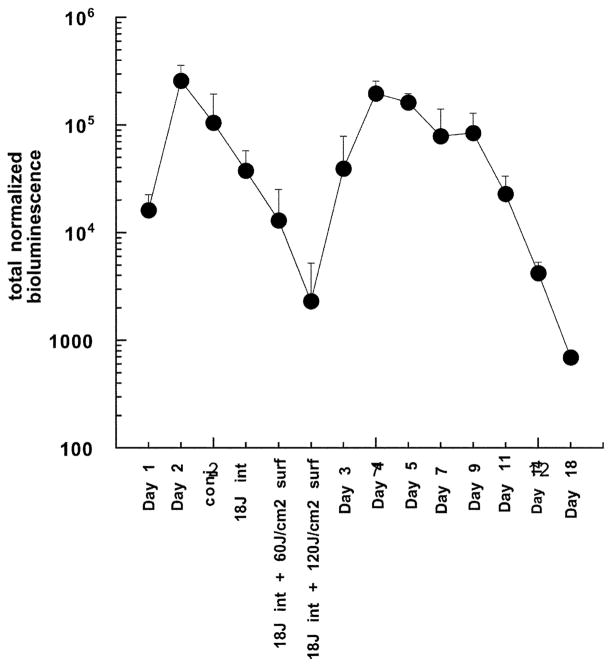
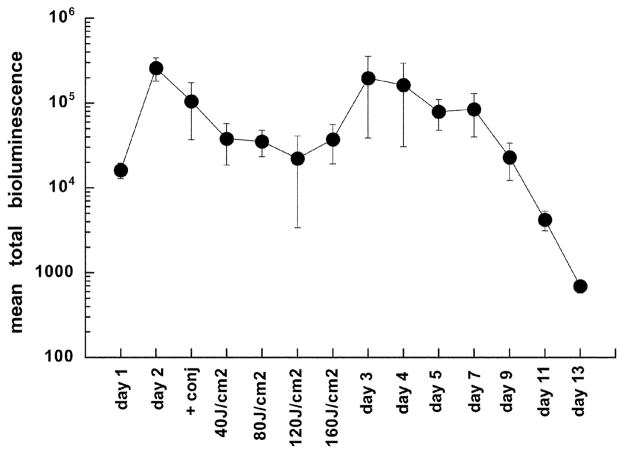
We investigated the delivery of light into the infected area with an interstitial fiber fitted with a spherical diffusing tip. We reasoned that the light was scattered by the overlying skin on top of the infection, thus decreasing the effective light dose that penetrated into the area containing the bacteria. In preliminary experiments, we tried interstitial illumination alone and delivered a defined fluence, removed the mouse, imaged it in the camera, re-inserted the fiber and delivered a further increment of light from the interstitial fiber. However, we observed that the reduction in bacterial bioluminescence seen after the first increment of interstitial light had been delivered was not replicated after the second and any subsequent increments. Although the reason for this observation is not obvious, it may be connected to the repeated puncturing of the infected area necessary to re-insert the fiber after imaging. We then decided to deliver the interstitial light in one increment of 18 J total out of the fiber at a power of 40 mW, withdraw the fiber, image the mice and then deliver two further increments of light (60 J cm−2) by surface illumination. This sequence gave superior results to those obtained with light delivery in the reverse order (surface first, followed by interstitial; data not shown). The mean bioluminescence values are shown in Fig. 3 and it can be seen that the mean bioluminescence signal was reduced by 99% after these three light increments. However, in all these 5 mice, there was re-growth of the bioluminescence signal on the day after PDT and by the next day, the intensity had almost reached pre-treatment levels. This re-growth of infection then slowly declined over the next 2 weeks.
Fig. 3.
Mean total normalized bioluminescence values from the thighs of 5 infected mice treated with PDT by injection of pL–ce6 conjugate into the infection, followed after 30 min by insertion of a diffusing tip fiber into the infection and delivery of 18 J of 665 nm light at a fluence rate of 40 mW, and then surface illumination as described. Error bars show the SEM.
Comparison with free ce6
In a previous publication 9 we showed that free ce6 was signifi-cantly better than pL–ce6 conjugate in mediating the light-dependent killing of S. aureus in vitro. To test our hypothesis that the selectivity of the polycationic PS conjugate for bacteria over the host tissue was important in PDT of soft-tissue infections, we repeated the experiment involving treatment of a single soft-tissue infection by injecting a solution of free ce6 into the infection, followed by superficial illumination. As depicted in Fig. 4, there was a reduction in luminescence observed 30 min after the PS had been injected into the infected area and the light dose dependent reduction in luminescence after each surface-delivered increment of 40 J cm−2 (although significant) was less than was seen with pL–ce6 (amounting to only approximately 80%). All the mice displayed an increase in the bioluminescence signal on the following day (Fig. 4).
Fig. 4.
Mean total normalized bioluminescence values from the thighs of 5 infected mice treated with free ce6 as described in Fig. 2. Error bars show the SEM.
Model with infections in both legs
In an attempt to control for significant variations between individual mice in the intensity of the bioluminescence signals from the infections, we studied a model where the mice received an equal bacterial inoculation in each thigh muscle. We reasoned that the PDT-dependent reduction in the bioluminescence signal should be easier to quantify if each mouse had a treated and an untreated infection. A typical mouse treated with an injection of conjugate into the right infected thigh, followed by illumination of the right thigh as described previously, is shown in Fig. 5(a), together with the mean bioluminescence values from both legs of 5 mice in Fig. 5(b). After 160 J cm−2 had been delivered, the bioluminescence of the treated infected legs had been reduced by >99% compared to the untreated contralateral legs. However 4 out of 5 of these treated legs suffered a recurrence of the bioluminescence on succeeding days (data not shown).
Fig. 5.
(a) Series of bioluminescence images (captured at a bit range of 2–4) from a neutropenic mouse infected on day 1 in both thighs, and treated on day 2 with injection of pL–ce6 into the right thigh, followed after 30 min by illumination of the right thigh with 665 nm light at a fluence rate of 100 mW cm−2. (b) Mean total normalized bioluminescence values from left (untreated) and right (PDT-treated) thighs of 5 mice infected in both thighs. Error bars show the SEM.
Discussion
The results of the present study have provided proof-of-principle that PDT can be effectively used to treat an established soft-tissue infection in living mice. However, the methodology contains considerable challenges and significant improvements will need to be made before it could be considered for clinical application. Although mice have been extensively employed as animal models of bacterial infection,18 they are not particularly susceptible to developing established soft-tissue infections. When Francis et al. studied 16 bioluminescent S. aureus 8325-4 (5 × 106 CFU containing a plasmid-based lux operon) injected into the thigh muscles of mice, they found that although the bioluminescence signal was higher at 4 h than immediately after injection, it subsequently declined over the next 20 h. Since we wanted to treat an established soft-tissue infection (i.e. one in which the bacteria had multiplied many times in the tissue using their virulence factors to adhere to tissue and obtain their nutrients), we tested the use of systemic cyclophosphamide to precondition the mice by inducing temporary granulocytopenia.19 The bacteria were injected into to the superficial layers of the thigh muscle and subsequently spread into the subcutaneous space; from days 2 to 5 bioluminescence imaging showed a greater than one hundredfold increase in both bacterial density and the overall size of the infection. The experiments illustrate the benefits of using bioluminescent bacteria and a low light imaging system to quantify bacterial numbers in tissue in real time, and the ability to longitudinally follow the progress of the infection in individual mice. The fact that the bacteria were stably transformed with the bacterial lux operon, as opposed to possessing the genes necessary for light generation on a plasmid, gave added assurance that variations in bioluminescence seen over extended periods were due to variations in bacterial numbers, rather than a slow loss of the plasmid due to pressure of growth selection.
Choosing the optimal parameters for treating an established soft-tissue infection by PDT is inherently complex. The identity of the PS, the concentration and volume of the injection into the infection and the number of injections needed to achieve an equal spatial spread of the PS throughout the infected area are crucial. In addition, the time between PS injection and illumination, the mode of light delivery (surface spot versus interstitial fiber placement), fluence rate and total delivered fluence are highly important variables. One major problem we encountered was the re-growth of the infections, as monitored by the bioluminescence signal, after PDT-mediated reductions greater that 95%. It could be argued that a rapid reduction in infectious bacterial burden is valuable in its own right and subsequent bacterial re-growth could be prevented by administration of systemic antibiotics, which are generally thought to be more efficient in achieving bacteriostasis than actively killing large numbers of bacteria. However, one of the main attractions of using PDT for infections, i.e. that it is effective against multiply antibiotic-resistant strains, would be somewhat negated. Although the results measuring the reduction of the lesion size and the improvement in leg-function scores did not show a dramatic benefit due to PDT, there was nevertheless a statistically significant improvement in both parameters in the PDT-treated infected legs compared to untreated controls. PDT alone carried out on non-infected cyclophosphamide-treated mice did not lead to any lesions or other adverse effects, thus demonstrating that under these conditions, damage to host tissue solely due to PDT was not observed. The comparative improvement in healing of the legs of infected mice treated with PDT, despite the observed recurrence of infection, may be due to the PDT-induced inflammatory response accelerating healing or to the ability of PDT to destroy secreted bacterial virulence factors that slow down healing.20
We have presented some evidence that the success of the treatment depends on killing bacteria without causing damage to host tissue. This is illustrated in the experiments using free ce6, which we have previously shown 9 to be superior to pL–ce6 in mediating PDI of S. aureus in vitro. In these cases, PDT after injection of ce6 into the infection led to only a small decrease in the bioluminescence in the infection and all mice subsequently showed a re-growth of the bacteria over the following days. One can speculate that free ce6 (although highly effective against S. aureus in vitro) does not show enough selectivity for bacteria over mammalian cells because it is a small molecule with an overall anionic character. When injected into the infection, if it was chiefly taken up by mouse cells and only to a lesser degree by the bacteria, ce6 would tend to cause host tissue damage upon illumination, together with a reduced antibacterial effect. Since fewer of the bacteria are killed and the host tissue is damaged, this combination would provide an ideal breeding ground for bacterial re-growth. However, further studies comparing leg function, healing time and histological analysis between PDT of infections mediated by pL–ce6 conjugate and free ce6 would be necessary to confirm this hypothesis. There was also more marked bacterial re-growth after the interstitial illumination followed by surface illumination. Again, it is possible that insertion of the fiber into the infection causes sufficient tissue damage to encourage re-growth of bacteria.
Berthiaume et al.21 evaluated the efficacy of antibody-targeted photolysis to kill bacteria in vivo using PS immun-conjugates against P. aeruginosa. Initially, they mixed the bacteria with the tin(IV) chlorin e6–monoclonal antibody conjugate in vitro and injected the mixture into the subcutaneous dorsal area in mice. After infection, both specific and non-specific conjugates were injected at the infection site. After a 15 min incubation period, the site was exposed to 630 nm light with a power density of 100 mW cm−2 for 1600 s. Illumination resulted in a greater than 75% decrease in the number of viable bacteria at sites treated with a specific conjugate, whereas normal bacterial growth was observed in animals that were untreated or treated with a non-specific conjugate. The only report of PDT being used to treat localized bacterial infections in patients was published by Lombard et al.22 They treated 5 patients with brain abscesses after craniotomy and surgical drainage by instilling hematoporphyrin into the abscess bed and illuminating 5 min afterwards, producing a positive clinical response.
S. aureus is responsible for diseases such as pyoderma, toxic shock syndrome, and wound and burn infections in hospital patients. It has attracted much attention recently due to its acquired antibiotic resistance. It is a major cause of infections in surgical patients and is frequently transmitted to wounds from colonies that have become established in the noses of either patients or hospital workers. Strains of S. aureus resistant to methicillin and other antibiotics are endemic in hospitals.23 Infection with methicillin-resistant S. aureus (MRSA) strains may also be increasing in non-hospital settings. Methicillin-resistant S. aureus strains with reduced susceptibility to vancomycin have emerged recently in Japan and the United States, and present a serious problem for physicians and patients.24 PDT could provide an alternative to surgical debridement and topical antimicrobials for these otherwise hard to treat wound and tissue infections.
Acknowledgments
This work was supported by the US National Institutes of Health (Grant R01AI050879 to M. R. H.) and the Department of Defense Medical Free Electron Laser Program (N00014-94-1-0927). We are grateful to David A. O’Donnell for technical assistance and to Christopher H. Contag for devising the system of bioluminescence monitoring of infections.
References
- 1.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Von Tappeiner H, Jodlbauer A. Ueber wirkung der photodynamischen (fluorescierenden) Stoffe auf Protozoan und enzyme. Dtsch Arch Klin Med. 1904;80:422–487. [Google Scholar]
- 3.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 4.Hinman AR. Global progress in infectious disease control. Vaccine. 1998;16:1116–1121. doi: 10.1016/s0264-410x(98)80107-2. [DOI] [PubMed] [Google Scholar]
- 5.Michel M, Gutmann L. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: therapeutic realities and possibilities. Lancet. 1997;349:1901–1906. doi: 10.1016/s0140-6736(96)11192-2. [DOI] [PubMed] [Google Scholar]
- 6.Wilson M, Yianni C. Killing of methicillin-resistant Staphylococcus aureus by low-power laser light. J Med Microbiol. 1995;42:62–66. doi: 10.1099/00222615-42-1-62. [DOI] [PubMed] [Google Scholar]
- 7.Wainwright M, Phoenix DA, Laycock SL, Wareing DR, Wright PA. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol Lett. 1998;160:177–181. doi: 10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- 8.Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. 1998;42:2595–2601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamblin MR, O’Donnell DA, Murthy N, Rajagopalan K, Michaud N, Sherwood ME, Hasan T. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J Antimicrob Chemother. 2002;49:941–951. doi: 10.1093/jac/dkf053. [DOI] [PubMed] [Google Scholar]
- 10.Hamblin MR, O’Donnell DA, Murthy N, Contag CH, Hasan T. Photodynamic treatment of wound infections in vivo monitored by luminescence imaging. Photochem Photobiol. 2002;75:51–57. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Rocchetta HL, Boylan CJ, Foley JW, Iversen PW, LeTourneau DL, McMillian CL, Contag PR, Jenkins DE, Parr TR., Jr Validation of a noninvasive, real-time imaging technology using bioluminescent escherichia coli in the neutropenic mouse thigh model of infection. Antimicrob Agents Chemother. 2001;45:129–137. doi: 10.1128/AAC.45.1.129-137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contag CH, Contag PR, Mullins JI, Spilman SD, Stevenson DK, Benaron DA. Photonic detection of bacterial pathogens in living hosts. Mol Microbiol. 1995;18:593–603. doi: 10.1111/j.1365-2958.1995.mmi_18040593.x. [DOI] [PubMed] [Google Scholar]
- 13.Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis. 2003;187:1717–1725. doi: 10.1086/375244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamblin MR, O’Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75:51–57. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Phonimdaeng P, O’Reilly M, Nowlan P, Bramley AJ, Foster TJ. The coagulase of Staphylococcus aureus 8325–4. Sequence analysis and virulence of site-specific coagulase-deficient mutants. Mol Microbiol. 1990;4:393–404. doi: 10.1111/j.1365-2958.1990.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 16.Francis KP, Joh D, Bellinger-Kawahara C, Hawkinson MJ, Purchio TF, Contag PR. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect Immun. 2000;68:3594–3600. doi: 10.1128/iai.68.6.3594-3600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis KP, Yu J, Bellinger-Kawahara C, Joh D, Hawkinson MJ, Xiao G, Purchio TF, Caparon MG, Lipsitch M, Contag PR. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect Immun. 2001;69:3350–3358. doi: 10.1128/IAI.69.5.3350-3358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zak O, O’Reilly T. Animal infection models and ethics: the perfect infection model. J Antimicrob Chemother. 1993;31:193–205. doi: 10.1093/jac/31.suppl_d.193. [DOI] [PubMed] [Google Scholar]
- 19.van der Meer JW, Barza M, Wolff SM, Dinarello CA. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci U S A. 1988;85:1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komerik N, Wilson M, Poole S. The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem Photobiol. 2000;72:676–680. doi: 10.1562/0031-8655(2000)072<0676:teopao>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Berthiaume F, Reiken S, Toner M, Tompkins R, Yarmush M. Antibody-targeted photolysis of bacteria in vivo. Biotechnology. 1994;12:703–706. doi: 10.1038/nbt0794-703. [DOI] [PubMed] [Google Scholar]
- 22.Lombard GF, Tealdi S, Lanotte MM. The treatment of neurosurgical infections by lasers and porphyrins. In: Jori G, Perria CA, editors. Photodynamic Therapy of Tumors and other Diseases. Edizione Libreria Progetto; Padova: 1985. pp. 363–366. [Google Scholar]
- 23.Perry C. Methicillin-resistant Staphylococcus aureus. J Wound Care. 1996;5:31–34. doi: 10.12968/jowc.1996.5.1.31. [DOI] [PubMed] [Google Scholar]
- 24.Smith TL, Pearson ML, Wilcox KR, Cruz C, Lancaster MV, Robinson-Dunn B, Tenover FC, Zervos MJ, Band JD, White E, Jarvis WR. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N Engl J Med. 1999;340:493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]