Abstract
Background
Chronic alcohol consumption reduces bone mass and strength, increasing fracture risk for alcohol abusers. Mechanisms underlying this vulnerability involve modulation of bone remodeling. Direct effects of alcohol on bone formation have been documented; those on bone resorption are less well studied. Skeletal effects of exposure to high blood alcohol concentrations (BAC’s) attained during binge drinking have not been studied. We examined the effects of repeated binge-like alcohol treatment on bone resorption, bone mineral density and vertebral compressive strength in adult male rats treated with the aminobisphosphonate, risedronate.
Methods
A binge alcohol exposure model was developed using intraperitoneal (IP) injection to administer a 20% (vol/vol) alcohol/saline solution (3 g/kg, 1X/day) on four consecutive days for 1, 2 or 3 weeks in 400 g rats, with and without weekly risedronate treatment (0.5 mg/kg, 1X/week). Total serum deoxypyridinoline (Dpd) a crosslink of bone type collagen released during resorption was measured by ELISA. Bone mineral density (BMD) was measured using peripheral quantitative computed tomography (pQCT). Vertebral compressive strength was determined using an Instron materials testing machine. Trabecular integrity was analyzed by computer-aided trabecular analysis system (TAS).
Results
Peak BAC’s averaged 308.5 ± 12 mg/dL; average BAC was 258.6 ± 28.7 mg/dL at time of euthanasia. No significant effects of treatment were observed after 1 or 2 weeks of binge alcohol exposure. At 3 weeks of alcohol treatment serum Dpd was significantly increased (205%, p < 0.05) over controls. Bone mineral density (BMD) in cancellous bone of distal femur and lumbar spine were significantly decreased (34% and 21% respectively, p < 0.01) after 3 weeks of binge treatment. Vertebral (L4) compressive strength (maximum load sustained before failure) also decreased (27%, p < 0.05) after 3 binge alcohol cycles. Risedronate maintained the Dpd level (p < 0.01), BMD (p < 0.001) and vertebral structural biomechanical properties (p < 0.01) of binge-treated rats at control levels (E vs ER). Indices of trabecular architectural integrity [Trabecular bone volume/tissue volume (BV/TV), bone area (BAR) and trabecular separation (Tb.Sp)] analyzed at week 3 showed (BV/TV) and (BAR) were significantly reduced in alcohol-binged rats (p < 0.01), while (Tb.Sp) was significantly increased (p < 0.01). Risedronate also maintained the trabecular architectural indices of binge-treated rats at control levels (E versus ER, p < 0.01).
Conclusions
In adult male rats, BAC’s reflective of those attained during alcoholic binge drinking may affect the skeleton in part by stimulating bone resorption, an effect mitigated by risedronate.
Keywords: Bone, Alcohol, Rat, Risedronate, Resorption
A history of alcohol abuse is an important risk factor for bone pathologies such as osteoporosis and fracture (Turner, 2000). Most alcoholics exhibit radiographic evidence of osteopenia (Bilke et al., 1985). The regulation of bone remodeling, which occurs throughout life and is essential for maintenance of normal bone mass and architecture in the adult skeleton, may be disrupted by alcohol exposure (Turner, 2000). Most studies examining the relationship between alcohol abuse and bone remodeling have focused on the formation phase of the remodeling cycle, leading to a hypothesis that reduced osteoblast activity resulting in under filling of resorptive lacunae is primarily responsible for alcohol-induced bone loss (Turner, 2000). There are reports in the literature supporting the concept that osteoblast proliferation and activity are negatively affected by alcohol treatment (Chavassieux et al., 1993; Klein et al., 1996). Chronic alcohol exposure also impairs skeletal development affecting bone mineral density, bone strength as well as perturbing osteoblast gene expression profiles (Wezeman et al., 1999). Thus, the process of bone formation appears to be a primary target of alcohol-induced bone loss (Turner, 2000).
Less is known about the effects of alcohol exposure on osteoclast function and resorption during bone remodeling and its potential contribution to alcohol-induced bone loss. Studies in this area have clinical relevance given the availability of effective inhibitors of bone resorption (Compston, 2002). Antiresorptive agents such as risedronate, a third generation aminobisphosphonate, reduce bone loss by inhibiting osteoclast-mediated bone resorption (Compston, 2002) and are commonly used in the treatment of osteoporosis (Russel and Rodgers, 1999). These compounds are also highly effective inhibitors of resorption in rat models of bone loss (Wezeman et al., 2000).
Human studies examining the relationship between alcohol abuse and markers of bone resorption have been somewhat contradictory. Several studies reported increased bone resorptive markers in both long-term alcoholic patients as well as during alcohol withdrawal (Laitinen et al., 1994; Nyquist et al., 1996; Pepersack et al., 1992). However, Bikle and coworkers (1985) reported decreased resorption in alcoholics with radiologic evidence of osteopenia, while others (Crilly et al., 1988; Diamond et al., 1989; Laitinen et al., 1992) reported no change in bone resorption markers in alcoholics.
Most animal studies have shown that alcohol exposure does increase markers of bone resorption both in vitro (Cheung et al., 1995; Farley et al., 1985) and in vivo (Zhang et al., 2002). Early studies of the effect of alcohol on bone remodeling found direct effects on both formation and resorption in an embryonic chick tibia in vitro model system (Farley et al., 1985), and suggested alcohol-induced alterations in bone cell membrane fluidity as a potential contributory mechanism. More recent reports using in vitro resorption assays found primary cultured osteoclasts exposed to different concentrations of alcohol exhibited greater in vitro resorption activity than control cultures (Cheung et al., 1995). Zhang and coworkers, using a model of chronic alcohol consumption in mice, observed both alcohol-induced bone loss and increased urinary markers of bone resorption (Zhang et al., 2002). This effect was diminished by concurrent treatment with osteoprotegerin (OPG), an osteoblast-derived factor that inhibits osteoclastogenesis, suggesting that alcohol has a stimulatory effect on bone resorption. However, decreases in bone resorption after chronic alcohol consumption in rats have also been reported (Preedy et al., 1991).
Approximately 90% of the organic matrix of bone is type 1 collagen helical protein (Seyed and Rosen, 1990). Type 1 collagen is cross-linked by specific molecules, pyridinolone and deoxypyridinolone, which provide rigidity and strength. During bone resorption type 1 collagen is degraded and the carboxyterminal cross-linked telopeptide region (ICTP) is liberated into the serum as an immunologic intact fragment, which resists further degradation (Eriksen et al., 1993). This trimeric antigen constitutes the collagen-derived precursor for pyridinolone and deoxypyridinolone (Dpd) crosslinks measurable in urine and serum (Eriksen et al., 1993). Current generation assays measure Dpd’s as a diagnostic for the increased bone resorptive activity that occurs in metabolic bone diseases such as osteoporosis (Robins, 1995).
Binge alcohol drinking is a pattern of alcohol consumption resulting in highly intoxicating blood alcohol concentrations (BAC’s) (Witt, 1994) coupled with repeated withdrawal phases (Lundquvist et al., 1995). Alcoholic binge drinkers can achieve extremely high BAC’s (Penland et al., 2001). Sober-appearing alcohol users seen in emergency rooms had BAC’s averaging close to 300 mg/dL, with some individuals exceeding 500 mg/dL (Urso et al., 1981). Cartlidge and Redmund (1990) reported approximately 10% of individuals admitted to an alcohol detoxification unit had BAC’s higher than 500 mg/dL, with one patient who was alert with a BAC of 894 mg/dL. Other studies have also reported high BAC’s in alcoholics (Lindblad and Olsson, 1976; Teplin et al., 1989). Thus, although very high BAC’s appear to be common among alcoholics (Penland et al., 2001), the effects of BAC’s of this magnitude on bone health parameters have not been studied to date. The consequences of binge alcohol consumption are not limited to alcoholics as weekend binge drinking of high alcohol content beverages by nonalcoholics is a commonly observed phenomenon in many cultures (Lundquvist et al., 1995). One recent study attempted to examine effects of more modest binge drinking episodes on bone in an actively growing rat model, but no definitive conclusions were reached (Sampson et al., 1999).
In the current study an alcohol exposure model system was developed using intraperitoneal (IP) injection to administer alcohol to adult male rats in a binge-like pattern, generating high blood alcohol concentrations seen in alcoholic humans. The model was used to examine the effects this level and pattern of alcohol exposure would have on markers of bone health. Our hypothesis was that binge alcohol exposure would induce bone loss, at least in part by increasing bone resorption, detectable by serum markers of bone collagen degradation and affecting bone mineral density (BMD) as well as the biomechanical properties and microarchitecture of bone. We further hypothesized that if bone resorption does play a role in alcohol-induced bone loss, concurrent treatment with an antiresorptive agent would attenuate these effects. As such, we examined the effect of concurrent treatment with an aminobisphosphonate (risedronate) and binge alcohol on bone health parameters.
METHODS
Binge Alcohol Model System
All animal studies were approved by the Loyola University Institutional Animal Care and Use Committee (IACUC). A model was developed to examine the effects of binge alcohol exposure on parameters of bone resorption, with and without concurrent administration of the aminobisphosphonate, risedronate. Seventy-two adult male Sprague-Dawley rats were obtained at 15 weeks of age (Harlan, Indianapolis, IN) and acclimated to the laboratory environment for 1 week. Animals were randomly assigned to one of 4 treatment groups: control (C), risedronate treated (CR), ethanol treated (E), and ethanol and risedronate treated (ER), and to 1, 2 or 3 weeks of intraperitoneal (IP) alcohol or isotonic saline administration. Animals were housed in pairs, with both paired animals assigned to the same treatment group. Animals were weighed twice weekly throughout the study period. Risedronate treatment began one week prior to ethanol treatment and was administered as a single weekly subcutaneous injection of an aqueous solution of risedronate sodium (Actonel, Aventis Pharmaceuticals, Kansas City, MO) at a dose of 0.5 mg/kg. This dose was calculated from the recommended human dose of 35 mg given once per week, assuming an average body weight of 70 kg. Alcohol administration was by a single daily IP injection of a 20% (vol./vol.) ethanol/saline solution at a dose of 3 g/kg. This dose was chosen to achieve peak BAC’s of approximately 300 mg/dL (Nation et al., 1993). Alcohol injections were given starting at 9:00 AM, 4 days/week. No injections were given on the remaining 3 days each week, to mimic a binge alcohol-drinking pattern. Six rats from each treatment group were euthanized from 1 to 3 hr after the final alcohol injection of each week by decapitation. Blood was collected at time of euthanasia for alcohol and biochemical analysis. Serum was collected by centrifugation of coagulated blood and stored at −70 degrees C. Proximal tibia bone samples were dissected free of soft tissue, snap frozen in liquid nitrogen and stored at −70 degrees C for subsequent molecular analysis. Lumbar spine and both femurs were placed in 70% ethanol for fixation following which the soft tissues were removed by dissection under a stereomicroscope.
Blood Alcohol Determination
Blood alcohol concentrations were determined by NAD+ reduction assay (Sigma Chemical Company, St. Louis MO) on blood samples collected from animals at time of euthanasia. Peak blood alcohol determination was measured in control animals that were euthanized 60 min after a singe IP injection of 20% (vol./vol.) ethanol/saline solution. The timing of peak BAC after IP injection was based on a previous study that evaluated the pharmacokinetic profile of IP ethanol metabolism (Nation et al., 1993).
Serum Dpd Analysis
Serum Dpd measurements were performed using an ELISA-based kit (Quidel Corporation, San Diego, CA) following the manufacturer’s suggested protocol for total Dpd quantification from serum samples. Serum samples were subjected to acid hydrolysis prior to Dpd analysis allowing measurement of both free and total Dpd. All samples were assayed in duplicate and Dpd amounts were quantified using MetraFIT version 1.1 (Metra Biosystem, Santa Clara, CA). Serum levels of Dpd were expressed as nmole/liter.
Bone Mineral Density Measurements
Cancellous and cortical bone mineral density (BMD) of each right femur and fourth lumbar vertebra was determined using quantitative computed tomography (QCT). Femur specimens and lumbar vertebral bodies were positioned in a Norland Stratec bone densitometer (Orthometrix, Inc., White Plains, NY). The bones were positioned uniformly on a support so that the instrument-scanning plane was perpendicular to the longitudinal axis of the bone. Scout views were obtained to determine the points of measurement. These were as follows: midpoint and 1/5 of the length of the femur, and the midpoint of the vertebral body. Three density measurements 1 millimeter apart were performed at each point on femur; two measurements were performed 1 millimeter apart at the midpoint of the vertebral body. The cancellous area of the bone was defined as 42% of the total bone cross-sectional area in the distal metaphysis of the femur, and as 45% of the total bone cross-sectional area at the midpoint of the vertebral body. The instrument was set to use threshold contour mode (soft-tissue threshold set at 220 mg/cm3) and concentric peel algorithm (58% and 55% peel-off, respectively). Scans were made at 50kV and 0.3mA. Data were stored electronically.
Biomechanical Structural Properties of Lumbar Vertebrae
Compression tests were performed on individual lumbar vertebrae (L4) from each rat using an Instron materials testing machine (model 1122, Canton, MA). The vertebral endplates were potted in methacrylate using a previously described method that resulted in two parallel loading surfaces necessary to perform a uniform compression test on individual vertebrae (Steinke et al., 1999). The specimens were prepared such that the posterior elements of the vertebra did not contact the loading platforms. All compression tests were performed in the displacement-control mode at a crosshead speed of 0.5 mm/min to eliminate any strain rate effects. A 50 kg load-cell was used to monitor the compressive load and a precision LVDT was used to measure the axial deformation of the specimen. The load-deformation data were analyzed to obtain the compressive strength (maximum load sustained before failure, N).
Trabecular Histomorphometric Analysis
Lumbar vertebrae were embedded in polymethylmethacrylate (PMMA, Sigma Aldrich, St. Luis, MO). Nondecalcified mid-sagittal 7 μm thick sections were obtained using a Jung Polycut E microtome (Reichert-Jung, Nussloch, Germany). Sections were deplastified, stained by the Von-Kossa method and analyzed using computerized Trabecular Analysis System (TAS), (Osteometrics, Atlanta, GA). Abbreviations used for reported histomorphometric indices were consistent the report from the Association of Bone and Mineral Research on standardization of histomorphometric nomenclature (Parfitt et al., 1987)
Statistical Analysis
All data obtained was analyzed using SPSS software package (SPSS Inc. Chicago, IL). Differences between groups were tested for statistical significance by one-way ANOVA and Tukey’s HSD multiple comparison procedure. Significance was noted at p ≤ 0.05.
RESULTS
Binge Alcohol Exposure Model System
An IP dose of 3 g/kg was chosen to produce peak blood alcohol concentrations of approximately 300 mg/dL (Nation et al., 1993). A once daily treatment regime was chosen to avoid alcohol withdrawal symptoms that can occur when high doses of alcohol are administered twice daily (Penland et al., 2001). All alcohol-treated animals were monitored throughout the study period and no obvious symptoms of alcohol withdrawal were observed during the three-day period each week when alcohol was not administered. Rats exhibited a short (approximately 1 hr) period of acute alcohol intoxication immediately following each IP injection. As can be seen in Fig. 1, no significant differences in body weights were observed between animals from each of the four treatment groups at any time point during the study. Necropsy performed after euthanasia revealed no obvious internal injury from IP injections; abdominal organs (including liver) of alcohol-treated animals were normal by gross inspection.
Fig. 1.
Body Weights of Alcohol Binge Treated Rats: Rats were weighed twice weekly throughout the study period, weights of animals after each week of alcohol treatment is shown above. Weights were not significantly affected by alcohol or risedronate treatment. Significance determined using one-way ANOVA and Tukey’s multiple comparisons procedure. (C) Control Group, (E), Ethanol Group, (CR) Control Risedronate Group, (ER) Ethanol-Risedronate Group.
BAC’s at time of euthanasia are shown in Table 1. Rats receiving 1 or 2 weeks of alcohol treatment were euthanized approximately 2 hr after the final alcohol injection given that week, while rats receiving 3 weeks of alcohol treatment group were euthanized approximately 1 hr after their final alcohol injection. This difference is reflected in BAC’s at time of euthanasia. Two alcohol naïve animals given a single 3 g/kg alcohol IP injection were assayed 1 hr post alcohol injection and represent approximate peak BAC’s achieved by this method (Nation et al., 1993). No differences in BAC were noted between risedronate-treated and nontreated animals.
Table 1.
Blood Alcohol Concentrations of Alcohol Binge-Treated Rats
| Binge cycle | BAC (mg/dL) |
|---|---|
| Week 1 | 241.5 ± 28.0 |
| Week 2 | 242.5 ± 27.5 |
| Week 3 | 291.75 ± 11.1 |
| 1 hour post-IP | 308.5 ± 8.5 |
Serum samples from four representative animals from each binge cycle were assayed for BAC. Mean BAC ± St. Dev. was calculated. BAC is representative of blood-alcohol content at time of sacrifice. No differences in BAC were noted between risedronate-treated and non-treated animals (data not shown). 1 hour post-IP BAC was measured in two alcohol-naïve rats and represents approximate peak BAC attained after a single IP injection of 3 g/kg alcohol (20% ethanol/saline solution), (Nation et al., 1993).
Serum testosterone levels decreased to 20.5% ± 7.1%, 7.4% ± 3.5% and 6.25% ± 5.3% of control levels after 1, 2 and 3 weeks respectively of binge alcohol treatment consistent with previous observations in a chronic alcohol feeding model (Wezeman et al., 1999). Risedronate treatment had no significant effect on serum testosterone levels of control or binge alcohol-treated rats (data not shown).
Serum Dpd Levels
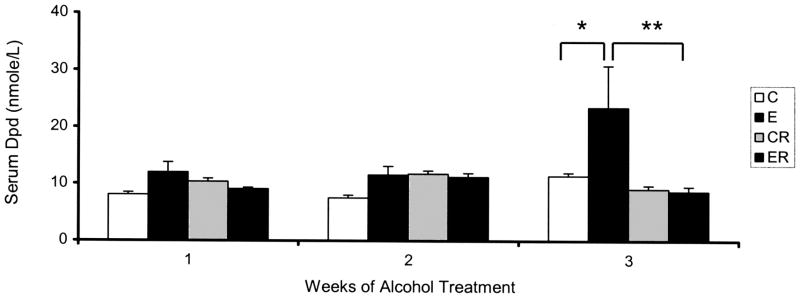
Baseline serum Dpd concentrations measured in control rats (C) did not change over the three-week study period (Fig. 2). Serum Dpd levels in rats subjected to binge-like alcohol treatments of one or two weeks (E1, E2) were slightly higher than matched controls, but this increase was not significant. After 3 weeks of alcohol exposure (E3), an approximately 2-fold increase in serum Dpd levels was observed in binge treated rats as compared to matched control animals (C versus E, p < 0.05). Risedronate treatment of nonalcohol treated (CR) rats did not significantly affect serum Dpd levels. However, risedronate had a noticeable effect on serum Dpd in animals treated with alcohol for three weeks. In this group (ER) risedronate treatment prevented the rise in serum Dpd, maintaining control levels. This effect was significant with the Dpd levels in the (ER) group being significantly lower than the matched alcohol (E) treatment group (E versus ER p ≤ 0.01).
Fig. 2.
Serum Dpd Levels of Alcohol Binge-Treated Rats: Serum Dpd levels assayed as described in the methods section. Significance determined using one-way ANOVA and Tukey’s multiple comparisons procedure. (C) Control Group,(E), Ethanol Group, (CR) Control Risedronate Group, (ER) Ethanol-Risedronate Group. * p < 0.05. ** p < 0.01.
Bone Mineral Density (BMD)
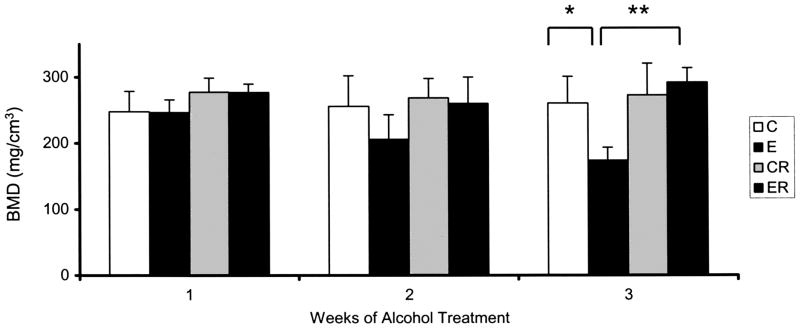
BMD was measured in the distal femur and fourth lumbar vertebra. No effect of alcohol treatment was observed in femur midshaft cortical bone BMD values (Fig. 3). However, an effect of treatment was observed in femur cancellous metaphyseal bone as shown in Fig. 4. Baseline BMD measured in control rats did not change over the three-week study period. BMD measured in cancellous bone from rats subjected to binge-like alcohol treatments of one or two weeks were lower than matched controls, but this decrease was not statistically significant. After 3 weeks of alcohol exposure, an approximately 34% decrease in cancellous BMD was observed in binge treated rats as compared to matched control animals (C versus E, p < 0.01). Risedronate treatment of non alcohol-treated rats did not significantly affect BMD values. Risedronate treatment of binge alcohol treated rats counteracted alcohol’s effect on cancellous BMD maintaining values at control values. The effect of risedronate treatment in eliminating alcohol-induced decreases in BMD was highly significant (E versus ER, p < 0.001).
Fig. 3.
Bone Mineral Density of Distal Femur Cortical Bone in Alcohol-Binge-Treated Rats: BMD was measured as described in Method Section. Significance determined using one-way ANOVA and Tukey’s multiple comparisons procedure. (C) Control Group,(E), Ethanol Group, (CR) Control Risedronate Group, (ER) Ethanol-Risedronate Group.
Fig. 4.
Bone Mineral Density of Distal Femur Cancellous Bone in Alcohol Binge-Treated Rats: BMD was measured as described in Method Section. Significance determined using one-way ANOVA and Tukey’s multiple comparisons procedure. (C) Control Group,(E), Ethanol Group, (CR) Control Risedronate Group, (ER) Ethanol-Risedronate Group. * p < 0.01. ** p < 0.001.
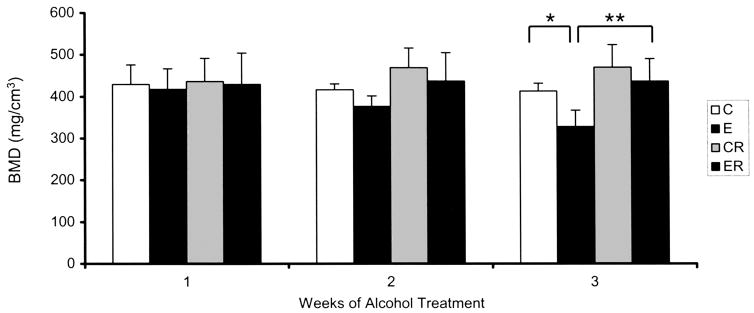
As with BMD measurements in the femur, no effect of alcohol treatment was observed on lumbar cortical BMD (data not shown). Binge alcohol treatment did have an effect in L4 cancellous bone as shown in Fig. 5. Lumbar BMD values decreased with each successive week of alcohol treatment; the most noticeable effect occurring after 3 weeks of binge exposure with an 21% decrease in BMD as compared to the matched control group (C versus E, p < 0.05). Treatment with risedronate eliminated this alcohol-induced decrease in BMD, maintaining values at control levels (E versus ER, p < 0.01).
Fig. 5.
Bone Mineral Density of Vertebral Cancellous Bone in Alcohol Binge-Treated Rats. BMD was measured as described in Method Section. Significance determined using one-way ANOVA and Tukey’s multiple comparisons procedure. (C) Control Group,(E), Ethanol Group, (CR) Control Risedronate Group, (ER) Ethanol-Risedronate Group. * p < 0.05. ** p < 0.01.
Biomechanical Properties of Lumbar Vertebrae
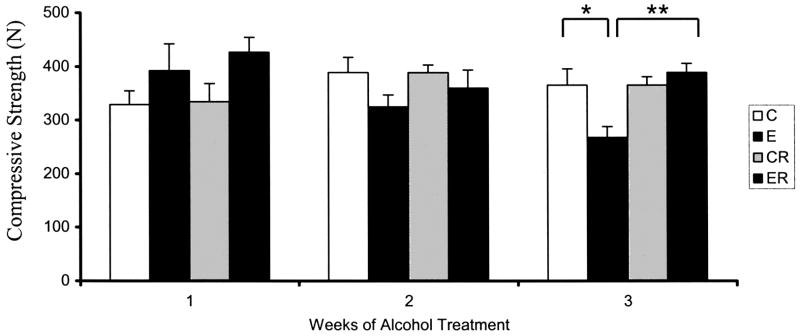
No significant effects of treatment on vertebral compressive strength were observed after 1 or 2 weeks of binge alcohol exposure (Figs. 6). However, after 3 weeks of treatment a significant effect on vertebral compressive strength (C versus E, 26% decrease, p < 0.05) was observed in binge alcohol-treated animals. Concurrent treatment with risedronate eliminated this alcohol-induced decrease in compressive strength, again maintaining values at control levels (E versus ER, p < 0.01). Analysis of total cross sectional area of each vertebral body showed no significant differences between treatment groups or between animals from weeks 1, 2 or 3 of the study, suggesting that effects of treatment on bone strength were not due to growth differences between the groups (data not shown).
Fig. 6.
Lumbar Spine L4 Compression Strength in Alcohol Binge-Treated Rats. L4 Vertebral Compressive Strength was measured as described in Method Section. Significance determined using one-way ANOVA and Tukey’s multiple comparisons procedure. (C) Control Group,(E), Ethanol Group, (CR) Control Risedronate Group, (ER) Ethanol-Risedronate Group. * p < 0.05. ** p < 0.01.
Bone Histomorphometric Analysis
Based on the results obtained from vertebral compressive strength studies of lumbar vertebrae, trabecular architecture of lumbar cancellous bone was examined specifically after 3 weeks of binge alcohol administration (Table 2). Bone histomorphometric parameters indicated structural changes were present in trabecular architecture of L3 after 3 binge alcohol cycles. Trabecular bone volume/tissue volume (BV/TV) and trabecular area (Tb.Ar) were significantly reduced (C vs. E, p < 0.001) in alcohol-binged rats, while trabecular separation (Tb.Sp) was significantly increased (C vs. E, p < 0.05). Risedronate prevented the alcohol-induced effects on these trabecular architectural indices, maintaining values at control levels (E vs. ER, p < 0.01).
Table 2.
Vertebral Trabecular Histomorphometric Parameters: Week 3 Alcohol Binge Model
| Parameter/Group | Units | C | CR | E | ER |
|---|---|---|---|---|---|
| BV/TV | % | 65.41 ± 4.10 | 59.08 ± 8.39 | 51.03 ± 3.06** | 64.98 ± 2.60 |
| Tb.Ar | mm2 | 0.40 ± 0.03 | 0.36 ± 0.05 | 0.31 ± 0.02** | 0.40 ± 0.02 |
| TbSp | μm | 29.28 ± 3.67 | 36.62 ± 12.48 | 44.68 ± 7.20* | 27.26 ± 4.97 |
Lumbar vertebrae sections were stained by the Von-Kossa method and analyzed using computerized Trabecular Analysis System (TAS) as described in methods section. Significance determined using one-way ANOVA and Tukey’s multiple comparisons procedure. (C) Control Group, (E), Ethanol Group, (CR) Control Risedronate Group, (ER) Ethanol-Risedronate Group.
p < 0.05;
p < 0.001.
DISCUSSION
The principal finding of this study was an increase in serum levels of Dpd, a marker for bone resorption, after multiple binge alcohol exposures of adult male rats. Serum Dpd levels significantly increased after 3 weeks of 4 days/week binge alcohol treatments. Treatment with the aminobisphosphonate risedronate, an antiresorptive agent, eliminated this alcohol-induced increase in serum Dpd. This data suggests that exposure to high doses of alcohol in a binge pattern may have a direct stimulatory effect on bone resorption in addition to its well-documented effects on bone formation. This finding is significant because it offers evidence that resorption is a contributing factor in alcohol induced bone loss and suggests the possible use of antiresorptive therapy for alcohol-induced osteopenia.
The results are in agreement with a previous rodent study that observed an increase in bone loss and urinary Dpd excretion after alcohol treatment (Zhang et al., 2002). OPG, an inhibitor of osteoclastogenesis, eliminated the alcohol-induced resorptive effects in this model. However, our results contrast with another earlier rodent study that observed decreased urinary excretion of total and conjugated deoxypyridinoline in rats consuming alcohol for 6 weeks (Preedy et al., 1991). The authors of this study concluded that alcohol treatment reduced the absolute rate of bone resorption in adolescent rats using a liquid diet model of alcohol exposure but did not provide information on BAC’s. Our study was performed in adult animals that received binge-like alcohol exposures resulting in BAC’s substantially higher than typical alcohol liquid diet models normally achieve. These fundamental differences may account for the contrasting results. Alcohol dose and duration of exposure are variables likely to influence the skeletal response to alcohol (Turner et al., 1998). The manner in which the adolescent and adult skeleton react to alcohol exposure is likely influenced by factors such as bone turnover rates and remodeling equilibrium, components of bone homeostasis that vary as the skeleton grows, matures and ages.
The findings in the current study are in agreement with many human studies on alcohol and bone resorption (Laitinen et al., 1994; Nyquist et al., 1996; Pepersack et al., 1992), although other human studies on alcohol consumption and bone resorption markers are contradictory (Bilke et al., 1985; Crilly et al., 1988; Diamond et al., 1989; Laitinen et al., 1992). The contrasting observations in human studies of alcohol and bone resorption might be explained by the inability to control for confounding variables such as smoking status, nutrition, liver disease and general health status that can influence experimental outcome. Another factor complicating interpretation is that in some studies patients were actively drinking at the time of the assay, while others looked at bone resorption during alcohol withdrawal. Alcoholic patients would presumably differ with respect to bone resorptive activity based on whether they were current drinkers or were abstaining from alcohol.
The current study also shows a clear association between alcohol binge-like exposures and decreased cancellous BMD of the distal femur and lumbar spine. Previous reports have found an association between alcohol consumption and decreased cortical and cancellous BMD using a chronic alcohol-feeding model in young rats (Nishiguchi et al., 2000). We observed reductions only in cancellous BMD, but since several differences exist between the two models, such as the age of animals used and the length and pattern of alcohol exposure, it is not surprising that our results differ.
We also observed significant decreases in lumbar vertebral body compressive strength after 3 weeks of binge alcohol exposure as well as a significant effect on selected lumbar histomorphometric parameters. The overall loss of trabecular bone volume and increased trabecular separation that occurred in binge-treated rats suggests that both trabecular plate thinning and decreased connectivity were present in lumbar cancellous bone from alcohol-exposed animals. These ethanol-induced alterations in trabecular architectural properties combined with reductions in BMD values in lumbar spine both likely contribute to the significant reductions in lumbar vertebral compressive strength we observed in binge treated animals. These finding are notable as they provide evidence suggesting that binge alcohol exposure has a negative impact on lumbar spine integrity. Vertebral compression fractures are common in osteoporosis (Genant and Jergas, 2003); our study suggests that alcohol may impart an additional or additive risk factor for vertebral fracture in susceptible individuals. It is again noteworthy that concurrent administration of risedronate maintained not only BMD values in femur and lumbar vertebra of alcohol-treated animals, but also maintained lumbar compressive strength and trabecular architectural indices of binge-alcohol treated rats at control levels. The fact that these parameters were maintained at control levels when binge alcohol treated animals were given risedronate strongly suggests a suppression of alcohol-induced, osteoclast-mediated bone loss in this group. This implies that alcohol-induced decreases in BMD, compressive strength and trabecular structural alterations result, at least in part, from an alcohol-induced increase in bone resorption. This data also suggests that antiresorptive therapy may prevent or lessen alcohol-induced bone loss. Future studies will determine if bisphosphonate treatment begun after alcohol-induced bone damage has already occurred is as effective in restoring BMD and compressive strength as preventative treatment was in this study.
The observed alcohol-induced decreases in femur and vertebral body BMD and vertebral strength correspond in timing to the elevation in serum Dpd levels, with both occurring after the third alcohol binge cycle. Although the indices of bone integrity examined in this study were not significantly influenced by alcohol binge treatment until the third binge cycle, all parameters show a trend toward an alcohol effect in the first and second binge cycles. There are several possible explanations for this observation. Alcohol-induced bone damage is likely cumulative and several alcohol insults may be required before bone remodeling is perturbed to a level that is significant. Our binge model allows a three-day rest period between alcohol exposure cycles, which might promote bone recovery from two binge cycles, but is not sufficient to overcome a third week of alcohol exposure. Bone resorption requires recruitment, differentiation and activation of osteoclasts before resorptive activity can begin (Teitelbaum, 2000). Any or all of the steps in this pathway may be modulated by alcohol exposure, perhaps some more so than others, resulting in different levels of resorptive activity based on alcohol dose and exposure duration.
Our rat model of binge-like alcohol consumption used IP injection as an alcohol administration route. There were several advantages to using this administration route to study effects of high dose alcohol treatment on bone health. First we were able to attain high BAC’s in a controlled, consistent manner with minimal effects on animal body weight. Protocols that rely on liquid diet administration of alcohol (36% of total caloric intake) are limited to an attainable BAC of approximately 150 mg/dL (Sampson, 1998; Wezeman et al., 2000). The target BAC chosen for this study is relevant to bone health effects in human alcoholics, because BAC’s near 300 mg/dL may be common in chronic alcohol abusers (Urso et al., 1981). 300 mg/dL was also the mean BAC in a study of alcohol-intoxicated patients with increased serum levels of collagen degradation products indicative of bone resorption (Laitinen et al., 1994). IP injection is well tolerated by rats and is accomplished with minimal stress. The pharmacokinetic profile of a 3 g/kg IP injection of a 20% (vol./vol.) ethanol/saline solution is well defined (Nation et al., 1993, 1994) and allows a controlled period of alcohol exposure each treatment day. Caloric intake was not matched between treatment groups, but no significant changes in body weight were observed between treatment groups.
Only one other study to date attempted to examine the effects of binge-like alcohol exposure on bone health in rats (Sampson et al., 1999). This study used a young growing rat model and gastric gavage to deliver alcohol in a binge-like pattern (2 or 5 days/week) over six weeks. This study found no adverse effects of either treatment regime on bone health parameters but showed increased bone length, weight and BMD in animals given alcohol 2 days/week. BAC’s attained by this protocol were between 40 and 100 mg/dL, which are more reflective of moderate alcohol consumption, and not in the range of BAC’s indicative of alcoholic binge drinking. The positive effects on bone health observed may actually reflect a beneficial effect of moderate alcohol consumption on BMD and fracture risk suggested in other studies (Angus et al., 1988; Felson et al., 1995; Laitinen et al., 1991). The benefits of moderate alcohol consumption on bone health have recently been challenged however by data suggesting that even low levels of alcohol consumption can suppress bone turnover and potentially damage the skeleton (Turner et al., 2001). Our alcohol treatment protocol attained BAC’s that are reflective of human alcoholic binge drinking, and the data suggests that these higher binge pattern BAC’s are clearly detrimental to bone health.
CONCLUSION
In conclusion, the current study suggests that binge alcohol exposure of adult male rats results in increased bone collagen degradation and lower bone mineral density, compressive strength and compromised trabecular structure due to increased bone resorption. The finding that concurrent treatment with risedronate eliminates these effects supports the hypothesis that bone resorption plays a significant role in alcohol-induced bone loss and suggests that therapeutic intervention may be possible. Studies are currently underway to examine the effects of binge alcohol treatment on the molecular mechanisms regulating osteoclast recruitment, differentiation and activity, which may clarify the role of bone resorption in alcohol-mediated osteopenia.
Acknowledgments
This work was supported by the National Institute of Health, National Institute on Alcohol Abuse and Alcoholism grants RO1 AA12576 and AA13527 (FHW).
The authors wish to thank all members of the Wezeman lab for their assistance in this study.
References
- Angus RM, Sambrook PN, Pocock NA. Dietary intake and bone mineral density. Osteopr Int. 1988;1:265–277. [PubMed] [Google Scholar]
- Bilke DD, Genant HK, Cann C, Recker RR, Halloran BP, Strewler GJ. Bone disease in alcohol abuse. Ann Inter Med. 1985;103:42–48. doi: 10.7326/0003-4819-103-1-42. [DOI] [PubMed] [Google Scholar]
- Cartlidge D, Redmond AD. Alcohol and conscious level. Biomed Pharmacother. 1990;44:205–208. doi: 10.1016/0753-3322(90)90025-5. [DOI] [PubMed] [Google Scholar]
- Chavassieux P, Serre CM, Vergnaud P, Delmas PD, Meunier PJ. In vitro evaluation of dose-effects of ethanol on human osteoblastic cells. Bone and Mineral. 1993;22:95–103. doi: 10.1016/s0169-6009(08)80221-8. [DOI] [PubMed] [Google Scholar]
- Cheung RC-Y, Gray C, Boyde A, Jones SJ. Effects of ethanol on bone cells in vitro resulting in increased resorption. Bone. 1995;16(1):143–147. [PubMed] [Google Scholar]
- Compston J. Mechanisms of bone loss and gain in untreated and treated osteoporosis. Endocrine. 2002;17(1):21–27. doi: 10.1385/ENDO:17:1:21. [DOI] [PubMed] [Google Scholar]
- Crilly RG, Anderson C, Hogan D, Delaquerriere-Richardson L. Bone histomorphometry, bone mass and related parameters in alcoholic males. Calcif Tissue Int. 1988;43:269–276. doi: 10.1007/BF02556634. [DOI] [PubMed] [Google Scholar]
- Diamond T, Stiel D, Lunzer M, Wilkinson M, Solomon P. Ethanol reduces bone formation and may cause osteoporosis. Am J Med. 1989;86:282–288. doi: 10.1016/0002-9343(89)90297-0. [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Charles P, Melsen F, Mosekilde L, Risteli L, Risteli J. Serum markers of type I collagen formation and degradation in metabolic bone disease; correlation with bone histomorphometry. J Bone Miner Res. 1993;8(2):127–132. doi: 10.1002/jbmr.5650080202. [DOI] [PubMed] [Google Scholar]
- Farley JR, Fitzsimmons R, Taylor AK, Jorch UM, Lau K-HW. Direct effects of ethanol on bone resorption and formation in vitro. Arch Biochem Biophys. 1985;238(1):305–314. doi: 10.1016/0003-9861(85)90169-9. [DOI] [PubMed] [Google Scholar]
- Felson DT, Zhang Y, Hannan MT, Kannal WB, Kiel DP. Alcohol intake and bone mineral density in elderly men and women. The Framingham Study. Am J Epidemiol. 1995;142(5):485–492. doi: 10.1093/oxfordjournals.aje.a117664. [DOI] [PubMed] [Google Scholar]
- Genant HK, Jergas M. Assessment of prevalent and incident vertebral fractures in osteoporosis research. Osteoporosis Int. 2003;14(Suppl 3):43–55. doi: 10.1007/s00198-002-1348-1. [DOI] [PubMed] [Google Scholar]
- Klein RF, Fausti KA, Carlos AS. Ethanol Inhibits human osteoblastic cell proliferation. Alcohol Clin Exp Res. 1996;20(3):572–578. doi: 10.1111/j.1530-0277.1996.tb01095.x. [DOI] [PubMed] [Google Scholar]
- Laitinen K, Lamberg-Allardt C, Tunninen R. Bone mineral density and abstention-induced changes in bone and mineral metabolism in noncirrhotic male alcoholics. Am J Med. 1992;93:642–650. doi: 10.1016/0002-9343(92)90197-j. [DOI] [PubMed] [Google Scholar]
- Laitinen K, Tahtela R, Luomanmaki K, Valimaki MJ. Mechanisms of hypocalcemia and markers of bone turnover in alcohol-intoxicated drinkers. Bone Miner. 1994;24:171–179. doi: 10.1016/s0169-6009(08)80134-1. [DOI] [PubMed] [Google Scholar]
- Laitinen K, Valimaki M, Keto P. Bone mineral density measured by duel-energy x-ray absorptiometry in health Finnish women. Calcif Tissues Int. 1991;48:224–231. doi: 10.1007/BF02556372. [DOI] [PubMed] [Google Scholar]
- Lindblad B, Olsson R. Unusually high levels of blood alcohol? JAMA. 1976;236:1600–1602. [PubMed] [Google Scholar]
- Lundquvist C, Alling C, Knoth R, Volk B. Intermittent ethanol exposure of adult rats: Hippocampal cell loss after one month of treatment. Alcohol Alcohol. 1995;30(6):737–748. [PubMed] [Google Scholar]
- Nation JR, Burkey RT, Grover CA. Lead/Ethanol interactions II. Pharacokinetics. Alcohol. 1993;10:363–367. doi: 10.1016/0741-8329(93)90021-f. [DOI] [PubMed] [Google Scholar]
- Nation JR, Burkey RT, Grover CA, Bratton GR. The effects of cadmium exposure on ethanol pharmacokinetics. Pharmacol Biochem Behav. 1994;48(2):543–546. doi: 10.1016/0091-3057(94)90568-1. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S, Shiomi S, Tamori A, Habu D, Takeda T, Tanaka T. Effect of ethanol on bone mineral density of rats evaluated by dual-photon X-ray absorptiometry. J Bone Miner Metab. 2000;18:317–320. doi: 10.1007/s007740070002. [DOI] [PubMed] [Google Scholar]
- Nyquist F, Ljunghall S, Berglund M, Obrant K. Biochemical markers of bone metabolism after short and long time ethanol withdrawal in alcoholics. Bone. 1996;19(1):51–54. doi: 10.1016/8756-3282(96)00110-x. [DOI] [PubMed] [Google Scholar]
- Parfitt MA, Drezner MK, Glorieux FH, Kanis JA, Malluche M, Meunier PJ, Ott Sm, Recker RR. Bone Histomorphometry: Standardization of nomenclature, symbols and units. J Bone Miner Res. 1987;2(6):595–609. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Penland S, Hoplight B, Obernier J, Crews FT. Effects of nicotine on ethanol dependence and brain damage. Alcohol. 2001;24:45–54. doi: 10.1016/s0741-8329(01)00142-2. [DOI] [PubMed] [Google Scholar]
- Pepersack T, Fuss M, Otero J, Bergmann P, Valsamis J, Corvilain J. Longitudinal study of bone metabolism after ethanol withdrawal in alcoholic patients. J Bone Miner Res. 1992;7(4):383–387. doi: 10.1002/jbmr.5650070405. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Sherwood RA, Akpoguma CO, Black D. The urinary excretion of the collagen degradation markers pyridinoline and deoxypridinoline in an experimental rat model of alcoholic bone disease. Alcohol Alcohol. 1991;26 (2):191–198. doi: 10.1093/oxfordjournals.alcalc.a045100. [DOI] [PubMed] [Google Scholar]
- Robins SP. Collagen crosslinks in metabolic bone disease. Acta Orthop Scand. 1995;66(Suppl 266):171–175. [PubMed] [Google Scholar]
- Russel RGG, Rodgers MJ. Bisphosphonates: From the laboratory to the clinic and back again. Bone. 1999;25:97–106. doi: 10.1016/s8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- Sampson HW. Effect of alcohol consumption on adult and aged bone: a histomorphometric study of the rat animal model. Alcohol Clin Exp Res. 1998;22(9):2029–2034. [PubMed] [Google Scholar]
- Sampson HW, Gallager S, Lange J, Chondra W, Hogan HA. Binge drinking and bone metabolism in a young actively growing rat model. Alcohol Clin Exp Res. 1999;23(7):1228–1231. doi: 10.1111/j.1530-0277.1999.tb04282.x. [DOI] [PubMed] [Google Scholar]
- Seyed SM, Rosen DM. Matrix proteins of the skeleton. Curr Opin Cell Biol. 1990;2:914–919. doi: 10.1016/0955-0674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Steinke B, Patwardhan AG, Havey R, King D. Human growth hormone transgene expression increases the biomechanical structural properties of mouse vertebrae. Spine. 1999;24:1–4. doi: 10.1097/00007632-199901010-00002. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Teplin LA, Abram KM, Michaels SK. Blood alcohol levels among emergency room patients: a multivariate analysis. J Stud Alcohol. 1989;50(5):441–447. doi: 10.15288/jsa.1989.50.441. [DOI] [PubMed] [Google Scholar]
- Turner RT. Skeletal response to alcohol. Alcohol Clin Exp Res. 2000;24(11):1693–1701. [PubMed] [Google Scholar]
- Turner RT, Kidder LS, Kennedy A, Evans GL, Sibonga JD. Moderate alcohol consumption suppresses bone turnover in adult female rats. J Bone Miner Res. 2001;16(3):589–594. doi: 10.1359/jbmr.2001.16.3.589. [DOI] [PubMed] [Google Scholar]
- Turner RT, Wronski TJ, Zhang M, Kidder LS, Bloomfield SA, Sibonga JD. Effects of ethanol on gene expression in rat bone: Transient dose-dependent changes in mRNA levels for bone matrix proteins, skeletal growth factors and cytokines are followed by reductions in bone formation. Alcohol Clin Exp Res. 1998;22:1591–1599. doi: 10.1111/j.1530-0277.1998.tb03953.x. [DOI] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sci. 1981;28:1053–1056. doi: 10.1016/0024-3205(81)90752-9. [DOI] [PubMed] [Google Scholar]
- Wezeman FH, Emanuele MA, Emanuele NV, Moskal SF, Woods M, Suri M, Steiner J, LaPaglia N. Chronic alcohol consumption during male rat adolescence impairs skeletal development through effects on osteoblast gene expression, bone mineral density and bone strength. Alcohol Clin Exp Res. 1999;23(9):1534–1542. [PubMed] [Google Scholar]
- Wezeman FH, Emanuele MA, Moskal SF, Steiner J, Lapaglia N. Alendronate administration and skeletal response during chronic alcohol intake in the adolescent male rat. J Bone Miner Res. 2000;15(1):2033–2041. doi: 10.1359/jbmr.2000.15.10.2033. [DOI] [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behav Neural Biol. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dai J, Lin D-L, Habib P, Smith P, Murtha J, Fu Z, Yao Z, Qi Y, Keller ET. Osteoprotegerin abrogates chronic alcohol ingestion-induced bone loss in mice. J Bone Min Res. 2002;17(7):1256–1263. doi: 10.1359/jbmr.2002.17.7.1256. [DOI] [PubMed] [Google Scholar]