Abstract
Background and Objective
Bacterial arthritis does not respond well to antibiotics and moreover multidrug resistance is spreading. We previously tested photodynamic therapy (PDT) mediated by systemic Photofrin® in a mouse model of methicillin-resistant Staphylococcus aureus (MRSA) arthritis, but found that neutrophils were killed by PDT and therefore the infection was potentiated.
Study Design/Materials and Methods
The present study used an intra-articular injection of Photofrin® and optimized the light dosimetry in order to maximize bacterial killing and minimize killing of host neutrophils. MRSA (5 × 107 CFU) was injected into the mouse knee followed 3 days later by 1 μg of Photofrin® and 635-nm diode laser illumination with a range of fluences within 5 minutes. Synovial fluid was sampled 6 hours or 1–3, 5, and 7 days after PDT to determine MRSA colony-forming units (CFU), neutrophil numbers, and levels of cytokines.
Results
A biphasic light dose response was observed with the greatest reduction of MRSA CFU seen with a fluence of 20 J cm−2, whereas lower antibacterial efficacy was observed with fluences that were either lower or higher. Consistent with these results, a significantly higher concentration of macrophage inflammatory protein-2, a CXC chemokine, and greater accumulation of neutrophils were seen in the infected knee joint after PDT with a fluence of 20 J cm−2 compared to fluences of 5 or 70 J cm−2.
Conclusion
PDT for murine MRSA arthritis requires appropriate light dosimetry to simultaneously maximize bacterial killing and neutrophil accumulation into the infected site, while too little light does not kill sufficient bacteria and too much light kills neutrophils and damages host tissue as well as bacteria and allows bacteria to grow unimpeded by host defense.
Keywords: photoinactivation, antimicrobial effect, neutrophil-mediated host defense, chemokine, macrophage inflammatory protein-2
INTRODUCTION
Treatments for bacterial infections in orthopedic fields, for example, arthritis and osteomyelitis, usually involve some difficulties because the blood supply to tissues is less in bone and cartilage. In addition, frequent use of artificial biomaterial implants made from metal or resin easily facilitates the formation of a biofilm [1,2]. Infections caused by multidrug-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) are particularly resistant to conventional therapies including conservative antibiotic therapy. Patients who suffer from these infections have to undergo invasive treatment such as surgical excision and curettage or continuous irrigation in addition to long-term antibiotic administration, resulting in prolonged hospitalization and diminution in the quality of life [3,4]. Therefore, alternative strategies to antibiotics are needed.
Photodynamic therapy (PDT) is a promising therapeutic modality for diseases caused by unwanted tissues or cells. A photosensitizer, such as Photofrin®, accumulates in proliferative tissues such as tumors. After accumulation, the photosensitizer is activated by visible light and induces the generation of singlet oxygen, which damages unwanted cells [5–10]. Over the past decade, there have been an increasing number of reports on therapeutic applications of PDT for bacterial infections, especially for infections with multidrug-resistant bacteria. However, although favorable results of in vitro PDT for cultured bacteria have been described in many reports, good results in vivo in animal models of localized infections have only been described in a few reports [11–16].
We recently demonstrated [17] that in vivo PDT using Photofrin® via a systemic (intravenous) injection for murine MRSA arthritis was not effective, although we confirmed bactericidal activity of the PDT for cultured MRSA in vitro. Pathological examination revealed that intra-articular leukocytes, mainly neutrophils, that had accumulated in the joint to control the MRSA infection died immediately after PDT using Photofrin®. These results suggest that the influence of PDT on leukocytes that have been accumulated in the local infectious site must be considered to achieve clinically effective PDT. Neutrophils have a potent phagocytic activity and thereby play a crucial role in the innate immune system in elimination of invading bacteria, especially S. aureus [18–20]. Malfunction of the phagocytic immune system therefore renders the host susceptible to bacterial infections [21]. If PDT injures neutrophils in the infectious foci, it will impair neutrophil-mediated host defense against bacterial infection, resulting in prolongation and deterioration of the focal infections. When considering a clinical situation of involving PDT of infection we should pay attention to how PDT affects immune cells, especially phagocytes such as neutrophils that may play a role in controlling the infections.
We have speculated that in vivo PDT can induce a sufficient antimicrobial effect in local infections when the damage to leukocytes, particularly neutrophils, by PDT is well controlled. The objective of this study was to verify this hypothesis. Hamblin and Dai [22] reported that direct administration of a photosensitizer to the infected lesion and immediate photoirradiation after administration of the photosensitizer are crucial for reducing damage to mammalian host cells in PDT for local bacterial infection. Along this line, in the present study, we changed the administration route of Photofrin® from intravenous injection to intra-articular direct injection and performed PDT immediately after Photofrin® administration to decrease the affinity of Photofrin® for leukocytes to the minimum level. PDT using Photofrin® via an intra-articular direct injection was carried out in a murine MRSA arthritis model to determine the optimal conditions, especially that of light dose, that minimize the damage to leukocytes and achieve the greatest bactericidal effect in vivo. Relationships between the kinetics of intra-articular leukocyte numbers after PDT and the therapeutic efficacy of PDT under various irradiation conditions were also investigated.
MATERIALS AND METHODS
This study was conducted in accordance with the guidelines of the Institutional Review Board for the Care of Animal Subjects at the National Defense Medical College.
MRSA Strain
MRSA isolated from clinical specimens of a patient in the National Defense Medical College Hospital was used [17]. MRSA was grown in brain heart infusion (BHI, Difco Laboratories, Detroit, MI) supplemented with oxacillin for 48 hours at 37°C under aerobic conditions. Cell density was monitored by absorbance at 600 nm (OD of 0.8 = 1× 108 cells ml−1).
PDT for Cultured MRSA
Photofrin®(Wyeth Lederle Japan, Tokyo) was dissolved in 1×Dulbecco’s phosphate-buffered saline (DPBS; Invitrogen, Japan, Tokyo) (final concentration of 100 μg ml−1). MRSA was suspended in DPBS (final cell density of 1 × 108 CFU ml−1). Then 0.5 ml Photofrin® solution and 0.5 ml MRSA suspension were mixed and transferred to wells of a 12-well plate (Asahi Glass, Tokyo, Japan) under dark conditions at 37°C, and the mixed solutions were immediately irradiated with continuous wave (CW) laser (wavelength of 635 nm; B&W Tec, Newark, NY) at a specified fluence rate for a time sufficient to deliver the required fluence (Table 1). A power meter (Field Master; Coherent, Santa Clara, CA) was used for adjustment of the light fluence rate. Immediately after the irradiation, each mixed solutions were cultured on BHI agar for 24 hours under dark conditions and the numbers of colonies were visually counted. The colony number (colony-forming units; CFU) of MRSA was estimated on the basis of dilution ratio.
TABLE 1.
Applied Light Doses of Photodynamic Therapy
| Fluence (J cm−2) | Fluence rate (mW cm−2) | Time (seconds) |
|---|---|---|
| 5 | 10 | 500 |
| 10 | 20 | 500 |
| 20 | 40 | 500 |
| 30 | 60 | 500 |
| 50 | 71.4 | 700 |
| 70 | 100 | 700 |
Murine MRSA Arthritis Model
Eight to nine-week-old male C57BL/6NCr mice (Japan SLC, Hamamatsu, Japan) were anesthetized using inhalation with isoflurane (Schering-Plough Animal Health, Tokyo, Japan) and the left knees were shaved. Ten microliters of MRSA suspension (5 × 109 CFU ml−1) was intra-articularly injected into the left knee joint through the midline of the patellar ligament using a syringe with a 29 G needle (Terumo, Tokyo, Japan).
PDT for Murine MRSA Arthritis Model in vivo
Under general anesthesia with isoflurane inhalation, 10 μl of Photofrin® solution (100 μg ml−1) was directly administered into the left knee joint 3 days after MRSA administration. The left knee was immediately (drug-light interval<1 minutes) irradiated using the CW laser using six different fluences ranging from 5 to 70 J cm−2 (Table 1). Four other groups of mice were prepared as controls: (1) a PS+IR− group, which received 10 μl of Photofrin® solution (100 μg ml−1) without subsequent photoirradiation, (2) a PS−IR+ group, which received 10 μl of DPBS without Photofrin® followed by photoirradiation, (3) a PS−IR− group, which received 10 μl of DPBS without subsequent photo-irradiation, and (4) a no treatment group (NT group), which did not receive Photofrin® or DPBS administration and received no photoirradiation.
Mice of the NT group were sacrificed 3 days after MRSA administration, and other groups were sacrificed 6 and 24 hours, 2, 3, 5, and 7 days after PDT for analysis of synovial fluid collected from the left knee joint using a syringe with a 27 G needle (Terumo).
Determination of MRSA CFU in Synovial Fluid
The whole collected fluid was immediately dissolved in DPBS. A series of dilutions of the synovial fluid solutions were prepared and cultured on BHI agar for 24 hours under dark conditions. The MRSA CFU in synovial fluid was estimated on the basis of dilution ratio.
Leukocyte Analysis in Synovial Fluid
The collected synovial fluid (2 μl) was immediately mixed with 38 μl of Hanks’ balanced salt solution (HBSS; Sigma–Aldrich, St. Louis, MO), and the number of leukocytes in each synovial fluid was measured using an automatic blood cell counter (PCE-210; Erma, Tokyo, Japan).
Another synovial fluid (2 μl) was immediately mixed with 10 μl of HBSS and 8 μl of 0.4%trypanbluesolution (Sigma–Aldrich), and 10 μl of the mixed solution was dropped onto a Burker-Turk hemacytometer (Erma) and leukocytes were observed using a bright field microscope (CX41, Olympus, Tokyo, Japan). Trypan blue-stained and unstained leukocytes were counted, and the viability of leukocytes (Trypan blue-unstained leukocytes/Total leukocytes per unit field) was calculated. We defined the number of viable leukocytes as the product of viability of leukocytes and total number of leukocytes.
Histopathological Evaluation of the Infected Knee Joint
Mice were sacrificed without collecting synovial fluid. Knee joints were extracted and fixed with 10%formaldehyde solution for 48 hours and then decalcified with 10% ethylenediaminetetraacetic acid (EDTA)-2Na solution (pH 7) for 14 days. The tissue samples were then processed for hematoxylin–eosin (HE) staining.
Measurement of Cytokine and Chemokine Levels in Synovial Fluid
Synovial fluid (2 μl) collected from the left knee joint was immediately mixed with 138 μl of DPBS (1:70 dilution) and centrifuged at 200 × g for 10 minutes. Hundred microliters of supernatant was collected. Concentrationsofinterleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and keratinocyte-derived chemokine (KC) in synovial fluid were determined using a Bio-Plex Suspension Alley System (Bio-Rad Laboratories Japan, Tokyo, Japan). The kits specific for each protein were purchased from Bio-Rad Laboratories and used according to the manufacturer’s instructions.
The supernatant was further diluted to 1:700 and the concentration of macrophage inflammatory protein-2 (MIP-2) in synovial fluid was determined by ELISA using Mouse CXCL2/MIP-2 Immunoassay (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Statistical Analysis
Data are expressed as means ± SE. We first tested variance homoscedasticity between data sets. For the data sets exhibiting equality of variance (homoscedastic), one-way analysis of variance (ANOVA) test followed by Fisher’s least significant difference (LSD) post-hoc test was used, and a nonparametric method using the Kruskal–Wallis test followed by Bonferroni’s post-hoc test was used for the datasets exhibiting unequal variance. Two-way repeated-measures ANOVA test was used for data analysis of time courses. SPSS ver.16 was used for each data analysis. P-values < 0.05 were considered statistically significant.
RESULTS
PDT for Cultured MRSA In Vitro
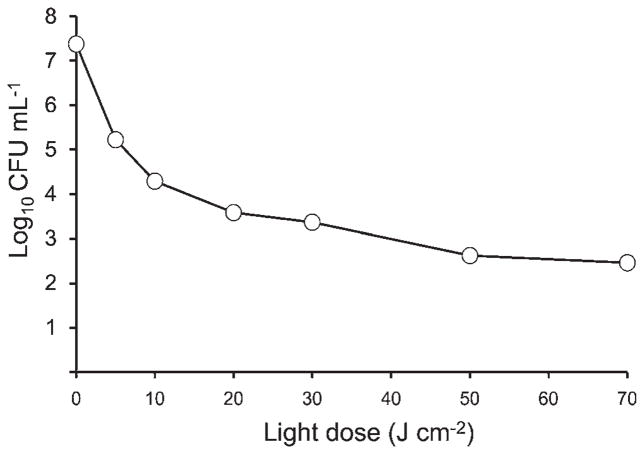
The results shown in Figure 1 demonstrate that PDT using Photofrin® for cultured MRSA exerted light-dose-dependent bactericidal effects with fluences ranging from 5 to 70 J cm−2. The results also demonstrate that there was an initial sharp decline in the numbers of CFU that became a shallower decline as the total fluence increased, which is consistent with photobleaching of Photofrin at higher fluences.
Fig. 1.
Correlation between light dose of PDT and number of MRSA colonies after PDT in vitro using Photofrin® (Log10 CFU ml−1). Concentration of Photofrin® was fixed at 100 μg ml−1. n = 1 each.
PDT for Murine MRSA Arthritis; MRSA Counts in Synovial Fluid
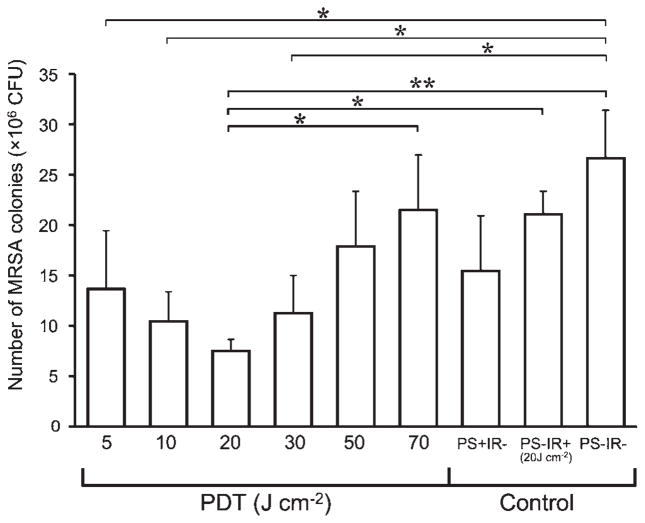
Next PDT was carried out for murine MRSA arthritis to examine the relationship between light dose of PDT and MRSA CFU in synovial fluid 24 hours after PDT (Fig. 2). Six different fluences were employed (5, 10, 20, 30, 50, and 70 J cm−2). The lowest three fluences (5, 10, and 20 J cm−2) showed an increasing antibacterial effect compared to the controls (no treatment, light alone, and Photofrin® alone). However, the next three fluences (30, 50, and 70 J cm−2) showed exactly the opposite effect with a dose-dependent increase in the number of CFU until after the highest (70 J cm−2) the CFU were the same as the control groups. The most effective therapeutic dose of in vivo PDT was 20 J cm−2 and the antimicrobial effect was lessened when the light dose was either lower or higher than 20 J cm−2. This remarkable biphasic light dose response curve has not to our knowledge been previously observed in PDT studies.
Fig. 2.
Effect of photodynamic therapy for murine MRSA arthritis models. PDT groups were photoirradiated at each fluence (J cm−2) immediately after Photofrin® administration intra-articularly into the left knee joint at the concentration of 100 μg ml−1. The PS+IR− group was not photoirradiated after Photofrin® administration. The PS−IR+ group was photoirradiated after administration of Dulbecco’s phosphate-buffered saline (DPBS) instead of Photofrin®. The PS−IR− group was not photoirradiated after DPBS administration. n = 5 in each group. *P < 0.05, **P < 0.01.
Leukocyte Counts and Viability in Synovial Fluid
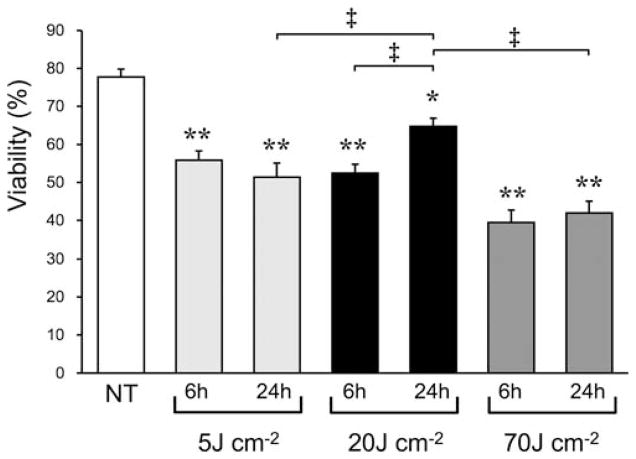
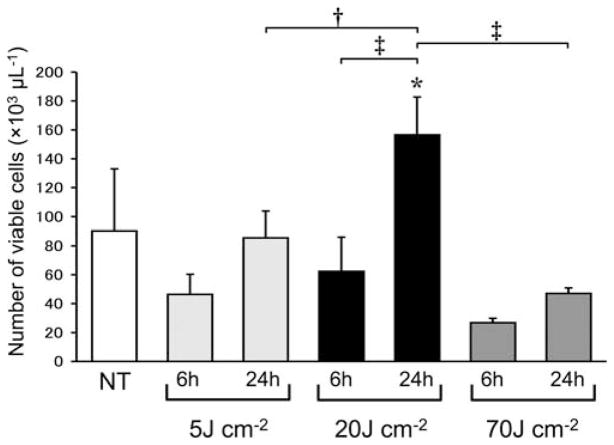
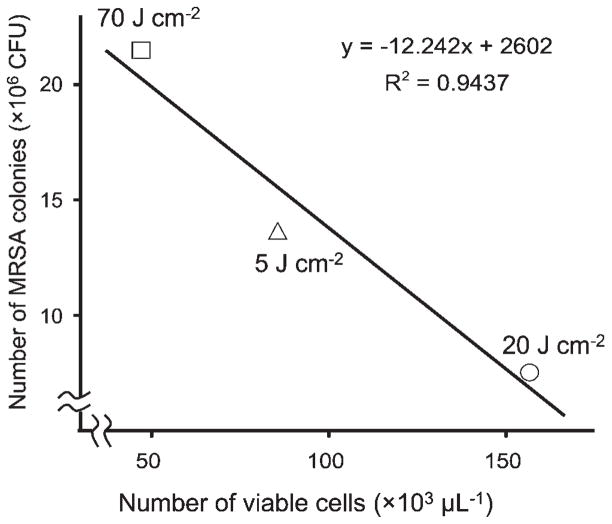
The number and viability of leukocytes in synovial fluid collected from the NT group, and from each PDT treated group (5, 20, and 70 J cm−2) collected 6 or 24 hours later were examined. A significant decrease in the viability of leukocytes was seen in all groups 6 hours after PDT (Fig. 3). However, the 20 J cm−2 group showed a significant increase 24 hours after PDT compared to that seen at 6 hours, while neither the 5 J cm−2 or group nor the 70 J cm−2 2 group showed such an increase (Fig. 3). Although each group showed a decrease in the number of viable leukocytes 6 hours after PDT, the 20 J cm−2 group showed a significant increase in the number of viable leukocytes at 24 hours, while neither the 5 J cm−2 group nor the 70 J cm−2 group showed a significant increase (Fig. 4). In the 24-hours post-PDT phase, the number of viable leukocytes in the 20 J cm−2 group reached 1.6 times the level of the NT group, while neither the 5 J cm−2 group nor the 70 J cm−2 group showed such a remarkable increase. In addition, a significant linear correlation (R2 = 0.9, P < 0.05) between the number of viable leukocytes and the MRSA CFU was seen at the 24-hours post-PDT phase (Fig. 5).
Fig. 3.
Viabilities of leukocytes in synovial fluid from knee joints of murine MRSA arthritis models without Photofrin® administration or photoirradiation (NT group), 6 hours after photoirradiation performed immediately after Photofrin® administration (6 hours groups) and 24 hours after photoirradiation (24 hours groups) at fluences of 5, 20, or 70 J cm−2. n = 5 each. *P < 0.05, **P < 0.01 compared to NT group; ‡P < 0.01.
Fig. 4.
Number of viable leukocytes in synovial fluid from knee joints of the same murine models as shown in Figure 3. n = 5 each. *P < 0.05 compared to NT group; †P < 0.05, ‡P < 0.01.
Fig. 5.
Correlation between number of viable leukocytes and number of MRSA colonies at 24-hours post-PDT phase. A significant correlation was found between the two parameters (R2 = 0.9437, P < 0.05).
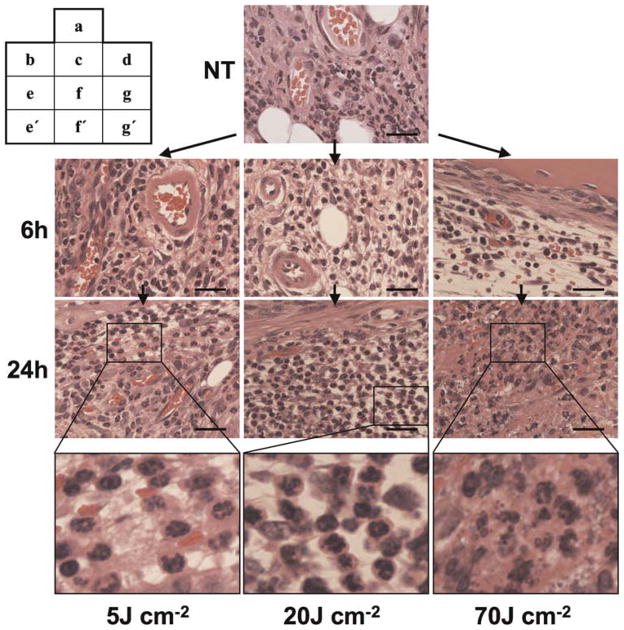
Histopathological Change in Infected Knee Joints
A large number of polymorphonuclear leukocytes, namely neutrophils, were accumulated in MRSA-infected knee joints after 3 days (the NT group), and these neutrophils appeared to be normal by inspection (Fig. 6a). In the 5 J cm−2 PDT group, neither an apparent morphological change in neutrophil appearance nor a change in their number was seen at 6 and 24 hours after PDT, and the structure of synovial microvessels remained intact (Fig. 6b,e,e′). In the 20 J cm−2 group, neither morphological change nor change in the number of neutrophils was seen at 6 hours after PDT (Fig. 6c), while neutrophils that were morphologically intact were markedly increased 24 hours after PDT and synovial microvessels showed normal structure (Fig. 6f and f′). In the 70 J cm−2 group, synovial microvessels were damaged and red blood cells were seen in the synovial tissue 6 hours after PDT (Fig. 6d), and the emergence of an atrophic synovium with hemorrhage and irregularly shaped neutrophils were observed 24 hours after PDT (Fig. 6g,g′).
Fig. 6.
Histopathological images of knee joints of murine MRSA arthritis models. The lesions were collected without Photofrin® administration or photoirradiation (NT group, a), 6 hours after photoirradiation (b–d) and 24 hours after photoirradiation (e–g, e′, f′, g′), HE staining. Before photoirradiation (a), many polymorphonuclear leukocytes (neutrophils) with normal appearance are seen in proliferated synovial tissue. No morphological change or variation in number of neutrophils is seen in the PDT-treated knee joints with a fluence of 5 J cm−2 at each time (b, e, e′), whereas no morphological change are seen in the PDT-treated knee joints with a fluence of 20 J cm−2 at 6 hours after photoirradiation (c), and a remarkable increase of morphologically intact neutrophils is seen 24 hours after photoirradiation (f, f′). However, in the case of PDT with a fluence of 70 J cm−2, blood vessel destruction complicated with hemorrhage is seen at 6 hours after irradiation (d), and nonspheroidal leukocytes and atrophic changes of the synovium are seen at 24 hours after irradiation (g, g′). Bar = 30 μm.
Quantitative Analysis of Cytokines and Chemokines in Synovial Fluid
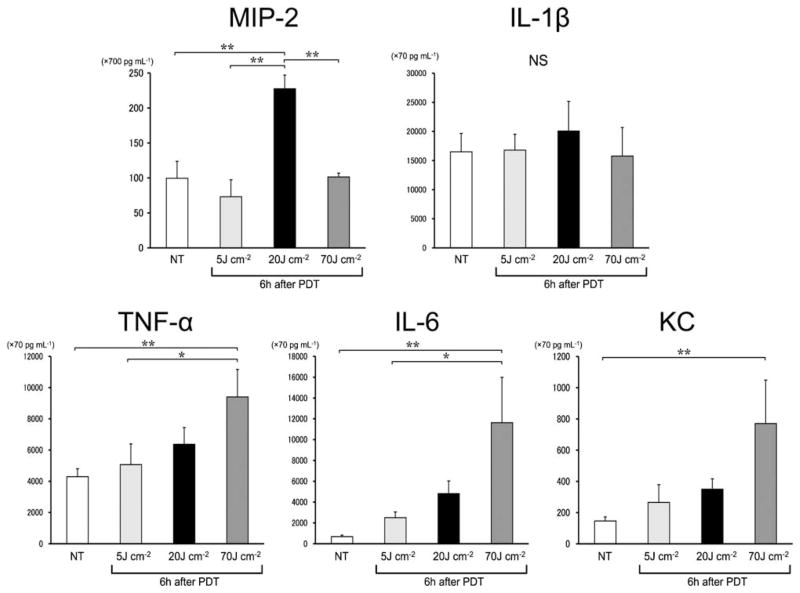
The level of MIP-2, a CXC chemokine that is closely involved in neutrophil chemotaxis [23] was significantly increased in the synovial fluid 6 hours after PDT in the 20 J cm−2 group but not in the 5 or 70 J cm−2 group. Significant increase in the IL-1β level of synovial fluid was not seen in any groups 6 hours after PDT. TNF-α, IL-6, and KC levels in the synovial fluid were correlated with light doses, thus indicating that these cytokines/chemokines reached the highest levels when the light dose was 70 J cm−2(Fig. 7). Twenty-four hours after PDT, however, the cytokines or chemokines did not show a significant elevation in each energy group when compared to the level of the NT group (data not shown).
Fig. 7.
Values of intra-articular cytokines and chemokines of murine MRSA models prior to PDT (NT) and 6 hours after PDT. IL-1β value shows no significant difference between the groups, whereas MIP-2 value shows a significantly higher level in the 20 J cm−2 group. The values of TNF-a, IL-6, and KC tend to be higher in proportion to the light doses. n = 5 each. *P < 0.05, **P < 0.01.
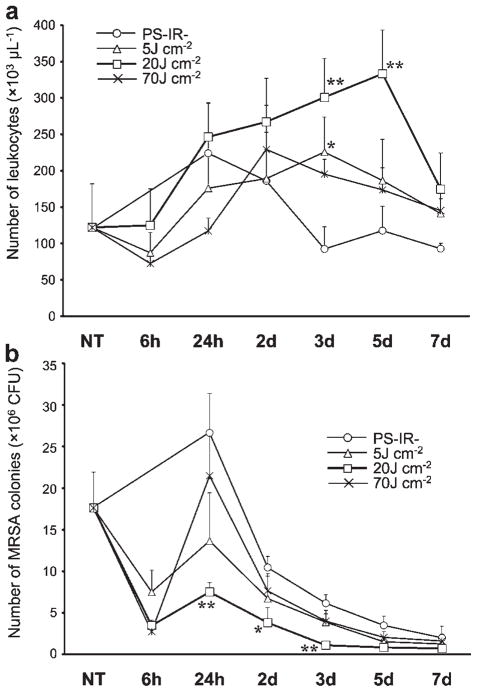
Time Courses of Intra-Articular MRSA and Leukocyte Counts
Intra-articular leukocytes in synovial fluid were increased in all samples taken from 24 hours to 5 days after PDT with a fluence of 20 J cm−2 (Fig. 8a). At this light dose, the MRSA CFU were remarkably decreased in the early period (from 24 hours to 3 days) after PDT compared to those in the control PS−IR− group at each time point (Fig. 8b). In the 5 J cm−2 group, an increase in leukocytes was only seen 3 days after PDT and this rise was transient (Fig. 8a); however, the MRSA CFU were not significantly different from those in the control group at each time point (Fig. 8b). In the 70 J cm−2 group, leukocytes did not significantly increase after PDT (Fig. 8a). Although MRSA CFU decreased to the minimum level 6 hours after PDT with a fluence of 70 J cm−2, the CFU markedly increased at 24 hours. As a result, the MRSA CFU was not significantly different from that in the control group at each time point in the period from 24 hours to 7 days after PDT (Fig. 8b).
Fig. 8.
Time courses of the numbers of intra-articular leukocytes (a) and MRSA colonies (b) after photodynamic therapy. n = 5 each. *P < 0.05, **P < 0.01 compared to the number in the same period of the control (PS−IR−) group.
DISCUSSION
Based on our previous results [17] and the suggestion by Hamblin and Dai [22], PDT using Photofrin® via an intra-articular direct injection was carried out immediately after administration of Photofrin® in murine MRSA arthritis models to decrease the affinity of Photofrin® for leukocytes and thus to reduce the damage to leukocytes caused by PDT. Although in vitro PDT using Photofrin® showed a light-dose-dependent bactericidal effect on cultured MRSA, in vivo PDT exhibited a remarkable biphasic light dose response of MRSACFU in synovial fluid. PDT with a fluence of 20 J cm−2 gave a clear maximal antibacterial effect on MRSA, while simultaneously maximizing the number and viability of leukocytes, mainly neutrophils, accumulated at the infection site. We speculated that PDT with this light dose might facilitate leukocyte accumulation to the infected knee joint as well as killing bacteria, resulting in a further favorable neutrophil-mediated therapeutic effect of PDT on MRSA arthritis. On the other hand, PDT with a fluence of 70 J cm−2 severely injured leukocytes/neutrophils in the infected tissues without a corresponding increase in the bacterial killing, and the overall effect was to produce a lesser antibacterial effect, which was similar to previous results we obtained using intravenously injected Photofrin®-PDT [17].
Di Poto et al. [24] have reported results from in vitro experiments of PDT on a staphylococcal biofilm: PDT followed by incubation of biofilms with phagocytes or with antibiotics produced a higher bactericidal effect compared to PDT alone, suggesting that in vivo PDT might also induce an effective bactericidal effect only with the cooperation of phagocytes or antibiotics as well. In general, spatially homogeneous photoirradiation on living tissue is difficult because of its anatomical complexity and optical inhomogeneity. Some bacteria inevitably survive even after a high-fluence PDT and resume proliferation and growth. Therefore, in vivo PDT requires the assistance/cooperation of a host defense system against bacteria, such as neutrophil-mediated defense; hence, regulation/suppression of the PDT-induced damage to neutrophils is important to maintain neutrophil-mediated host defense and exert effective bactericidal activity of PDT.
Although many studies have demonstrated neutrophil accumulation after anti-tumor PDT [25–30], the influence of neutrophil accumulation after PDT on the anti-tumor effect has remained controversial: some studies have shown a strong correlation between accumulated neutrophils and anti-tumor effect [25–28], while such a correlation was not found in other studies [29,30]. In the case of bacterial infection, neutrophils are crucial for host defense against bacterial invasion and proliferation [18–20]. The present study confirmed for the first time a strong correlation between accumulated neutrophils and bactericidal effect after PDT. PDT using an appropriate fluence (20 J cm−2) facilitated neutrophil accumulation into the local infection site, leading to an enhanced therapeutic effect of PDT against focal bacterial infection.
PDT using Photofrin® with a fluence of 20 J cm−2 increased synovial MIP-2 level and also increased leukocyte/neutrophil count in the infected knee joint. MIP-2 is a CXC chemokine secreted from macrophages and synovial fibroblasts as a signal for attracting neutrophils into the infectious site by its concentration gradient [23]. Göllnick et al. [25] have demonstrated that MIP-2 is closely related to neutrophil accumulation in a PDT-treated malignant tumor lesion. Although synovial MIP-2 level had returned to the initial level at 24 hours after PDT, a significant increase of leukocytes in the PDT-treated knee joint was observed for 5 days, suggesting that the initial elevation of MIP-2 in the infectious site is important to induce neutrophil chemotaxis.
Although PDT using Photofrin® with a fluence of 70 J cm−2 remarkably reduced the colony number of MRSA 6 hours after PDT, MRSA rapidly re-increased at 24 hours. Not only synovial tissue including microvessels but also intra-articular leukocytes were severely injured 6 hours after PDT with a fluence of 70 J cm−2, and atrophic synovium with hemorrhage and irregularly shaped neutrophils were found at 24 hours. TNF-α, IL-6, and KC (butnotMIP-2) levels, which reflect tissue injuries, were significantly increased 6 hours after PDT, suggesting that PDT with an excessive light dose injures synovial tissue and also accumulated leukocytes. These findings are reinforced by results of studies regarding PDT-induced injury to synovial tissue and/or blood vessels [31–34]. Degenerative atrophy of the synovium and destruction of blood vessels in synovial tissue were observed by histological examination after PDT. Thus, reduction of the therapeutic efficacy of PDT with a fluence of 70 cm−2 was presumably due to damage to (1) intra-articular neutrophils, (2) macrophages and fibroblasts, which are MIP-2-producing cells, and (3) synovial tissue and vessels, which are routes of neutrophil supply.
The therapeutic window of Photofrin®-PDT is narrow because Photofrin lacks selectivity for bacteria over host cells. Even when the PDT parameters are optimized, it is likely that a good therapeutic effect would be difficult to obtain clinically. The present study suggests that an ideal photosensitizer for this disease must have properties of selective accumulation in bacteria and of avoiding accumulation in immune cells or host tissue. A photosensitizer that shows high selectivity for bacteria and low accumulation in host cells may allow effective treatment of bacterial arthritis.
With regard to clinical applicability, besides the use of a selective photosensitizer, the development of a device that enables uniform and efficient light irradiation is required to a site that has a complicated anatomical structure such as a knee joint. A device using diffuse irradiation-type optical fiber or a surrounding cuff type irradiation equipment should be considered.
In conclusion, directly administrated Photofrin®-PDT raised synovial MIP-2 level and facilitated neutrophil accumulation into a local infection site, leading to an optimal therapeutic effect of PDT when the light dose was 20 J cm−2. The therapeutic efficacy of Photofrin®-PDT was closely related to the preservation of intra-articular leukocytes and tissue structure including microvessels after PDT. When PDT is applied for in vivo local bacterial infections, irradiation conditions, particularly with regard to the state of immunocompetent cells, should be considered to elicit a favorable therapeutic effect.
Acknowledgments
This work was supported by the Young Scholar Encouragement Program of National Defense Medical College. M. R. H. was supported by US NIH (grant RO1AI050875), US Air Force MFEL program (contract FA9550-04-1-0079), Center for Integration of Medicine and Innovative Technology (DAMD17-02-2-0006), Congressionally Directed Medical Research Program (W81XWH-09-1-0514).
Contract grant sponsor: US NIH; Contract grant number: RO1AI050875; Contract grant sponsor: US Air Force MFEL program; Contract grant number: FA9550-04-1-0079; Contract grant sponsor: Center for Integration of Medicine and Innovative Technology; Contract grant number: DAMD17-02-2-0006; Contract grant sponsor: Congressionally Directed Medical Research Program; Contract grant number: W81XWH-09-1-0514.
References
- 1.Neut D, van der Mei HC, Bulstra SK, Busscher HJ. The role of small-colony variants in failure to diagnose and treat biofilm infections in orthopedics. Acta Orthop. 2007;78:299–308. doi: 10.1080/17453670710013843. [DOI] [PubMed] [Google Scholar]
- 2.Gallo J, Kolar M, Novotny R, Rihakova P, Ticha V. Pathogenesis of prosthesis-related infection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147:27–35. doi: 10.5507/bp.2003.004. [DOI] [PubMed] [Google Scholar]
- 3.Patel A, Calfee RP, Plante M, Fischer SA, Arcand N, Born C. Methicillin-resistant Staphylococcus aureus in orthopedic surgery. J Bone Joint Surg Br. 2008;90:1401–1406. doi: 10.1302/0301-620X.90B11.20771. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill E, Pozzi C, Houston P, Smyth D, Humphreys H, Robinson DA, O’Gara JP. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J Clin Microbiol. 2007;45:1379–1388. doi: 10.1128/JCM.02280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loewen GM, Pandey R, Bellnier D, Henderson B, Dougherty T. Endobronchial photodynamic therapy for lung cancer. Lasers Surg Med. 2006;38:364–370. doi: 10.1002/lsm.20354. [DOI] [PubMed] [Google Scholar]
- 6.Mimura S, Narahara H, Otani T, Okuda S. Progress of photodynamic therapy in gastric cancer. Diagn Ther Endosc. 1999;5:175–182. doi: 10.1155/DTE.5.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: What, where, why, and how. Photochem Photobiol Sci. 2002;1:1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad N, Mukhtar H. Mechanism of photodynamic therapy-induced cell death. Methods Enzymol. 2000;319:342–358. doi: 10.1016/s0076-6879(00)19034-2. [DOI] [PubMed] [Google Scholar]
- 9.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagishi Y, Morimoto Y, Fujita M, Morimoto N, Ozeki Y, Maehara T, Kikuchi M. Photodynamic therapy for airway stenosis in rabbit models. Chest. 2008;133:123–130. doi: 10.1378/chest.07-1410. [DOI] [PubMed] [Google Scholar]
- 11.Berthiaume F, Reiken SR, Toner M, Tompkins RG, Yarmush ML. Antibody-targeted photolysis of bacteria in vivo. Biotechnology (NY) 1994;12:703–706. doi: 10.1038/nbt0794-703. [DOI] [PubMed] [Google Scholar]
- 12.Gad F, Zahra T, Francis KP, Hasan T, Hamblin MR. Targeted photodynamic therapy of established soft-tissue infections in mice. Photochem Photobiol Sci. 2004;3:451–458. doi: 10.1039/b311901g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambrechts SA, Demidova TN, Aalders MC, Hasan T, Hamblin MR. Photodynamic therapy for Staphylococcus aureus infected burn wounds in mice. Photochem Photobiol Sci. 2005;4:503–509. doi: 10.1039/b502125a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisland SK, Chien C, Wilson BC, Burch S. Pre-clinical in vitro and in vivo studies to examine the potential use of photodynamic therapy in the treatment of osteomyelitis. Photochem Photobiol Sci. 2006;5:31–38. doi: 10.1039/b507082a. [DOI] [PubMed] [Google Scholar]
- 15.Zolfaghari PS, Packer S, Singer M, Nair SP, Bennett J, Street C, Wilson M. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:27. doi: 10.1186/1471-2180-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg Med. 2010;42:38–44. doi: 10.1002/lsm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka M, Kinoshita M, Yoshihara Y, Shinomiya N, Seki S, Nemoto K, Morimoto Y. Influence of intra-articular neutrophils on the effects of photodynamic therapy for murine MRSA arthritis. Photochem Photobiol. 2010;86:403–409. doi: 10.1111/j.1751-1097.2009.00658.x. [DOI] [PubMed] [Google Scholar]
- 18.DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am. 2009;23:17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21:215–226. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun. 1997;65:2517–2521. doi: 10.1128/iai.65.7.2517-2521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massey E, Paulus U, Doree C, Stanworth S. Granulocyte transfusions for preventing infections in patients with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev. 2009;21:CD005341. doi: 10.1002/14651858.CD005341.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Hamblin MR, Dai T. Can surgical site infections be treated by photodynamic therapy? Photodiagnosis Photodyn Ther. 2010;7:136–138. doi: 10.1016/j.pdpdt.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsujimoto H, Ono S, Majima T, Kawarabayashi N, Takayama E, Kinoshita M, Seki S, Hiraide H, Moldawer LL, Mochizuki H. Neutrophil elastase, MIP-2, and TLR-4 expression during human and experimental sepsis. Shock. 2005;23:39–44. doi: 10.1097/01.shk.0000145936.31967.d7. [DOI] [PubMed] [Google Scholar]
- 24.Di Poto A, Sbarra MS, Provenza G, Visai L, Speziale P. The effect of photodynamic treatment combined with antibiotic action or host defense mechanisms on Staphylococcus aureus biofilms. Biomaterials. 2009;30:3158–3166. doi: 10.1016/j.biomaterials.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 25.Göllnick SO, Evans SS, Baumann H, Owczarczak B, Maier P, Vaughan L, Wang WC, Unger E, Henderson BW. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br J Cancer. 2003;88:1772–1779. doi: 10.1038/sj.bjc.6600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vree WJ, Essers MC, de Bruijn HS, Star WM, Koster JF, Sluiter W. Evidence for an important role of neutrophils in the efficacy of photodynamic therapy in vivo. Cancer Res. 1996;56:2908–2911. [PubMed] [Google Scholar]
- 27.Sun J, Cecic I, Parkins CS, Korbelik M. Neutrophils as inflammatory and immune effectors in photodynamic therapy-treated mouse SCCVII tumours. Photochem Photobiol Sci. 2002;1:690–695. doi: 10.1039/b204254a. [DOI] [PubMed] [Google Scholar]
- 28.Kousis PC, Henderson BW, Maier PG, Gollnick SO. Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Res. 2007;67:10501–10510. doi: 10.1158/0008-5472.CAN-07-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuitmaker JJ, de Koster BM, Elferink JG. The effects of photodynamic therapy on human neutrophil migration using bacteriochlorin a. Photochem Photobiol. 1998;68:841–845. [PubMed] [Google Scholar]
- 30.de Bruijn HS, Sluiter W, van der Ploeg-van den Heuvel A, Sterenborg HJ, Robinson DJ. Evidence for a bystander role of neutrophils in the response to systemic 5-aminolevulinic acid-based photodynamic therapy. Photodermatol Photoimmunol Photomed. 2006;22:238–246. doi: 10.1111/j.1600-0781.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- 31.Torikai E, Kageyama Y, Kohno E, Hirano T, Koide Y, Terakawa S, Nagano A. Photodynamic therapy using talaporfin sodium for synovial membrane from rheumatoid arthritis patients and collagen-induced arthritis rats. Clin Rheumatol. 2008;27:751–761. doi: 10.1007/s10067-007-0794-8. [DOI] [PubMed] [Google Scholar]
- 32.Bagdonas S, Kirdaite G, Streckyte G, Graziene V, Leonaviciene L, Bradunaite R, Venalis A, Rotomskis R. Spectroscopic study of ALA-induced endogenous porphyrins in arthritic knee tissues: Targeting rheumatoid arthritis PDT. Photochem Photobiol Sci. 2005;4:497–502. doi: 10.1039/b503790e. [DOI] [PubMed] [Google Scholar]
- 33.Beischer AD, Bhathal P, deSteiger R, Penn D, Stylli S. Synovial ablation in a rabbit rheumatoid arthritis model using photodynamic therapy. ANZ J Surg. 2002;72:517–522. doi: 10.1046/j.1445-2197.2002.02451.x. [DOI] [PubMed] [Google Scholar]
- 34.Fingar VH, Wieman TJ, Wiehle SA, Cerrito PB. The role of microvascular damage in photodynamic therapy: The effect of treatment on vessel constriction, permeability, and leukocyte adhesion. Cancer Res. 1992;52:4914–4921. [PubMed] [Google Scholar]