Abstract
One of the most fascinating examples of parasite-induced host manipulation is that of hairworms, first, because they induce a spectacular “suicide” water-seeking behavior in their terrestrial insect hosts and, second, because the emergence of the parasite is not lethal per se for the host that can live several months following parasite release. The mechanisms hairworms use to increase the encounter rate between their host and water remain, however, poorly understood. Considering the selective landscape in which nematomorph manipulation has evolved as well as previously obtained proteomics data, we predicted that crickets harboring mature hairworms would display a modified behavioral response to light. Since following parasite emergence in water, the cricket host and parasitic worm do not interact physiologically anymore, we also predicted that the host would recover from the modified behaviors. We examined the effect of hairworm infection on different behavioral responses of the host when stimulated by light to record responses from uninfected, infected, and ex-infected crickets. We showed that hairworm infection fundamentally modifies cricket behavior by inducing directed responses to light, a condition from which they mostly recover once the parasite is released. This study supports the idea that host manipulation by parasites is subtle, complex, and multidimensional.
Keywords: behavior, insects, nematomorph, parasite manipulation, parasitism, phototaxis
Many parasitic organisms manipulate the phenotype of their host to increase their transmission (for review, see Moore 2002; Poulin 2007; Thomas, Rigaud, and Brodeur 2010). Phenotypic alterations can vary greatly in diversity, extending from morphological to physiological and behavioral modifications, and also in magnitude, from subtle changes in the percentage of time spent performing a given activity to the display of spectacular morphologies or behaviors (Lefèvre et al. 2009; Thomas, Poulin, and Brodeur 2010). Although in most cases parasitized hosts are manipulated until their death (e.g., Lafferty 1999; Wellnitz 2005), there are few intriguing situations in which host manipulation is only temporary and hosts apparently recover afterward. However, in spite of extensive research on manipulative parasites, recoveries of manipulations remain poorly studied (see Eberhard 2010).
Nematomorphs are one of the most impressive examples of manipulating parasites that activate an original behavior in their host. As juveniles, freshwater hairworms are mostly parasites of insects but, once adult, they are free living and need to enter water to mate, oviposit, and produce infective stages (Schmidt-Rhaesa 2002). Insects harboring mature hairworms display a new and original behavior: they seek water to immerse themselves (Begon et al. 1990; Thomas et al. 2002; Sánchez et al. 2008). This behavior, originally absent from the host's repertoire, allows the parasite to emerge from its host into the aquatic environment it needs to continue its life cycle. Interestingly, emergence of hairworms is not lethal per se for the host, and crickets of both sexes, when they do not drown, can live several months after having released their parasite (Biron, Ponton, et al. 2005).
The mechanisms used by hairworms to increase the encounter rate with water remain a poorly understood aspect of this host manipulation (Sánchez et al. 2008). Although water detection from long distances through humidity gradients is not involved (Thomas et al. 2002), it has been argued that phototaxis alterations (changes in the responses to light stimuli) could be a part of the wider strategy of hairworms for the completion of their life cycle (Biron, Marche, et al. 2005; Biron et al. 2006). Specifically, parasite-induced positive phototaxis could improve the encounter rate with water (Biron et al. 2006). These assumptions were derived from 2 kinds of arguments. First, in the native forest of southern of France, water areas such as ponds and rivers are, at night, luminous openings contrasting with the dense surrounding forest. Indeed, the combination of direct light from the sky and reflected light from the water potentially provides more exposure to light. Light could be then a sensory cue (see Henze and Labhart 2007) that leads infected arthropods to an aquatic environment. Besides this ecological reasoning, proteomics data indicated a differential expression of protein families (i.e., CRAL_TRIO) that may be functional components of the visual cycle in the central nervous system of Nemobius sylvestris harboring Paragordius tricuspidatus (Biron et al. 2006). Interestingly, the modified expressions of these proteins were only observed at a key period of the manipulative process, which is when crickets harbor a mature hairworm and when they attempt to enter water, but not in ex-infected insects (Biron et al. 2006). A differential expression of protein from the CRAL_TRIO family has also been found in amphipods infected with cerebral trematodes (Ponton et al. 2006) and, indeed, infected amphipods were attracted to light. Altered photic behavior is one of the most documented forms of parasite-induced altered behavior in invertebrates (for review, see Moore 2002), and these facts also support an eventual modification of phototaxis in crickets infected by nematomorphs.
Considering the selective landscape in which nematomorph's manipulation has evolved, in addition to former proteomics data, we predicted that crickets harboring mature hairworms would display a positive phototaxis compared with uninfected individuals. Furthermore, we predicted that the host would recover from the modified phototaxis after parasite emergence owing to the fact that the host cricket and parasitic worm do not physiologically interact anymore. We experimentally tested this hypothesis for the wood-cricket N. sylvestris (Bosc 1792) (Orthoptera: Gryllidae: Nemobiinae) infected by the nematomorph P. tricuspidatus (Dufour 1828) (Nemotomorpha: Gordioida: Chordodidae). We evaluated the orientation behavior of uninfected and infected crickets, as well as ex-infected crickets at 3, 20, and 35 h after parasite's emergence. Finally, because it has been hypothesized that nematomorph parasites cause some kind of “thirst” in the host that leads it to approach water (e.g., Blunk 1922; Thomas et al. 2003), we examined the behavior of water-deprived individuals.
MATERIALS AND METHODS
Collection and maintenance of insects
Nemobius sylvestris crickets were collected at Avènes les Bains (lat 43°45′N, long 3°06′E, Southern France) in July 2005 between 10 PM and 1 AM following the same methodology as described by Thomas et al. (2002). Because uninfected N. sylvestris are exclusively found in the forest, they could only be collected in this habitat. Conversely, manipulated crickets could not be sampled in the same way because the frequency of infected individuals in the forest is very low (usually, 5%, Thomas F, unpublished data) and only concerns crickets that harbor immature worms. However, it is common to find specimens harboring mature and manipulative worms in atypical habitats surrounding the forest (e.g., areas made of concrete). We thus collected infected crickets on concrete areas around the forest as 99% of these crickets (i.e., 69 of 70) were infected by a nematomorph. Insects were kept in plastic boxes with food ad libitum (in equal proportions: cereals, fish food Tetra AniMin, dry gammarids, and dry tubifex) and humidified cotton at a temperature between 20 and 25 °C. Individuals were shipped from France to Switzerland by express delivery (48 h).
Experimental design
Servosphere
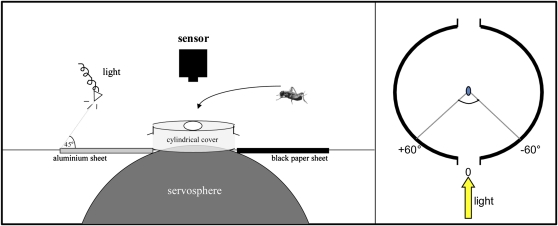
A locomotion compensator or servosphere (Kramer 1976; Otálora-Luna et al. 2004) was used to evaluate the behavioral response of walking crickets to light (Figure 1). This tracking system allowed a free animal to walk unimpeded in all directions on the upper pole of a 50-cm diameter black sphere. The x, y coordinates of displacements caused by the moving animal on the sphere were recorded (as in Taneja and Guerin 1995) at a rate of 0.1 s with an accuracy of 0.1 mm on a computer where the track described by the animal was reconstructed. The behavior of each walking insect was also registered by an infrared sensitive CCD video camera (Canon Ci 20PR, Japan and PCO Computer Optics 77CE, Germany) placed above the apex of the servosphere.
Figure 1.
Experimental design used to test light attraction of crickets (left) and view of the servosphere set up from above (right).
Stimulus delivery
A cold halogen light (tungsten lamp, 150 W, 20 KHz, Schott-KL 1500) was pointed into a horizontal aluminum foil (15 × 10 cm) that reflected the light to the insect (intensity = 137 lux), simulating the reflection of nocturnal lights by the surface of a reservoir of water (Figure 1). The lamp located over the aluminum foil produced a broad cone of light pointed at 45° relative to the horizontal axis of the aluminum sheet (Figure 1A). Therefore, the light reaching the cricket was so diffuse that it could not distinguish the shape of the light cone. The light produced by the tungsten lamp has a wide wavelength spectrum that goes from ultraviolet to far infrared. The light was white (according to human perception) and had a high color temperature (i.e., cool-colored light). The experimental room was darkened during each experiment, and a cylindrical metal black cover was placed on the top of the servosphere to prevent any residual light interfering with the set up of experiments (see Figure 1B). The light stimulus entered through an aperture (37 mm wide and 32 mm high) on the side of the cover. Another aperture on the other side of the cover was opened on to a black sheet of paper.
Treatments
The effect of reflected light on the orientation behavior of uninfected and infected crickets was evaluated on the servosphere. Additionally, for a group of infected crickets, worm emergence was experimentally induced by placing infected crickets in water. Such crickets were named “ex-infected.” To follow the effect of the light stimulus on the behavior of ex-infected insects over time, crickets were tested on the servosphere 3, 20, and 35 h after the parasite emerged. We also tested blind individuals in order to verify that our results were only due to light and not to another sensory modality that we might not have been able to control during the experiments. For this, we painted the eyes of several infected and uninfected crickets black (Plaka-lack, Schwarz Black 70; Pelikan, Schindellegi, Switzerland) and tested them in the same conditions as sighted individuals 1 day after painting. Finally, several water-deprived uninfected crickets were tested with light to assess if this stimulus could serve as an indication of water for these insects. For this treatment, individuals were placed in plastic boxes deprived of wet cotton for 2 days and tested in the same conditions as the other insects.
Experiments started at least 5 min after the animal had been placed on the servosphere to allow it to acclimate to the experimental condition. For each cricket, an experiment consisted of 3 consecutive 2-min periods: 1) dark (control period), 2) light stimulus (test period), and 3) dark again (end-control period). Observation of the crickets on the servosphere and turning the light stimulus on and off were performed from outside of the experimental room to avoid disturbing the animals during the experiments. Insects that did not walk during the test period were discarded from the analysis.
Track analysis and statistics
The basic x and y spatial coordinates of displacements provided by the servosphere at intervals of 0.1 s were merged in step sizes of 3 units for efficient summary of the tracks (Kitching and Zalucki 1982; Otálora-Luna et al. 2004). The provided step-size intervals of 0.3 s allowed the insect to move at least 50% of its length before recording its next position, taking into account that N. sylvestris mean length is approximately 8.5 mm and its average speed (referred to hereafter as velocity) is approximately 15 mm/s. Average cricket velocity was measured from uninfected, infected, and ex-infected insects walking during the 3 consecutive 2-min periods. Subsequently, a running mean was calculated over 5 successive (0.3 s) intervals to smoothen the data in order to remove some noise related to the insect's gait that was picked up by the servosphere. To identify stops, 3 mm/s was considered the minimum speed the animal had to achieve to be considered walking. The maximum velocity recorded was 35.15 mm/s, observed for an infected insect during the stimulation period. We evaluated the behavioral responses to the light stimulus in the following cricket classes: “uninfected crickets” (N = 27), “infected” (N = 27), “3-h ex-infected” (N = 11), “20-h ex-infected” (N = 19), “35-h ex-infected” (N = 13), “blind-uninfected” (N = 11), “blind-infected” (N = 11), and water-deprived uninfected crickets (N = 12).
In order to measure cricket behavioral responses to the light source, the following parameters were calculated for each 2-min track (i.e., for the control period, test period, end-control period): velocity, displacement, displacement in a cone toward the light source (see below), and path straightness. Path straightness is the resultant magnitude of the circular mean vector averaged for each 2-min track derived from instantaneous angles recorded every 0.3 s. Path straightness serves as an index that varies from 0 to 1, where 1 indicates that the insect walked very straight and 0 that the insect walked very tortuously (for more details, see Batschelet 1981). We considered that a cricket was attracted to light (i.e., phototaxis) when it walked into an arbitrary assigned cone, 60° either side of the center of the aperture on the illuminated side of the servosphere cover (i.e., displacement in the cone, see Figure 1B). We considered that a cricket increased its walking activity when it increased its velocity and overall displacement.
The effect of the light stimulus on all measured parameters (i.e., velocity, displacement, displacement in the cone, and path straightness) was evaluated by calculating indices representing the difference between the data obtained for the test period and the control periods. Indices were calculated for each cricket class. When residuals of our model followed a normal distribution and showed homoscedasticity, they were analyzed with one-way analyses of variance (ANOVAs) and Tukey–Kramer post hoc test; otherwise, indices were analyzed with Kruskal–Wallis tests and Dunn's post hoc test. The data of the 2 control periods were compared by repeated-measures ANOVA or Friedman's ANOVA (when data deviated from normality), with the period as the repeated factor. Displacements were square root transformed. Data were analyzed using Systat version 12 for Windows, SYSTAT software Inc. (San Jose, CA). Equality of variance was tested with Levene's test, and normality of the residuals of models was analyzed within each treatment with Shapiro–Wilk W test. Post hoc multiple comparisons were done following Ruxton and Beauchamp (2008). All results were considered significant at the 5% level.
RESULTS
Walking activity
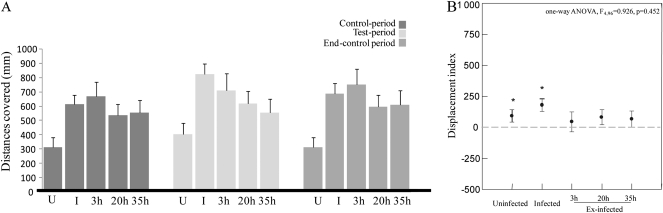
Figure 2A illustrates the distances covered by the 5 cricket classes during the 3 experimental periods. Distances covered by insects before and after stimulation (the 2 control periods) were significantly influenced by the cricket's status (repeated-measures ANOVA, see Table 1 and Supplementary Table 1) with infected and 3-h ex-infected crickets walking longer distances than uninfected insects during both control periods (“control period”: one-way ANOVA F4,96 = 3.755, P = 0.007, “end-control period”: one-way ANOVA F4,94 = 5.497, P = 0.001, Tukey–Kramer post hoc tests P < 0.05). The distances covered by 20- and 35-h ex-infected crickets during the first control period were intermediate between distances covered by infected and uninfected crickets (i.e., not significantly different from infected and uninfected crickets, Tukey–Kramer post hoc tests P > 0.05). Displacement indices (calculated as the difference between the square root transformed distance covered during the test period and square root transformed distance covered during the first control period) were not significantly different between the 5 cricket classes (see Figure 2B and Supplementary Table 1). Nevertheless, infected and uninfected crickets walked slightly more during the test periods compared with the control periods (i.e., indices are significantly higher than zero, Figures 2A,B).
Figure 2.
(A) Distances covered (millimeter) by uninfected (U), infected (I), and ex-infected (3, 20, and 35 h) crickets during the 3 periods of experiments. (B) Displacement index (mean ± standard error) in relation to parasitism status. Asterisks indicate means that are significantly different from zero (paired t-test, P < 0.05).
Table 1.
Repeated-measures ANOVA comparing distances covered and average velocity for the 5 cricket categories (uninfected; infected; 3, 20, and 35 h ex-infected) during the 2 control periods (control and end-control period)
| Source | Sum of Squares | df | F | P |
| Distances covered | ||||
| Among cricket categories | ||||
| Status | 2191.991 | 4 | 5.134 | 0.001 |
| Error | 9500.574 | 89 | ||
| Between the 3 different periods | ||||
| Period | 46.011 | 1 | 3.785 | 0.055 |
| Period × status | 28.902 | 4 | 0.594 | 0.668 |
| Error | 1081.882 | 89 | ||
| Average velocity | ||||
| Among cricket categories | ||||
| Status | 420.918 | 4 | 3.770 | 0.008 |
| Error | 2093.368 | 75 | ||
| Between the 3 different periods | ||||
| Period | 11.101 | 1 | 3.861 | 0.053 |
| Period × status | 23.542 | 4 | 2.047 | 0.096 |
| Error | 215.630 | 75 | ||
| Path straightness | ||||
| Among cricket categories | ||||
| Status | 0.016 | 4 | 0.702 | 0.593 |
| Error | 0.418 | 74 | ||
| Between the 3 different periods | ||||
| Period | 0.000 | 1 | 0.007 | 0.934 |
| Period × status | 0.038 | 4 | 2.491 | 0.050 |
| Error | 0.284 | 74 | ||
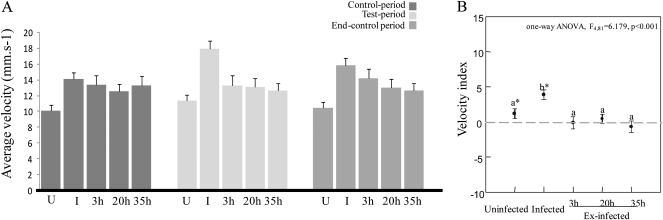
Similar results to those obtained for distance covered were found for the average velocity (see Figure 3A and Supplementary Table 1). Velocity was significantly influenced before and after stimulation (the 2 control periods) by the cricket's status (repeated-measures ANOVA, see Table 1 and Supplementary Table 1). Infected crickets walked faster than uninfected insects during both control periods (control period: one-way ANOVA F4,80 = 2.759, P = 0.034, end-control period: one-way ANOVA F4,80 = 4.410, P = 0.003, Tukey–Kramer post hoc tests P < 0.05). Velocities of 20- and 35-h ex-infected crickets were intermediate between velocities of infected and uninfected crickets for both control periods (i.e., not significantly different from infected and uninfected crickets, Tukey–Kramer post hoc tests P > 0.05). Moreover, infected crickets responded to the light stimulus by increasing their velocity (Figure 3B). Uninfected insects also slightly increased their velocity after being stimulated (Figure 3B). However, we did not record any response to the light for ex-infected (3, 20, and 35 h) insects (i.e., indices are not significantly different from zero, Figure 3B).
Figure 3.
(A) Average velocity (mm/s) of uninfected (U), infected (I), and ex-infected (3, 20, and 35 h) crickets during the 3 periods of experiments. (B) Index of speed (mean ± standard error) in relation to parasitism status. Asterisks indicate means that are significantly different from zero (paired t-test, P < 0.05). Different letters indicate significant pairwise differences (Tukey–Kramer post hoc tests) across the different cricket classes for the distances walked over the whole experiment.
Path straightness
We did not find any significant differences for path straightness between the 5 cricket categories during the 2 control periods (repeated-measures ANOVA, see Table 1 and Supplementary Table 1). Indices of path straightness were also not significantly different between the 5 cricket classes (Kruskal–Wallis test, χ2 = 1.667, degrees of freedom [df] = 4, P = 0.797, see Supplementary Table 1). We nevertheless found that infected crickets responded to the light stimulus with a slight increase in path straightness (i.e., infected insects walked straighter when stimulated, index of path straightness was significantly different and higher than zero, Wilcoxon signed-rank test, P = 0.05, see Supplementary Table 1 and Supplementary Figure 1).
Phototaxis
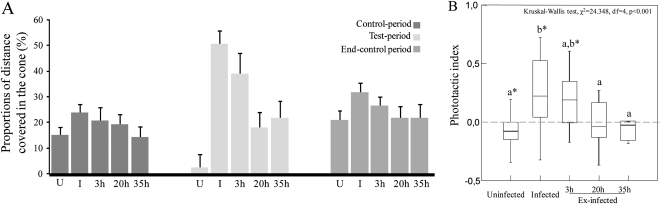
Nematomorphs induced a clear phototactic behavior in their hosts as indicated by the proportion of distance covered in a cone 60° either side of the incoming light: crickets directed their walk to the light stimulus when infected, a behavior not observed from uninfected crickets (Figure 4; see also Supplementary Table 1). Indeed, during stimulation with light, 100% of infected and 3-h ex-infected crickets walked in the cone 60° either side of the incoming light, 52% of uninfected crickets never walked in the cone, whereas 20- and 35-h ex-infected crickets showed intermediate responses, and 21% of 20-h ex-infected and 31% of 35-h ex-infected crickets never walked in the cone (Fisher Exact test r × k contingency table, P < 0.001). The proportions of distance covered in the cone were slightly greater during the end-control period for all cricket categories compared with the first control period (Friedman's ANOVA between the 2 control periods, χ2 = 5, df = 1, P = 0.025, Figure 4A and Supplementary Table 1). We nevertheless did not find any significant difference in the proportion of distance covered in the cone between the 2 control periods when considering cricket classes separately (paired t-test, P > 0.05). The phototactic index (calculated as the proportion of distance covered in the cone during the test period minus the proportion of the distance covered in the cone during the first control period, see Supplementary Table 1) was significantly different between the 5 cricket classes with infected individuals being more attracted to light than uninfected and 20- and 35-h ex-infected crickets (Figure 4B). Three-hour ex-infected crickets showed a positive phototactic index indicating that they were still attracted by light (Figure 4B). Uninfected crickets showed a negative index and this could indicate that, when uninfected, crickets stay away from light (Figure 4B). The phototactic index of 20- and 35-h ex-infected crickets was not significantly different from zero (Figure 4B).
Figure 4.
(A) Proportions of distance covered in the cone (%) during the 3 experimental periods in relation to parasitism status (U = uninfected, I = infected, and 3/20/35 h = ex-infected). (B) Phototactic index (median, quartiles, and range) in relation to parasitism status. A positive index reflects a positive phototaxis. Asterisks indicate means that are significantly different from zero (Wilcoxon signed-rank test, P > 0.05). Different letters indicate significant pairwise differences across the different cricket classes (Dunn's post hoc test).
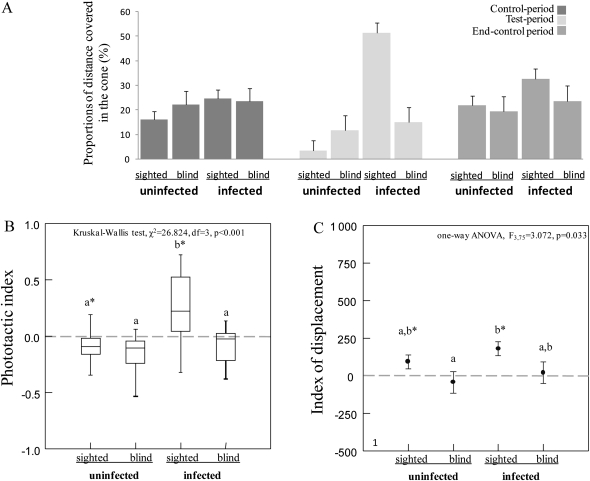
Blind crickets
Figure 5A shows the orientation behavior of sighted-uninfected and sighted-infected compared with blind-uninfected and blind-infected crickets over the 3 experimental periods (see also Supplementary Table 2). Sighted crickets (infected or not) and blind crickets (infected or not) covered similar distances in the cone during the 2 control periods (Friedman's ANOVA between the 2 control periods, χ2 = 1, df = 1, P = 0.317). Moreover, blind-infected crickets did not show any phototactic behavior and behaved significantly differently to sighted infected insects (Figure 5B and Supplementary Table 1). Finally, blind-infected and blind-uninfected crickets did not show any increase in walking activity when the light was on (Figure 5C and Supplementary Table 1). These results demonstrate that light was the stimulus that made crickets (infected or not) walk more.
Figure 5.
(A) Proportions of the distance covered in the cone (%) for infected (blind and sighted) and uninfected (blind and sighted) crickets during the 3 experimental periods (i.e., control period, test period, and end-control period). (B) Phototactic index (median, quartiles, and range) for infected (blind and sighted) and uninfected (blind and sighted) crickets. Asterisks indicate means that are significantly different from zero (Wilcoxon signed-rank test, P > 0.05). Different letters indicate significant pairwise differences across the different cricket categories (Dunn's post hoc tests). (C) Displacement index (mean ± standard error) in relation to parasitism status. Asterisks indicate means that are significantly different from zero (paired t-test, P < 0.05). Different letters indicate significant pairwise differences across the different cricket categories (Tukey–Kramer post hoc tests).
Thirst and phototactism
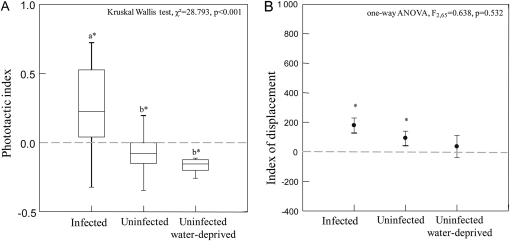
Water-deprived uninfected crickets did not orientate to the light stimulus showing the same behavior as uninfected controls during the light stimulation period (Figure 6A and Supplementary Table 3). Moreover, the displacement index was not significantly different for infected, uninfected, and uninfected water-deprived individuals (Figure 6B and Supplementary Table 1).
Figure 6.
(A) Phototactic index (median, quartiles, and range) for infected, uninfected, and uninfected water-deprived crickets. A positive index reflects a positive phototaxis. Asterisks indicate means that are significantly different from zero (Wilcoxon signed-rank test, P > 0.05). Different letters indicate significant pairwise differences across the different cricket categories (Dunn's post hoc tests). (B) Displacement index (mean ± standard error) in relation to parasitism status. Asterisks indicate means that are significantly different from zero (paired t-test, P < 0.05). Different letters indicate significant pairwise differences across the different cricket categories (Tukey–Kramer post hoc tests).
DISCUSSION
Our results show that mature hairworms induce positive phototaxis in their insect host (i.e., infected insects orientate to light), a behavior not observed in uninfected crickets. Additionally, infected crickets depicted less tortuous trajectories when stimulated with light, a behavior that renders the approach to light more efficient. A similar increase in path straightness has been reported recently on Leptinotarsa decemlineata when stimulated with light (Otálora-Luna and Dickens 2010). It is intriguing that even though photostimulation induced both infected and uninfected crickets to walk faster and for longer distances, such responses were not observed on ex-infected crickets. As blind-infected crickets were not attracted to light, it was confirmed that vision was the only sensory modality involved in the phototaxis we observed on the servosphere. Moreover, our data suggest that the parasite-induced change in host phototaxis is reversible, that is, once the nematomorph parasite is released, crickets are no longer attracted to light. This recovery is progressive and a 20-h period after parasite release is enough to “erase” the light attraction characterizing manipulated hosts. Our results also indicate that the nematomorph induced crickets to walk longer and faster, for even 35 h after having released their parasite we observed a tendency of ex-infected crickets to do so. Therefore, ex-infected crickets did not totally recover the behavioral phenotype characteristic of healthy individuals up to 35 h after parasite emergence. Additionally, water deprivation did not modify the behavior of uninfected individuals toward light as these did not show any positive phototaxis in our study. This might reveal that normal crickets do not look for water surfaces using a light cue even after water deprivation, a finding that counters the widespread notion that hairworms cause host thirstiness to induce them to approach water (see Thomas et al. 2003). However, should 2 days of water deprivation not be sufficient to induce water stress in these crickets, then our results do not exclude a potential effect of thirst if the deprivation period was too short.
Complexity of parasitic manipulation
Inducing a terrestrial insect to orientate toward the water, immerse itself, and stay in the water until parasite emergence is complete represents a major challenge for the hairworm, especially because both host and parasite face increased odds of being preyed on during the sequence of behavior leading to the release of the parasite. Nematomorphs are able to meet this challenge successfully by altering 2 phenotypic traits that occur in sequential steps (i.e., an erratic behavior and a suicidal behavior when confronted with water, e.g., Thomas et al. 2002; Ponton et al. 2006; Sánchez et al. 2008). Crickets tested here did not show any particular signs of erratic behavior. Indeed, they showed a strong attraction to light and walked comparatively straight to this stimulus. In the present study, nematomorph-manipulated crickets showed an alteration of 2 different behaviors: phototaxis (i.e., a straight walk to light) and walking activity (i.e., an increase in distance covered and speed). Numerous phylogenetically unrelated manipulative parasites have been shown to alter these 2 host traits (i.e., phototactic behavior, e.g., Bethel and Holmes 1973; Helluy 1981; Bakker et al. 1997 and activity, e.g., Helluy 1983; Edwards 1987; Hechtel et al. 1993; Mouritsen and Jensen 1997) even though known examples mostly concern trophically transmitted parasites. Because of the sampling technique used (see MATERIALS AND METHODS), we cannot reject the hypothesis that infected specimens used could corresponded to photophilic individuals. However, it seems very unlikely here that the photophilic behavior could be the cause rather than the consequence of the infection. Indeed, although the worm needs several months to develop inside the crickets, only mature worms are found in crickets frequenting concrete areas. In addition, the fact that the photophilic behavior is not maintained once the worm has emerged strongly supports the idea that the infection was the cause and not the consequence of the photophilic behavior.
Following our findings here and those of others (Sánchez et al. 2008), we propose the following scenario which is, in our view, the most likely to explain how nematomorphs induce their host to find water. Nematomorphs might modify the walking activity of their host (as shown by increased distances walked and velocity of infected crickets in our experiment) and induce an erratic behavior of the cricket before the hairworm is completely mature. This might last till water has been reached. Then, once mature and ready to enter water, nematomorphs may manipulate light responses of their hosts to cause them to orientate toward ponds and rivers. However, our investigations are undoubtedly not exhaustive and infected crickets are certainly manipulated in a more complex way that induces a wider range of phenotypic modifications than we know of. For example, in this study, only the direction of light was considered, but light can be a source of other information, for example, polarization, color, and shape (see Henze and Labhart 2007). It would be interesting to determine if polarized light, particular wavelengths, or shapes can induce the observed phototactic response or if direction of light alone is responsible for the water-seeking behavior observed for manipulated crickets. A full understanding of the manipulative processes undeniably requires the study of a larger range of behavioral traits in infected hosts than just the most obviously modified one, that is, orientation behavior (Thomas et al. 2005; Thomas, Rigaud, and Brodeur 2010).
Recovery of ex-infected crickets: complex molecular mechanisms involved in parasitic manipulation?
Another noteworthy finding from the present study was that ex-infected crickets progressively lost their positive phototaxis and 20- and 35-h ex-infected crickets were no longer attracted by light. On the other hand, 3-, 20-, and 35-h ex-infected insects did not completely recover the typical “noninfected behavior” as they showed a tendency to remain more active than uninfected crickets (i.e., they walk longer distances and faster). They thus partially kept some properties of their former infected status such as the enhanced walking activity. The difference in time course of the recovery for the 2 types of activity may reflect differences in the physiology of the pathways involved in the modification of host behavior. In addition, it is possible that locomotor patterns revert to normal more slowly than photic responses and that tests performed more than 35 h after the release of the hairworm would find the motor activity of ex-infected crickets to have returned to normal as well.
On the other hand, one might expect that the parasite-induced behavioral alterations observed in infected crickets are not only due to molecular processes restricted to the alteration of certain behaviors but also to other mechanisms, direct or indirect, as consequences of internal physical damage. For instance, in the central nervous system of manipulated N. sylvestris differential expression of proteins (from the BIR; 2 family) involved in the inhibition of apoptosis was found (see Biron et al. 2006). This suggests perturbation of a cellular process, which could lead to a modified neural circuitry (see also Thomas et al. 2003). This phenomenon is certainly long term, and ex-infected individuals would be liable to keep some symptoms of the infection resulting in a modified behavior even after the parasite's emergence. A cocktail of molecular mechanisms (direct and indirect) is thus certainly used by parasites to interfere with the normal functioning of the host's central nervous system (Lefèvre et al. 2009; Libersat et al. 2009), which could lead to irreversible effects on host behavior. Given that crickets can live up to 130 days after the emergence of their parasite (Biron, Ponton, et al. 2005), further studies would be necessary to precisely determine how the different postinfection alterations are maintained.
To conclude, this work shows for the first time how the orientation behavior of crickets is manipulated by nematomorphs. Nematomorph infection fundamentally modifies cricket orientation behavior, a condition from which they can apparently nearly recover once the parasite is released, nevertheless retaining some characteristics of manipulated insects. This study, together with previous ones, supports the idea that the manipulation exerted by hairworms on their insect hosts is both subtle and multidimensional. This study also confirms the idea that proteomics approaches are useful in the study of host manipulation by parasites (see Biron, Marche, et al. 2005; Biron, Ponton, et al. 2005; Lefèvre et al. 2009) because the present work was initiated following the discovery of differential expression of protein linked to the visual system in manipulated crickets (Biron et al. 2006).
SUPPLEMENTARY MATERIAL
Supplementary material can be found at http://www.beheco.oxfordjournals.org/.
FUNDING
French Ministry of Research during the experiments and the Human Frontier Science Program during the writing of the manuscript to F.P.; French Ministry of Research to T.L.; and Agence Nationale de la Recherche, projet blanc to F.T. (FI.071215.01.01); "Marcel Roche" Library - Instituto Venezolano de Investigaciones Cientificas (part of the Venezuelan Ministry of People's Power for Science, Technology and Medium-Sized Industry) to F.O.L. for the Open Access publication of this article.
Supplementary Material
Acknowledgments
We thank the thermal station of Avènes les Bains for their cooperation during the field study. We wish to acknowledge Ingrid Inciarte from the Laboratory of Sensory Ecology, CEIF, IVIC-Mérida for her valuable comments on the optical methods used here. The experiments were performed at the Institute of Biology, University of Neuchâtel, rue Emile-Argand 11, 2000 Neuchâtel, Switzerland. All experiments comply with the current laws of the country in which they were performed.
References
- Bakker TCM, Mazzi D, Zala S. Parasite-induced changes in behavior and color make Gammarus pulex more prone to fish predation. Ecology. 1997;78:1098–1104. [Google Scholar]
- Batschelet E. Circular statistics in biology. London: Academic Press; 1981. [Google Scholar]
- Begon M, Harper JL, Townsend CR. Ecology—individuals, populations, communities. 2nd ed. Oxford: Blackwell Scientific Publications; 1990. [Google Scholar]
- Bethel WM, Holmes JC. Altered evasive behavior and responses to light in amphipods harboring acanthocephalan cystacanths. J Parasitol. 1973;59:945–956. [PubMed] [Google Scholar]
- Biron DG, Marché L, Ponton F, Loxdale HD, Galéotti N, Renault L, Joly C, Thomas F. Behavioral manipulation in a grasshopper harbouring hairworm: a proteomics approach. Proc R Soc Lond B Biol Sci. 2005;272:2117–2126. doi: 10.1098/rspb.2005.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron DG, Ponton F, Joly C, Menigoz A, Hanelt B, Thomas F. Water-seeking behavior in insects harbouring hairworms: should the host collaborate? Behav Ecol. 2005;16:656–660. [Google Scholar]
- Biron DG, Ponton F, Marché L, Galéotti N, Renault L, Demey-Thomas E, Poncet J, Brown SP, Jouin P, Thomas F. ‘Suicide’ of crickets harbouring hairworms: a proteomics investigation. Insect Mol Biol. 2006;15:731–742. doi: 10.1111/j.1365-2583.2006.00671.x. [DOI] [PubMed] [Google Scholar]
- Blunk H. Die Lebensgeschichte der im Gelbrand schmarotzenden Saitenwürmer I. Zool Anz. 1922;54:110–132. [Google Scholar]
- Eberhard WG. Recovery of spiders from the effects of parasitic wasps: implications for fine-tuned mechanisms of manipulation. Anim Behav. 2010;79(2):375–383. [Google Scholar]
- Edwards JC. Parasite-induced changes in host behavior [PhD thesis] Nottingham (UK): University of Nottingham; 1987. [Google Scholar]
- Hechtel LJ, Johnson CL, Juliano SA. Modification of antipredator behavior of Caecidotea intermedius by its parasite Acanthocephalus dirus. Ecology. 1993;74:710–713. [Google Scholar]
- Helluy S. Parasitisme et comportement. Etude de la metacercaire de Microphallus papillorobustus (Rankin 1940) et de son influence sur les gammares [PhD thesis] Montpellier (France): USTL Montpellier; 1981. [Google Scholar]
- Helluy S. Relations hotes–parasites du trematode Microphallus papillorobustus (Rankin 1940). II. Modifications du comportement des Gammarus hotes intermediaires et localisation des metacercaires. Ann Parasitol Hum Comp. 1983;58:1–17. [PubMed] [Google Scholar]
- Henze MJ, Labhart T. Haze, clouds and limited sky visibility: polarotactic orientation of crickets under difficult stimulus conditions. J Exp Biol. 2007;210(18):3266–3276. doi: 10.1242/jeb.007831. [DOI] [PubMed] [Google Scholar]
- Kitching RL, Zalucki MP. Component analysis of the movement process: analysis of simple tracks. Res Popul Ecol. 1982;24:224–238. [Google Scholar]
- Kramer E. The orientation of walking honeybees in odour fields with small concentration gradients. Physiol Entomol. 1976;1:27–37. [Google Scholar]
- Lafferty KD. The evolution of trophic transmission. Parasitol Today. 1999;15:111–115. doi: 10.1016/s0169-4758(99)01397-6. [DOI] [PubMed] [Google Scholar]
- Lefèvre T, Adamo SA, Biron DG, Missé D, Hughes D, Thomas F. Invasion of the body snatchers: the diversity and evolution of manipulative strategies in host-parasite interactions. Adv Parasitol. 2009;68:45–83. doi: 10.1016/S0065-308X(08)00603-9. [DOI] [PubMed] [Google Scholar]
- Libersat F, Delgado A, Gal R. Manipulation of host behavior by parasitic insects and insect parasites. Annu Rev Entomol. 2009;54:189–207. doi: 10.1146/annurev.ento.54.110807.090556. [DOI] [PubMed] [Google Scholar]
- Moore J. Oxford series in ecology and evolution. London (UK): Oxford University Press; 2002. Parasites and the behavior of animals. [Google Scholar]
- Mouritsen KN, Jensen KT. Parasite transmission between soft-bottom invertebrates: temperature mediated infection rates and mortality in Corophium volutator. Mar Ecol Prog Ser. 1997;151:123–134. [Google Scholar]
- Otálora-Luna F, Dickens JC. Spectral preference and temporal modulation of photic orientation by Colorado potato beetle on a servosphere. Entomol Exp Appl. 2011;138:93–103. [Google Scholar]
- Otálora-Luna F, Perret JL, Guerin PM. Appetence behaviors of the triatomine bug Rhodnius prolixius on a servosphere in response to the host metabolites carbon dioxide and ammonia. J Comp Physiol A. 2004;190:847–854. doi: 10.1007/s00359-004-0540-5. [DOI] [PubMed] [Google Scholar]
- Ponton F, Lebarbenchon C, Lefèvre T, Biron DG, Duneau D, Hughes DP, Thomas F. Parasite survives predation on its host. Nature. 2006;440:756. doi: 10.1038/440756a. [DOI] [PubMed] [Google Scholar]
- Poulin R. Evolutionary ecology of parasites. Princeton (NJ): Princeton University Press; 2007. [Google Scholar]
- Ruxton GD, Beauchamp G. Time for some a priori thinking about post hoc testing. Behav Ecol. 2008;19:690–693. [Google Scholar]
- Sánchez MI, Ponton F, Schmidt-Rhaesa A, Hughes DP, Misse D, Thomas F. Two-steps to suicide in crickets harbouring hairworms. Anim Behav. 2008;76:1621–1624. [Google Scholar]
- Schmidt-Rhaesa A. Two dimensions of biodiversity research exemplified by nematomorpha and gastrotricha. Integr Comp Biol. 2002;42:633–640. doi: 10.1093/icb/42.3.633. [DOI] [PubMed] [Google Scholar]
- Taneja J, Guerin PM. Oriented responses of the triatomine bugs Rhodnius prolixius and Triatoma infestans to vertebrate odours on a servosphere. J Comp Physiol A. 1995;176:455–464. [Google Scholar]
- Thomas F, Moore J, Adamo S. Parasitic manipulation: where are we and where should we go? Behav Process. 2005;68:185–199. doi: 10.1016/j.beproc.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Thomas F, Poulin R, Brodeur J. Host manipulation by parasites: a multidimensional approach. Oikos. 2010;119:1217–1223. [Google Scholar]
- Thomas F, Rigaud T, Brodeur J. Evolution of parasite-induced behavioural alteration. San Diego (CA): The Academic Press; Forthcoming 2010. [Google Scholar]
- Thomas F, Schmidt-Rhaesa A, Martin G, Manu C, Durand P, Renaud F. Do hairworms (Nematomorpha) manipulate the water seeking behavior of their terrestrial hosts? J Evol Biol. 2002;15:356–361. [Google Scholar]
- Thomas F, Ulitsky P, Augier R, Dusticier N, Samuel D, Strambi C, Biron DG, Cayre M. Biochemical and histological changes in the brain of the crickets Nemobius sylvestris infected by the manipulative parasite Paragordius tricuspidatus (Nematomorpha) Int J Parasitol. 2003;33:435–443. doi: 10.1016/s0020-7519(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Wellnitz T. Parasite-host conflicts: winners and losers or negotiated settlements? Behav Process. 2005;68:245–246. doi: 10.1016/j.beproc.2004.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.