Abstract
Background
The β2-adrenergic receptor (ADRB2) gene has been widely researched as a candidate gene for essential hypertension (EH), but no consensus has been reached in different ethnicities. The aim of the present study was to evaluate the possible association between the ADRB2 gene polymorphisms and the EH risk in the Northern Han Chinese population.
Methodology/Principal Findings
This study included 747 hypertensive subjects and 390 healthy volunteers as control subjects in the Northern Han Chinese. Genotyping was performed to identify the C-47T, A46G and C79G polymorphisms of the ADRB2 gene. G allelic frequency of A46G polymorphism was significantly higher in hypertensive subjects (P = 0.011, OR = 1.287, 95%CI [1.059–1.565]) than that in controls. Significant association could also be found in dominant genetic model (GG+AG vs. AA, P = 0.006, OR = 1.497, 95%CI [1.121–1.998]), in homozygote comparison (GG vs. AA, P = 0.025, OR = 1.568, 95%CI [1.059–2.322]), and in additive genetic model (GG vs. AG vs. AA, P = 0.012, OR = 1.282, 95%CI [1.056–1.555]). Subgroup analyses performed by gender suggested that this association could be found in male, but not in female. Stratification analyses by obesity showed that A46G polymorphism was related to the prevalence of hypertension in the obese population (GG vs. AG vs. AA, P<0.001, OR = 1.645, 95%CI [1.258–2.151]). Significant interaction was found between A46G genotypes and body mass index on EH risk. No significant association could be found between C-47T or C79G polymorphism and EH risk. Linkage disequilibrium was detected between the C-47T, A46G and C79G polymorphisms. Haplotype analyses observed that the T-47-A46-C79 haplotype was a protective haplotype for EH, while the T-47-G46-C79 haplotype increased the risk.
Conclusions/Significances
We revealed that the ADRB2 A46G polymorphism might increase the risk for EH in the Northern Han Chinese population.
Introduction
Essential hypertension (EH) is a worldwide escalating problem. In China, it was reported that 27.2% of the adult population age 35 to 74 years suffered from it [1]. EH is a highly heterogeneous disorder, which points to a multi-factorial aetiology and polygenic abnormalities [2]. As a consequence, a lot of gene polymorphisms have been assessed as candidate determinants of the risk of hypertension. In the search for the inheritable determinants of EH phenotype in humans, the gene encoding for the β2-adrenergic receptor (ADRB2) has been investigated worldwide, since in hypertension vascular responses to ADRB2 stimulation are impaired [3], [4] and the ADRB2 gene polymorphisms appear to affect vasodilation [5], [6].
At the molecular level, the role of the ADRB2 gene in hypertension has been extensively evaluated. In vitro, the research [7] on the single-nucleotide polymorphisms (SNPs) in the coding region suggested that Arg16→Gly (rs1042713, A46G), Gln27→Glu (rs1042714, C79G), and Arg16→Gly +Gln27→Glu, compared to wild-type ADRB2, displayed normal agonist binding and functional coupling to the stimulatory form of G protein (Gs), resulting in the stimulation of adenylyl cyclase activity. In vivo, it was reported that both polymorphisms might contribute to enhanced vascular reactivity to isoproterenol in capacitance vessel, which played a part in the regulation of blood pressure [5]. Another SNP at nucleotide position -47, C-47T (Arg-19Cys, rs1042711), is functionally important since it is located within a short open reading frame, the 5′-leader cistron, and affects ADRB2 expression at a translational level [8]. Studies have also identified that C-47T polymorphism was in linkage disequilibrium (LD) with the A46G and C79G polymorphisms in the coding region [9]–[11].
A substantial number of studies have previously investigated the association between the A46G, C79G and C−47T polymorphisms and EH risk. Several of these failed to identify any association [11]–[18]. Other studies have found significant associations [9], [19]–[24], but there is no consensus regarding which allele was associated with hypertension or related traits. It has reported that the GG46 genotype remarkably increased the risk for EH in Japanese [19]. Interestingly, another study on the East Asian population suggested that the GG46 genotype played a protective role for EH in the Yi minority of Chinese [9]. Also, in the Han Chinese population, consistent results have rarely been found for particular candidate SNPs [23]–[25], as the Han Chinese population was considered be intricately substructured, corresponding roughly to Northern Han, Central Han, and Southern Han, based on the populations of the geographic origins [26]. To clarify the effect of these 3 polymorphisms on the risk of hypertension in the Northern Han Chinese population, we conducted a case-control study in middle-aged and older humans.
Results
Characteristics of the participants
A total of 1,137 unrelated participated subjects comprising 747 hypertensive patients (479 men and 268 women; mean age = 51.52; SD±9.46) and 390 normotensive control subjects (233 men and 157 women; mean age = 51.02; SD±7.66) were recruited for the present study. The clinical and laboratory parameters of cases and controls were summarized in Table 1. Aside from blood pressure measurements, significant differences in body mass index (BMI), total chelesterol, high-density lipoprotein cholesterol, triglyceride, glucose, the ratio of drinkers were observed between the hypertensives and the normotensives.
Table 1. Characteristics of study participants.
| Hypertension | Normotension | ||
| (n = 747) | (n = 390) | P | |
| gender, M/F | 479/268 | 233/157 | NS |
| age(years) | 51.52±9.46 | 51.02±7.66 | NS |
| SBP(mmHg) | 153.41±19.65 | 114.33±11.07 | <0.001 |
| DBP(mmHg) | 99.18±14.20 | 74.04±8.26 | <0.001 |
| BMI(kg/m2) | 26.88±3.45 | 24.90±3.20 | <0.001 |
| TC(mmol/L) | 5.56±2.98 | 5.13±1.07 | <0.001 |
| HDL-C(mmol/L) | 1.26±0.62 | 1.44±1.17 | 0.006 |
| LDL-C(mmol/L) | 3.38±0.87 | 3.45±0.78 | NS |
| TG(mmol/L) | 2.11±1.36 | 1.69±1.05 | <0.001 |
| Glu(mmol/L) | 5.38±0.61 | 5.08±0.59 | <0.001 |
| Cr(µmol/L) | 78.97±18.70 | 77.67±14.68 | NS |
| ALT(U/L) | 25.62±13.19 | 24.48±12.44 | NS |
| HR(bpm) | 71.30±9.61 | 70.73±9.20 | NS |
| Smokers(n) | 193 | 102 | NS |
| Drinkers(n) | 223 | 53 | <0.001 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; Glu, glucose; Cr, creatinine; ALT, alanine aminotransferase; HR, heart rate.
Values are mean±SD.
NS indicates not significant.
Association analyses
Among all the participants, 99.3% samples of C-47T polymorphism, 98.3% samples of A46G polymorphism and 97.7% samples of C79G polymorphism were successfully tested in the laboratory. No deviation from Hardy-Weinberg expectation was observed for C-47T, A46G or C79G polymorphism in either hypertensives or normotensives. The genotype frequencies for the 3 polymorphisms in ADRB2 were shown in Table 2. Univariate analyses indicated that the A46G polymorphism was significantly associated with EH. The significantly higher prevalence of G allelic frequencies was observed in the hypertensives than in the normotensives.
Table 2. The frequencies of the β2-adrenergic receptor gene C-47T, A46G and C79G polymorphisms genotypes.
| Genotype (frequency,%) | P* | Allele (frequency,%) | P** | |||||
| C-47T | TT | CT | CC | T allele | C allele | |||
| Case (Total) | 571(77.2) | 163(22.0) | 6(0.8) | 1305(88.2) | 175(11.8) | |||
| Control (Total) | 314(80.7) | 68(17.5) | 7(1.8) | 0.358 | 696(89.5) | 82(10.5) | 0.361 | |
| Case (Male) | 371(78.3) | 100(21.1) | 3(0.6) | 842(88.8) | 106(11.2) | |||
| Control (Male) | 187(80.6) | 41(17.7) | 4(1.7) | 0.723 | 415(89.4) | 49(10.6) | 0.726 | |
| Case (Female) | 200(75.2) | 63(23.7) | 3(1.1) | 463(87.0) | 69(13.0) | |||
| Control (Female) | 127(80.9) | 27(17.2) | 3(1.9) | 0.289 | 281(89.5) | 33(10.5) | 0.288 | |
| A46G | AA | AG | GG | A allele | G allele | |||
| Case (Total) | 208(28.3) | 369(50.2) | 158(21.5) | 785(53.4) | 685(46.6) | |||
| Control (Total) | 143(37.3) | 174(45.4) | 66(17.2) | 0.003 | 460(60.1) | 306(39.9) | 0.003 | |
| Case (Male) | 135(28.8) | 239(51.0) | 95(20.3) | 509(54.3) | 429(45.7) | |||
| Control (Male) | 94(40.9) | 98(42.6) | 38(16.5) | 0.006 | 286(62.2) | 174(37.8) | 0.005 | |
| Case (Female) | 73(27.4) | 130(48.9) | 63(23.7) | 276(51.9) | 256(48.1) | |||
| Control (Female) | 49(32.0) | 76(49.7) | 28(18.3) | 0.167 | 174(56.9) | 132(43.1) | 0.164 | |
| C79G | CC | CG | GG | C allele | G allele | |||
| Case (Total) | 566(76.8) | 164(22.3) | 7(0.9) | 1296(87.9) | 178(12.1) | |||
| Control (Total) | 301(80.5) | 66(17.6) | 7(1.9) | 0.335 | 668(89.3) | 80(10.7) | 0.337 | |
| Case (Male) | 367(77.9) | 100(21.2) | 4(0.8) | 834(88.5) | 108(11.5) | |||
| Control (Male) | 185(80.4) | 41(17.8) | 4(1.7) | 0.649 | 411(89.3) | 49(10.7) | 0.650 | |
| Case (Female) | 199(74.8) | 64(24.1) | 3(1.1) | 462(86.8) | 70(13.2) | |||
| Control (Female) | 116(80.6) | 25(17.4) | 3(2.1) | 0.319 | 257(89.2) | 31(10.8) | 0.319 | |
*P value of the comparison of the additive genetic model using the generalized linear model.
**P value of the comparison of allelic frequencies.
Logistic regression analysis was performed after adjusting for confounding risk variables, that is, gender, age, BMI, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, serum triglyceride levels, plasma glucose level, smoking habits and drinking habits. For A46G polymorphism, we observed a significantly higher prevalence of G allelic frequencies (P = 0.011, OR = 1.287, 95%CI [1.059–1.565]) in the hypertensives than the normotensives, which suggests that G allele might be a risk factor for hypertension in the Northern Han Chinese. Significant association could also be found in dominant genetic model (GG+AG vs. AA, P = 0.006, OR = 1.497, 95%CI [1.121–1.998]), in homozygote comparison (GG vs. AA, P = 0.025, OR = 1.568, 95%CI [1.059–2.322]), and in additive genetic model (GG vs. AG vs. AA, P = 0.012, OR = 1.282, 95%CI [1.056–1.555]). No significant association could be found between C-47T or C79G polymorphism and EH risk. (Table 3) Subgroup analyses were performed by gender and showed that significant effect between A46G polymorphism and EH risk could be found in male, but not in the subgroup of female. The P-value for A46G genotype-gender interaction was 0.617, which suggested that no significant interaction was found. As for the C-47T or C79G polymorphism, no significant association was found in either subgroup. (Table 3)
Table 3. Odds ratios of additive genetic model comparison for each single-nucleotide polymorphism genotype associated with essential hypertension in the Northern Han Chinese population.
| Overall | Male* | Female* | |||||
| SNP | Contrast | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| C-47T | CC vs. CT vs. TT | 1.012(0.746–1.374) | 0.940 | 0.910(0.611–1.355) | 0.642 | 1.156(0.709–1.887) | 0.560 |
| A46G | GG vs. AG vs. AA | 1.282(1.056–1.555) | 0.012 | 1.332(1.037–1.709) | 0.025 | 1.176(0.854–1.623) | 0.319 |
| C79G | GG vs. CG vs. CC | 1.032(0.760–1.401) | 0.841 | 0.956(0.645–1.418) | 0.825 | 1.138(0.692–1.873) | 0.611 |
ORs adjusted for gender, age, body mass index, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, serum triglyceride levels, plasma glucose level, smoking habits and drinking habits. OR, odds ratio; CI, confidence interval; SNP, single-nucleotide polymorphism.
*ORs adjusted for gender was not performed in Male and Female.
P-value for the interaction between A46G genotype and gender on hypertension was 0.617.
Interactive effect of obesity and the ADRB2 gene polymorphisms on hypertension
To explore the interactive effect of obesity and the ADRB2 gene polymorphisms on hypertension, we analyzed the relation between C-47T, A46G and C79G polymorphisms and hypertension by stratification analyses. All the participants were divided into the obese subgroup and the non-obese subgroup according to BMI. The case-control study was further performed in both subgroups. Table 4 shows the genotype distributions and allele frequencies in obese and non-obese. As shown in Table 5, A46G polymorphism was related to the prevalence of hypertension in the obese (GG vs. AG vs. AA, P<0.001, OR = 1.645, 95%CI [1.258–2.151]). The association could also be found in the subgroup of obese men (GG vs. AG vs. AA, P = 0.005, OR = 1.603, 95%CI [1.153–2.227]) and obese women (GG vs. AG vs. AA, P = 0.028, OR = 1.739, 95%CI [1.062–2.849]). Whereas in the non-obese subjects, no significant association could be found between A46G polymorphism and EH risk. The P-value for A46G genotype-BMI interaction was 0.010. This result indicated that significant interaction existed between A46G genotypes and obesity on hypertension. There was no difference in genotype distribution of C-47T or C79G polymorphism between the hypertensive and the normotensive, neither in the subgroup of the obese subjects, nor in the non-obese participants (P>0.05).
Table 4. The genotype distributions and allele frequencies of the β2-adrenergic receptor gene C-47T, A46G and C79G polymorphisms in obese and non-obese.
| Polymorphism | Total (n = 1137) | Male (n = 712) | Female (n = 425) | |||||||||
| Obese (n = 713) | Non-obese (n = 424) | Obese (n = 487) | Non-obese (n = 225) | Obese (n = 226) | Non-obese (n = 199) | |||||||
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
| (n = 533) | (n = 180) | (n = 214) | (n = 210) | (n = 365) | (n = 122) | (n = 114) | (n = 111) | (n = 168) | (n = 58) | (n = 100) | (n = 99) | |
| C-47T | ||||||||||||
| TT (n) | 397 | 145 | 174 | 169 | 277 | 98 | 94 | 89 | 120 | 47 | 80 | 80 |
| CT (n) | 125 | 32 | 38 | 36 | 81 | 23 | 19 | 18 | 44 | 9 | 19 | 18 |
| CC (n) | 6 | 3 | 0 | 4 | 3 | 1 | 0 | 3 | 3 | 2 | 0 | 1 |
| C allele frequency | 0.130 | 0.106 | 0.090 | 0.105 | 0.120 | 0.102 | 0.084 | 0.109 | 0.150 | 0.112 | 0.096 | 0.101 |
| A46G | ||||||||||||
| AA (n) | 142 | 74 | 66 | 69 | 101 | 53 | 34 | 41 | 41 | 21 | 32 | 28 |
| AG (n) | 258 | 82 | 111 | 92 | 176 | 53 | 63 | 45 | 82 | 29 | 48 | 47 |
| GG (n) | 124 | 22 | 34 | 44 | 80 | 15 | 15 | 23 | 44 | 7 | 19 | 21 |
| G allele frequency | 0.483 | 0.354 | 0.424 | 0.439 | 0.471 | 0.343 | 0.415 | 0.417 | 0.509 | 0.377 | 0.434 | 0.464 |
| C79G | ||||||||||||
| CC (n) | 395 | 141 | 171 | 160 | 275 | 97 | 92 | 88 | 120 | 44 | 79 | 72 |
| CG (n) | 126 | 32 | 38 | 34 | 81 | 23 | 19 | 18 | 45 | 9 | 19 | 16 |
| GG (n) | 6 | 3 | 1 | 4 | 3 | 1 | 1 | 3 | 3 | 2 | 0 | 1 |
| G allele frequency | 0.131 | 0.108 | 0.095 | 0.106 | 0.121 | 0.103 | 0.094 | 0.110 | 0.152 | 0.118 | 0.097 | 0.101 |
Table 5. Stratified analyses of association between the genotypes and risk of essential hypertension in the obese and the non-obese participants.
| Total | Male* | Female* | ||||||
| SNP | Population | Contrast | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| C-47T | Obese | CC vs. CT vs. TT | 1.300(0.861–1.961) | 0.211 | 1.138(0.672–1.923) | 0.631 | 1.727(0.850–3.509) | 0.130 |
| Non-obese | CC vs. CT vs. TT | 0.708(0.430–1.166) | 0.174 | 0.634(0.322–1.247) | 0.187 | 0.753(0.351–1.618) | 0.467 | |
| A46G | Obese | GG vs. AG vs. AA | 1.645(1.258–2.151) | <0.001 | 1.603(1.153–2.227) | 0.005 | 1.739(1.062–2.849) | 0.028 |
| Non-obese | GG vs. AG vs. AA | 0.927(0.688–1.248) | 0.616 | 0.942(0.618–1.433) | 0.777 | 0.839(0.539–1.305) | 0.436 | |
| C79G | Obese | GG vs. CG vs. CC | 1.287(0.851–1.942) | 0.232 | 1.147(0.678–1.942) | 0.609 | 1.647(0.810–3.356) | 0.169 |
| Non-obese | GG vs. CG vs. CC | 0.745(0.457–1.214) | 0.236 | 0.722(0.380–1.374) | 0.320 | 0.745(0.341–1.626) | 0.459 | |
ORs adjusted for gender, age, body mass index, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, serum triglyceride levels, plasma glucose level, smoking habits and drinking habits. OR, odds ratio; CI, confidence interval; SNP, single-nucleotide.
*ORs adjusted for gender was not performed in sub-group analyses of Male and Female.
P-value for the interaction between A46G genotype and body mass index on hypertension was 0.010.
Haplotype analyses
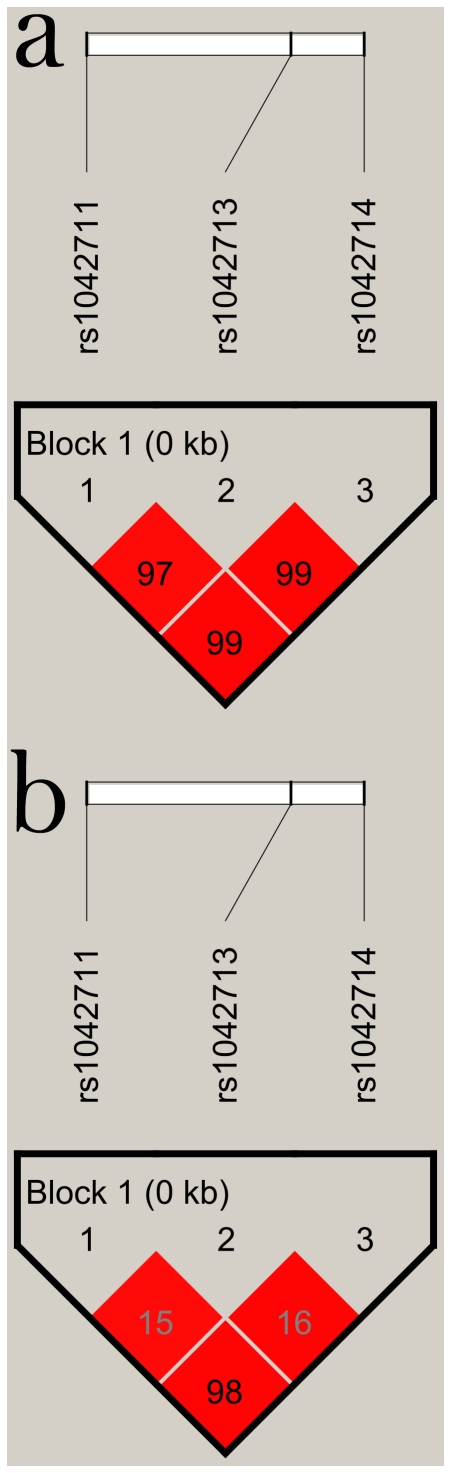
As shown in Figure 1, the C-47T and C79G were almost in complete LD (D' = 0.99, r2 = 0.98). LD could also be found in C-47T and A46G (D' = 0.97, r2 = 0.15), as well as in A46G and C79G (D' = 0.99, r2 = 0.16). The Haploview program revealed C-47T, A46G and C79G polymorphisms in the same LD block. The haplotype analyses of the 3 polymorphisms of ADRB2 in hypertension and control subjects were shown in Table 6. Only three of eight possible haplotypes (T-A-C, T-G-C and C-G-G) with frequency greater than 1% were detected in the haplotype analyses. Global analysis revealed that significant differences in haplotype distributions of ADRB2 gene between cases and controls (X 2 = 8.49, df = 2, Global P = 0.014). Haplotype specific (HS) testing showed that the T-A-C haplotype was a protective haplotype (P = 0.003, OR = 0.763, 95%CI [0.639–0.912]) while the T-G-C haplotype was observed to be a risk (P = 0.015, OR = 1.265, 95%CI [1.047-1.530]). The C-G-G haplotype was not associated with EH in the Northern Han Chinese (P = 0.292).
Figure 1. Linkage disequilibrium (LD) block defined by the Haploview program based on the confidence interval method.
a represents LD measure of D'. b represents LD measure of r2.
Table 6. Haplotype analyses of the β2-adrenergic receptor gene polymorphisms in hypertension and control subjects.
| Haplotype frequency | |||||||||
| C-47T | A46G | C79G | Cases | Controls | HS test P valuea | OR [95% CI]a | P valueb | OR [95% CI]b | Global P valuec |
| T | A | C | 0.534 | 0.600 | 0.003 | 0.763 [0.639–0.912] | - | - | 0.014 |
| T | G | C | 0.345 | 0.294 | 0.015 | 1.265 [1.047–1.530] | 0.005 | 1.319 [1.085–1.604] | |
| C | G | G | 0.121 | 0.106 | 0.292 | 1.162 [0.879–1.537] | 0.089 | 1.284 [0.963–1.713] | |
All haplotypes with frequency greater than 1% detected in the haplotype analyses are shown in the table.
HS test, haplotype specific testing; OR, odds ratio; CI, confidence interval.
P values and OR values derived from comparing of a specific haplotype with the other two.
P values and OR values derived from comparing each haplotype with the base-line haplotype (T-A-C).
P value for global test comparing model with haplotypes to model without.
The relative effects of the haplotypes were evaluated by the logistic regression. As the most highly prevalent haplotype, the T-A-C haplotype was defined as the base-line haplotype. Comparing with the base-line haplotype, the T-G-C haplotype was associated with a risk to EH (P = 0.005, OR = 1.319, 95%CI [1.085-1.604]). No association could be found between the C-G-G haplotype and EH (P = 0.089).
Discussion
The present study found that the A46G polymorphism of the ADRB2 gene was associated with EH in the Northern Han Chinese. G46 allele had a significantly higher representation in the cases than in the controls. These findings were consistent with another study among the Northern Han Chinese on stage 2 hypertension diagnosed on systolic blood pressure (SBP)≥160 mmHg and/or diastolic blood pressure (DBP)≥100 mmHg reported by Gu et al. and Ge et al. [21], [23] Compared with their study, we failed to find significant association between C79G polymorphism and EH risk. As the diagnostic standard of Gu et al. and Ge et al.'s study [21], [23] was higher than our current study, we considered that C79G polymorphism might associate with more severe hypertension. Our previous meta-analysis [27] also found the similar results, which suggested that C79G polymorphism was associated with ‘severe hypertension' defined on SBP≥160 mmHg and/or DBP≥95 mmHg, but no significant association could be found based on the current clinical diagnostic standard of hypertension on SBP≥140 mmHg and/or DBP≥90 mmHg [2]. Interestingly, further research was conducted to compare with Gu et al. and Ge et al.'s study [21], [23] and different results were acquired. We also defined hypertensive participants according to the stage 2 definition and 441 patients were included. The patients' genotypes were compared with that of the normotensive subjects and logistic regression analysis was also performed after adjusting for confounding risk variables. There was no difference in genotype distribution of C79G between the cases and the controls (GG vs. CG vs. CC, P = 0.454, OR = 0.873, 95%CI [0.613–1.244]). We thought that this discrepancy might be resulted from 2 main reasons. Firstly, the participants of the stage 2 hypertension subgroup in the current study were fewer than that of Gu et al. and Ge et al.'s. For the low frequency of the uncommon allele of C79G, extremely large trial size was required for enough statistical power to detect real differences between groups[28]. The P-values of the case-control studies might have been a statistical fluctuation by chance owing to the nature of random sampling, dependent on the sample size and the low minor allele frequency of C79G. Secondly, different results might be partially attributed to the complex genetic differentiation among the Northern Han Chinese population[29]. We found almost complete LD between C79G and C-47T polymorphisms, whereas weak LD was found in Gu et al. and Ge et al.'s study. As for the C-47T polymorphism, both studies got negative results. Negative results were also found on C79G polymorphism in our case-control study since it was almost in complete LD with C-47T, which was different from Gu et al. and Ge et al.'s. Previous research also reported different degree of LD between C79G and C-47T in different ethnic background subjects[22], [30]–[32]. With the human migration in the history, the Northern Han Chinese population was under strong genetic influences from other populations[29]. The samples from our study and Gu et al. and Ge et al.'s were not entirely enrolled from the same area, the participants were not in a single homogenous population[26], [29], which might result in different association in these studies.
Stratification analyses showed that A46G polymorphism was association with EH risk in male, but not in the female population. No significant interaction existed between A46G genotype and gender. Yet we suggested that the different results between men and women and the P-value for genotype-gender interaction should be treated cautiously, as the women participants in this study were considerably smaller than men, which might limit the power to detect differences in OR estimates between the subgroups. Stratification analyses on obesity found the association between A46G polymorphism and EH risk in the obese subgroup, both in obese men and in obese women, whereas in the non-obese population, no association could be found. Significant interaction could also be found between A46G genotypes and BMI on EH risk. These findings suggested that obesity might have an effect on the association between genetic factors and EH risk. Mo et al. [25] compared the obese hypertensive population and the non-obese normotensive controls in the Southern Han Chinese population and indicated that the association could be found on C79G polymorphism in male, whereas no association was found on A46G polymorphism. We considered that the deviation between these 2 studies was mainly on the obesity effect. We performed stratification analyses and compared on the obese hypertension and the obese normotension, which was different from Mo et al.'s comparison on the control subjects. When both research studied on the same kind of participants, the non-obese population, and compared between hypertension and normotension, our findings were consistent with Mo et al.'s result. Furthermore, with the genetic background difference [26], [33]–[35] and the different environmental factors, such as eating habits, lifestyle, geographical localities and climate between the Northern and the Southern Han Chinese population, the results of these 2 studies might also be different.
Compared with the studies on Caucasians [11], [12], [15]–[17], most of them indicated ‘negative’ results on A46G polymorphism and EH risk. It was reported that the allele frequencies of the C-47T, A46G and C79G polymorphisms were quite different among ethnicities [36]. In the Asian populations, the allele frequencies of C-47, G46 and G79 were significantly lower than that of the Caucasians and the Blacks. The heterogeneity of allelic frequencies among the ethnicities might be the main point of the different results in the case-control studies. Beside this, the environment risk factors should also be taken into consideration. In the present study, A46G polymorphism was associated with EH risk in the obese population. This might be related to the living habits and environments of the obese. So we considered that the association might be the result of gene-environment interaction.
Haplotype analyses suggested that only 3 haplotypes with frequency greater than 1% were found in the study as previously described [30], [37]. As the haplotype frequencies listed in Table 6, the haplotype frequencies summed to 100%, both in cases and in controls, which meant that there was almost no rare haplotypes detected in the current study. This was attributed to the close LD of the C-47T, A46G and C79G polymorphisms. It was reported that subjects homozygous for G79 were nearly always homozygous G46, whereas A46/G79 occurred naturally extremely rare [38]–[40]. In our study, no AA46/GG79 combined genotype was found. As for the LD of C-47T and C79G, Lima et al. [30] reported that these two polymorphisms were in complete LD in whites and African Americans. In the current research, similar result was observed in Northern Han Chinese. In the 1105 subjects (97.2% of all the participants), whose genotypes of both the C-47T and C79G polymorphisms were successfully detected, only 4 subjects were deviated from the consistent genotype of mutant with mutant or wild-type with wild-type on these 2 tight LD polymorphisms. For the reasons of the high consistency of the C-47T and C79G polymorphisms, and of the low frequencies of both uncommon alleles, in the case-control study, the results of the haplotype analyses on the C-47T, A46G and C79G polymorphisms might be particularly determined by the allelic variants of the A46G polymorphism. Haplotype analyses showed that the T-A-C haplotype was a protective haplotype and the T-G-C haplotype was a risk, which was consistent with the findings of the association analyses between the A46G polymorphism and EH risk. For only 3 haplotypes detected, we could hardly perform further comparisons on multiple hypotype combinations.
As Green et al. reported [7], either the G46 allele or the G79 allele, compared to their wild allele, displayed normal agonist binding and functional coupling to Gs, resulting in the stimulation of adenylyl cyclase activity. In the downregulation of ADRB2, the A46G and the C79G polymorphisms played roles in the different degree. As compared to the wild-type AA46, the GG46 underwent a greater degree of downregulation of ADRB2 in response to the β2-agonist isoproterenol. These findings suggested that for the reason of decrease agonist sensitivity, an increased blood pressure might be produced by the GG46. On the contrary, the GG79, compared to the wild-type CC79, displayed no downregulation of ADRB2. Interestingly, the receptor with the GG46/GG79 combined genotype also displayed a greater degree of downregulation as compared to that found with the AA46/CC79 combined wild-type. Our results were in agreement with these findings. However, Wu et al. [9] studied on two Southern Chinese minority human population and got the different conclusions. They found that both the A46G and the C79G polymorphisms could play a role in the blood pressure regulation in the Yi, not in the Hani, minority group. In the Yi minority, the G46 allele and the G79 allele were more frequent in the control groups than in the hypertensive participants. Compared to Han Chinese, Yi and Hani minority groups have different ethnic origins [41], and living in a particular geographical environment. So we considered that the different results were probably due to the differences in ethnicity and geographical conditions.
It was reported [42] that the C-47T variant in the promoter region of the ADRB2 gene controlled the ADRB2 expression. The T-47 allele resulted in increased expression of the ADRB2 as compared with the C-47 allele. As the LD between the C-47T polymorphism and the ADRB2 gene coding block polymorphisms A46G and C79G, it suggested that the allele frequency of the C-47T polymorphism associated with that of the A46G and C79G polymorphisms. In other words, the A46G and the C79G variants within the coding region were thought to affect the ADRB2 gene expression. The current study found that the A46G polymorphism, but not the C79G or C-47T polymorphism, associated with the risk of EH in the Northern Han Chinese population. However, for the tight LD between multiple polymorphisms on the ADRB2 gene, we believe that the A46G polymorphism should not be independently considered as the impact factor of the ADRB2 gene on the pathogenesis of EH. Further research on the role of the multiple polymorphisms of different regions within the ADRB2 gene in the pathophysiologic of EH are important to clarify the genetic mechanism of EH.
Compared with early studies, the sample size of the present study was moderate. However, for the minor allele frequencies of C-47T, A46G and C79G of the Asians were significantly lower than that of the Caucasians and the Blacks as previously reported [36], and the allele frequencies of C-47 and G79 were much lower than G46, the statistical power to detect a significant association on C-47T and C79G polymorphisms were limited. The current study found that the 3 polymorphisms were in tight LD, but in the association study of EH, the results were quite different, both in all the participants and in the subgroups analyses. With the limited statistical power, the conclusions on C-47T and C79G polymorphisms should be treated cautiously. Considering the vastly different allele frequencies of the ADRB2 gene polymorphisms in different ethnicities and the low minor allele frequencies of C-47T and C79G polymorphisms, larger case-control studies stratified for different ethnicities should be performed to clarify the association between the ADRB2 gene polymorphisms and EH risk in the future.
In conclusion, we found that the A46G polymorphism in the ADRB2 gene significantly associated with EH risk among the Northern Han Chinese population. The G46 allele was a risk factor for EH. Subgroup analyses performed by gender suggested that this association could be found in male, but not in female. Stratification analyses by obesity showed that G46 allele was related to the prevalence of hypertension in the obese population. Significant interaction existed between A46G genotypes and BMI on EH. Haplotype analyses suggested that the T-47-A46-C79 haplotype was a protective haplotype for EH, while the T-47-G46-C79 haplotype increased the risk of EH. Furthermore, for the tight LD between the polymorphisms in the ADRB2 gene, further research is warranted to elucidate the role of the multiple polymorphisms within the ADRB2 gene in the mechanism of EH.
Materials and Methods
Ethics Statement
The study complies with the Declaration of Helsinki, the local ethics committee of Beijing Anzhen Hospital of the Capital University of Medical Sciences has approved the research protocol. Written informed consent was obtained from each participant.
Subjects
All individuals were of Northern Han Chinese origin and from the Beijing area recruited range from June 2008 to June 2009 in this study. All the participants were ascertained and identified via Anzhen Hospital of the Capital University of Medical Sciences, Beijing, China (n = 859), and two examination centers at local health stations, Liuliqiao (n = 71) and Guozhuang (n = 207), in Beijing suburbs.
Blood pressure was measured by trained and certified observers according to a common protocol adapted from procedures recommended by European Society of Hypertension [2]. After sitting for 30 min in a quiet room, three measurements with a standardized mercury sphygmomanometer were performed at least 5 min intervals. One of the 4 bladders (standard, larger, smaller, pediatric) was chosen and all readings were obtained from the right arm. SBP and DBP were defined according to Korotkoff I and V. Heart rate was measured by pulse palpation 30 sec after the measurement of blood pressure. Hypertension was defined as the average SBP>140 mmHg and/or the average DBP>90 mmHg and/or self-reported current treatment for hypertension with antihypertensive medication. The control subjects had systolic and diastolic blood pressures <140 mmHg and <90 mmHg, respectively, and should never been treated for hypertension [2]. Subjects with secondary hypertension, primary renal disease, diabetes mellitus, hepatic disorders, cancer, endocrine diseases such as hyperthyroidism were excluded. Physical examination, a questionnaire and serum biochemical profile were administered to each of the participants. Information on smoking and drinking habits was obtained by interview. Smoker was defined as the cigarette consumer who has smoked ≥100 cigarettes, and drinker was defined as the alcohol consumer who drank ≥12 times during the year [21], [23]. Obese was defined as a BMI ≥25 kg/m2 according to the World Health Organization obesity guidelines on Asians [25], [43], [44].
Genotyping
Blood was taken into EDTA-containing receptacles. Genomic DNA was extracted from peripheral blood according to standard phenol-chloroform methods. We genotyped SNPs using the TaqMan assay. The ADRB2 SNP Taqman probes and primers were obtained from Applied Biosystems Assay-by-Design Service for SNP genotyping. The sample DNA was amplified by PCR following the recommendations of the manufacturer. Thermal cycling was done on a GeneAmp PCR System 9700 thermal cycler (Applied Biosystems, 850 Lincoln Centre Drive, Foster City, CA 94404 USA). Genotypes were differentiated by analyzing the fluorescence levels of PCR products using an ABI PRISM 7900HT Sequence Detector (Applied Biosystems). Genotyping was performed blindly to all other data.
Statistical Analyses for Patient–Control Study
We used SPSS (Version 17.0; SPSS, Chicago, Illinois) for database management and statistical analyses. All comparisons between specific groups for continuous variables were made using a two-sample t-test. Allelic and genotypic frequencies were compared between the hypertensive cases and the normotensive controls by using the chi-square test. Genotype frequency of the subjects specified by different genetic models (additive, dominant, recessive and homozygote comparison) was analyzed by multivariate logistic regression adjusted for covariates. Analyses used two-tailed estimation of significance. The statistical significance was defined P<0.05.
The presence of Hardy–Weinberg equilibrium was tested by the chi-square test for goodness of fit based on a web program (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
Construction of the LDmap and haplotype blocks within C-47T, A46G and C79G polymorphisms of the ADRB2 gene was based on genotypes and utilized Haploview software (version 4.1) (http://www.broad.mit.edu/mpg/haploview/) [45]. The expectation maximization algorithm [46] was performed to estimate haplotype frequencies and to obtain the best haplotype configuration for each multi-locus genotype. All haplotypes with frequency greater than 1% in the combined case and control samples were examined. The chi-square test was conducted to compare the haplotype distributions between the hypertensives and the normotensives. HS testing was performed to compare a specific haplotype with the others. Assuming the highly prevalent haplotype as the base line, each of the other haplotype was also compared with the base-line haplotype using a logistic regression model. A global test statistic comparing the model with genetic data to the model without genetic data was also performed to check for the overall association of haplotypes with the disease outcome, using an online computer platform SHEsis (http://analysis.bio-x.cn/myAnalysis.php) [47], [48].
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by grants 2008AA02Z441 of the National High Technology Research and Development Program (http://program.most.gov.cn/page/pds/mian1_AA.htm?planId=AA), and grants 2008BAI52B03 of the National Eleventh Five-year Plan Program from the Ministry of Science and Technology (http://program.most.gov.cn/page/pds/mian1_BA.htm?planId=BA) of the People's Republic of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gu D, Reynolds K, Wu X, Chen J, Duan X, et al. The International Collaborative Study of Cardiovascular Disease in ASIA. Prevalence, awareness, treatment, and control of hypertension in china. Hypertension. 2002;40:920–927. doi: 10.1161/01.hyp.0000040263.94619.d5. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 3.Stein CM, Nelson R, Deegan R, He H, Wood M, et al. Forearm beta adrenergic receptor-mediated vasodilation is impaired, without alteration of forearm norepinephrine spillover, in borderline hypertension. J Clin Invest. 1995;96:579–585. doi: 10.1172/JCI118070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman RD, Gros R. Impaired vasodilator function in hypertension: the role of alterations in receptor-G protein coupling. Trends Cardiovasc Med. 1998;8:297–305. doi: 10.1016/s1050-1738(98)00022-x. [DOI] [PubMed] [Google Scholar]
- 5.Cockcroft JR, Gazis AG, Cross DJ, Wheatley A, Dewar J, et al. β2-adrenoceptor polymorphism determines vascular reactivity in humans. Hypertension. 2000;36:371–375. doi: 10.1161/01.hyp.36.3.371. [DOI] [PubMed] [Google Scholar]
- 6.Dishy V, Sofowora GG, Xie HG, Kim RB, Byrne DW, et al. The effect of common polymorphisms of the beta2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001;345:1030–1035. doi: 10.1056/NEJMoa010819. [DOI] [PubMed] [Google Scholar]
- 7.Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 8.Scott MG, Swan C, Wheatley AP, Hall IP. Identification of novel polymorphisms within the promoter region of the human beta2 adrenergic receptor gene. Br J Pharmacol. 1999;126:841–844. doi: 10.1038/sj.bjp.0702385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu H, Tang W, Li H, Zhou X, Yang Y, et al. Association of the beta2-adrenergic receptor gene with essential hypertension in the non-Han Chinese Yi minority human population. J Hypertens. 2006;24:1041–1047. doi: 10.1097/01.hjh.0000226193.21311.e1. [DOI] [PubMed] [Google Scholar]
- 10.Petrone A, Zavarella S, Iacobellis G, Zampetti S, Vania A, et al. Association of beta2 adrenergic receptor polymorphisms and related haplotypes with triglyceride and LDL-cholesterol levels. Eur J Hum Genet. 2006;14:94–100. doi: 10.1038/sj.ejhg.5201521. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann SM, Nicaud V, Tiret L, Evans A, Kee F, et al. Polymorphisms of the beta2 -adrenoceptor (ADRB2) gene and essential hypertension: the ECTIM and PEGASE studies. J Hypertens. 2002;20:229–235. doi: 10.1097/00004872-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Xie HG, Stein CM, Kim RB, Gainer JV, Sofowora G, et al. Human beta2-adrenergic receptor polymorphisms: no association with essential hypertension in black or white Americans. Clin Pharmacol Ther. 2000;67:670–675. doi: 10.1067/mcp.2000.106293. [DOI] [PubMed] [Google Scholar]
- 13.Candy G, Samani N, Norton G, Woodiwiss A, Radevski I, et al. Association analysis of beta2 adrenoceptor polymorphisms with hypertension in a Black African population. J Hypertens. 2000;18:167–172. doi: 10.1097/00004872-200018020-00006. [DOI] [PubMed] [Google Scholar]
- 14.Jia H, Sharma P, Hopper R, Dickerson C, Lloyd DD, et al. beta2-adrenoceptor gene polymorphisms and blood pressure variations in East Anglian Caucasians. J Hypertens. 2000;18:687–693. doi: 10.1097/00004872-200018060-00005. [DOI] [PubMed] [Google Scholar]
- 15.Gjesing AP, Andersen G, Burgdorf KS, Borch-Johnsen K, Jørgensen T, et al. Studies of the associations between functional beta2-adrenergic receptor variants and obesity, hypertension and type 2 diabetes in 7808 white subjects. Diabetologia. 2007;50:563–568. doi: 10.1007/s00125-006-0578-8. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann V, Büscher R, Go MM, Ring KM, Hofer JK, et al. Beta2-adrenergic receptor polymorphisms at codon 16, cardiovascular phenotypes and essential hypertension in whites and African Americans. Am J Hypertens. 2000;13:1021–1026. doi: 10.1016/s0895-7061(00)01188-2. [DOI] [PubMed] [Google Scholar]
- 17.Bartels NK, Börgel J, Wieczorek S, Büchner N, Hanefeld C, et al. Risk factors and myocardial infarction in patients with obstructive sleep apnea: impact of beta2-adrenergic receptor polymorphisms. BMC Med. 2007;5:1. doi: 10.1186/1741-7015-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu CJ, Wang CH, Lee JH, Hsieh CM, Cheng CC, et al. Association between polymorphisms of ACE, B2AR, ANP and ENOS and cardiovascular diseases: a community-based study in the Matsu area. Clin Chem Lab Med. 2007;45:20–25. doi: 10.1515/CCLM.2007.014. [DOI] [PubMed] [Google Scholar]
- 19.Misono M, Maeda S, Iemitsu M, Nakata Y, Otsuki T, et al. Combination of polymorphisms in the beta2-adrenergic receptor and nitric oxide synthase 3 genes increases the risk for hypertension. J Hypertens. 2009;27:1377–1383. doi: 10.1097/HJH.0b013e32832b7ead. [DOI] [PubMed] [Google Scholar]
- 20.Pojoga L, Kolatkar NS, Williams JS, Perlstein TS, Jeunemaitre X, et al. Beta-2 adrenergic receptor diplotype defines a subset of salt-sensitive hypertension. Hypertension. 2006;48:892–900. doi: 10.1161/01.HYP.0000244688.45472.95. [DOI] [PubMed] [Google Scholar]
- 21.Gu D, Su S, Ge D, Chen S, Huang J, et al. Association study with 33 single-nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension. 2006;47:1147–1154. doi: 10.1161/01.HYP.0000219041.66702.45. [DOI] [PubMed] [Google Scholar]
- 22.Kato N, Sugiyama T, Morita H, Kurihara H, Sato T, et al. Association analysis of beta(2)-adrenergic receptor polymorphisms with hypertension in Japanese. Hypertension. 2001;37:286–292. doi: 10.1161/01.hyp.37.2.286. [DOI] [PubMed] [Google Scholar]
- 23.Ge D, Huang J, He J, Li B, Duan X, et al. beta2-Adrenergic receptor gene variations associated with stage-2 hypertension in northern Han Chinese. Ann Hum Genet. 2005;69:36–44. doi: 10.1046/j.1529-8817.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 24.Yu SF, Zhou WH, Jiang KY, Gu GZ, Wang S. Job stress, gene polymorphism of beta2-AR, and prevalence of hypertension. Biomed Environ Sci. 2008;21:239–246. doi: 10.1016/S0895-3988(08)60036-7. [DOI] [PubMed] [Google Scholar]
- 25.Mo W, Zhang GG, Yang TL, Dai XP, Li HH, et al. The genetic polymorphisms of beta3-adrenergic receptor (AR) Trp64Arg and beta2-AR Gln27Glu are associated with obesity in Chinese male hypertensive patients. Clin Chem Lab Med. 2007;45:493–498. doi: 10.1515/CCLM.2007.089. [DOI] [PubMed] [Google Scholar]
- 26.Xu S, Yin X, Li S, Jin W, Lou H, et al. Genomic dissection of population substructure of Han Chinese and its implication in association studies. Am J Hum Genet. 2009;85:762–774. doi: 10.1016/j.ajhg.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lou Y, Liu J, Huang Y, Liu J, Wang Z, et al. A46G and C79G polymorphisms in the β2-adrenergic receptor gene (ADRB2) and essential hypertension risk: A meta-analysis. Hypertens Res. 2010;33:1114–1123. doi: 10.1038/hr.2010.151. [DOI] [PubMed] [Google Scholar]
- 28.Kao WH, Arking DE, Post W, Rea TD, Sotoodehnia N, et al. Genetic Variations in Nitric Oxide Synthase 1 Adaptor Protein Are Associated With Sudden Cardiac Death in US White Community-Based Populations. Circulation. 2009;119:940–951. doi: 10.1161/CIRCULATIONAHA.108.791723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu JY, Huang W, Kuang SQ, Wang JM, Xu JJ, et al. Genetic relationship of populations in China. Proc Natl Acad Sci USA. 1998;95:11763–11768. doi: 10.1073/pnas.95.20.11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lima JJ, Feng H, Duckworth L, Wang J, Sylvester JE, et al. Association analyses of adrenergic receptor polymorphisms with obesity and metabolic alterations. Metabolism. 2007;56:757–765. doi: 10.1016/j.metabol.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szczepankiewicz A, Breborowicz A, Sobkowiak P, Kramer L, Popiel A. Role of ADRB2 gene polymorphism in asthma and response to beta(2)-agonists in Polish children. J Appl Genet. 2009;50:275–281. doi: 10.1007/BF03195683. [DOI] [PubMed] [Google Scholar]
- 32.Busjahn A, Li GH, Faulhaber HD, Rosenthal M, Becker A, et al. β-2 adrenergic receptor gene variations, blood pressure, and heart size in normal twins. Hypertension. 2000;35:555–560. doi: 10.1161/01.hyp.35.2.555. [DOI] [PubMed] [Google Scholar]
- 33.Wen B, Li H, Lu D, Song X, Zhang F, et al. Genetic evidence supports demic diffusion of Han culture. Nature. 2004;431:302–305. doi: 10.1038/nature02878. [DOI] [PubMed] [Google Scholar]
- 34.Shi H, Dong YL, Wen B, Xiao CJ, Underhill PA, et al. Y-chromosome evidence of southern origin of the East Asian-specific haplogroup O3-M122. Am J Hum Genet. 2005;77:408–419. doi: 10.1086/444436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang HG, Chen YF, Ding M, Jin L, Case DT, et al. Dermatoglyphics from all Chinese ethnic groups reveal geographic patterning. PLoS One. 2010;5:e8783. doi: 10.1371/journal.pone.0008783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brodde O, Leineweber K. β2-adrenoceptor gene polymorphisms. Pharmacogenet Genom. 2005;15:267–275. doi: 10.1097/01213011-200505000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Hawkins GA, Tantisira K, Meyers DA, Ampleford EJ, Moore WC et al. Sequence, haplotype, and association analysis of ADRbeta2 in a multiethnic asthma case-control study. Am J Respir Crit Care Med. 2006;174:1101–1109. doi: 10.1164/rccm.200509-1405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol. 2003;43:381–411. doi: 10.1146/annurev.pharmtox.43.100901.135823. [DOI] [PubMed] [Google Scholar]
- 39.Leineweber K, Buscher R, Bruck H, Brodde OE. Beta-adrenoceptor polymorphisms. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:1–22. doi: 10.1007/s00210-003-0824-2. [DOI] [PubMed] [Google Scholar]
- 40.Kirstein SL, Insel PA. Autonomic nervous system pharmacogenomics: a progress report. Pharmacol Rev. 2004;56:31–52. doi: 10.1124/pr.56.1.2. [DOI] [PubMed] [Google Scholar]
- 41.Deng W, Shi B, He X, Zhang Z, Xu J, et al. Evolution and migration history of the Chinese population inferred from Chinese Y-chromosome evidence. J Hum Genet. 2004;49:339–348. doi: 10.1007/s10038-004-0154-3. [DOI] [PubMed] [Google Scholar]
- 42.McGraw DW, Forbes SL, Kramer LA, Liggett SB. Polymorphisms of the 5′ leader cistron of the human beta2-adrenergic receptor regulate receptor expression. J Clin Invest. 1998;102:1927–1932. doi: 10.1172/JCI4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. WHO Regional Office for the Western Pacific, International Association for the study of obesity. 2000. International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment. Melbourne, Health Communications Australia.
- 44.Smith SC, Jr, Clark LT, Cooper RS, Daniels SR, Kumanyika SK, et al. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: report of the Obesity, Metabolic Syndrome, and Hypertension Writing Group. Circulation. 2005;111:e134–e139. doi: 10.1161/01.CIR.0000157743.54710.04. [DOI] [PubMed] [Google Scholar]
- 45.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 46.Qin ZS, Niu T, Liu JS. Partition-ligation-expectation-maximization algorithm for haplotype inference with single-nucleotide polymorphisms. Am J Hum Genet. 2002;71:1242–1247. doi: 10.1086/344207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15:97–98. doi: 10.1038/sj.cr.7290272. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Zhang Z, He Z, Tang W, Li T, et al. Cell Res. 2009;19:519–523. doi: 10.1038/cr.2009.33. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis ( http://analysis.bio-x.cn). [DOI] [PubMed] [Google Scholar]