Abstract
This study investigates variation in collective behavior in a natural population of colonies of the harvester ant, Pogonomyrmex barbatus. Harvester ant colonies regulate foraging activity to adjust to current food availability; the rate at which inactive foragers leave the nest on the next trip depends on the rate at which successful foragers return with food. This study investigates differences among colonies in foraging activity and how these differences are associated with variation among colonies in the regulation of foraging. Colonies differ in the baseline rate at which patrollers leave the nest, without stimulation from returning ants. This baseline rate predicts a colony's foraging activity, suggesting there is a colony-specific activity level that influences how quickly any ant leaves the nest. When a colony's foraging activity is high, the colony is more likely to regulate foraging. Moreover, colonies differ in the propensity to adjust the rate of outgoing foragers to the rate of forager return. Naturally occurring variation in the regulation of foraging may lead to variation in colony survival and reproductive success.
Keywords: behavioral reaction norm, behavioral syndrome, individual variation
A first step in understanding the evolution of behavior is to investigate variation among individuals in their responses to changing conditions (Sih et al. 2004; Dingemanse et al. 2009). Many social animals engage in collective decision making (Conradt et al. 2009). In social insect colonies, individual workers, using local information, perform and regulate colony tasks collectively (e.g., Pratt and Sumpter 2006; Gordon 2010). Because social insect colonies produce new colonies, the colony can be considered to be a reproductive individual. Ant colonies vary in the distribution of worker sizes (e.g., Beshers and Traniello 1994; Powell 2008), and differences among colonies in genetic diversity or number of patrilines (e.g., Sundstrom 1993; Ross and Keller 1995; Crozier and Pamilo 1996; DeHeer and Herbers 2004) are associated with differences in behavior (Snyder 1993; Wiernasz et al. 2008). However, little is known about differences among colonies in collective behavior, such as foraging.
How ant colonies regulate foraging varies among species, depending on the kind of food a species collects. When food sources are large or clumped, so that many ants are needed to retrieve the food, the problem for the colony is to direct the appropriate number of ants to the location with the richest food supply. For example, elegant laboratory experiments and models show how trail-laying species such as Lasius niger or Tetramorium caespitum solve this problem (e.g., Beckers et al. 1990, 1993; Collignon and Detrain 2010). The number of ants laying trail pheromone is related to the quality of food encountered; this sets the probability that inactive foragers will encounter pheromone and be recruited to the food (Detrain and Deneubourg 2008). Leaf-cutter ants, which forage for leaves to feed the fungus that they consume, regulate foraging using the quality of leaves found (Saverschek et al. 2010) and the interactions on the trail (Burd 2000; Farji-Brener et al. 2010).
By contrast, ant species that forage for scattered food sources, each of which can be retrieved by a single ant, face a different problem. The colony obtains more food not by directing more ants to the best food sources but by covering more ground with ants searching individually. For example, the red harvester ant (Pogonomyrmex barbatus), common in arid chaparral habitats in the southwestern US and Mexico, forages for seeds widely scattered by wind and flooding (Gordon 1993) and does not lay pheromone trails to particular food sources. Colonies store food inside the nest for many months (Gordon 1992, 1993). The colony must balance the costs of desiccation while foraging and the need to claim foraging area in competition with neighboring colonies (Adler and Gordon 2003). Ants foraging in hot dry conditions lose water, but obtain water from metabolizing fats in the seeds that they eat (Lighton and Bartholomew 1988; Lighton and Feener 1989). Thus, ants must spend water to get water. But if a colony's foraging area is not occupied by its foragers, a neighboring colony is likely to use it (Gordon 1992; Gordon and Kulig 1996), and intraspecific competition is more intense in dry conditions (Sanders and Gordon 2004).
Here we examine, in a field study, variation among harvester ant colonies in the minute-to-minute regulation of foraging behavior in response to current food availability. An inactive forager is stimulated to leave the nest on its next trip by the return of foragers with food (Schafer et al. 2006; Gordon et al. 2008). Each forager travels quickly for up to 20 m from the nest in a stream of foragers, then searches individually, and returns directly to the nest as soon as it finds food (Gordon and Kulig 1996). The duration of a foraging trip depends on search time, not on the distance traveled (Beverly et al. 2009). The more food is available, the less time is needed to search and the more quickly a forager returns with food. Thus, the overall rate of return of successful foragers reflects the availability of food on that day. This system produces recruitment without any specified location. Each forager returns to the same site on successive trips (Beverly et al. 2009), and the return of foragers with food from one direction can stimulate other foragers to search in other directions.
Colonies of P. barbatus show consistent differences in how much they forage. No colony forages every day, but some colonies tend to forage on more days than others, and these differences persist from year to year (Gordon 1991). In several species in this genus, there is variation among colonies in the times that foraging activity begins and ends (Gordon 1984; Cole et al. 2010); in P. occidentalis, the timing of foraging activity is associated with genetic diversity (Wiernasz et al. 2008).
Differences among colonies in foraging activity may arise in part from differences in how soon a forager leaves the nest on its next trip once foraging is underway. Two processes may influence how quickly a forager leaves the nest on its next trip. First, we hypothesize that there is a baseline rate at which a forager leaves the nest, independently of encounters with returning foragers. Second, as previous work shows, when a forager leaves the nest depends on its recent rate of encounter with incoming ants (Greene and Gordon 2003; Schafer et al. 2006).
Differences among colonies in the baseline rate at which ants leave the nest, and in the response to returning foragers, could lead to variation among colonies in how closely the colony adjusts to changing food availability. The more quickly each forager in a colony is likely to leave the nest on its next trip, independently of the rate at which others return, the higher the colony's foraging activity. In turn, a colony's level of foraging activity could influence how closely it adjusts the numbers foraging from minute to minute. The more ants are returning to the nest, and the more that inactive foragers respond to the return of foragers with food by going out to forage, the more the rate of outgoing foragers will reflect changes in the rate of forager return.
Here, we consider how colonies differ in foraging activity and in the propensity to regulate foraging. We ask:
Do colonies differ in the baseline probability that an ant will leave the nest? We measured the rate at which patrollers emerge in the absence of any stimulation from returning ants. Patrollers are a group of ants (about 50 in a mature colony), distinct from the foragers (Gordon 1989), that are the first to emerge in the morning (Gordon 1991). They leave the nest, walk around the foraging area, and then return to the nest. Encounters with returning patrollers stimulate the foragers to leave the nest on the first trip of the day (Greene and Gordon 2003, 2007). Such encounters do not provide information about food supply; glass beads with the odor of patrollers are sufficient to stimulate foraging. Here, we captured returning patrollers and measured the rate at which more patrollers continued to leave the nest.
Is variation among colonies in the baseline rate at which patrollers emerge associated with variation in foraging activity? We consider whether there may be a colony-specific baseline rate at which both patrollers and foragers leave the nest, independently of the return of other ants. To do this, we examined the association between the rate at which patrollers leave the nest early in the morning and the rate at which foragers leave the nest later in the day.
Do colonies differ in their propensity to regulate foraging? We examined variation among colonies in whether they adjust the rate of outgoing foragers in response to an experimentally induced change in the forager return rate.
Does the current rate of foraging influence the response to a change in forager return rate? We asked if the rate of foraging before an experimentally induced change in forager return rate predicts whether the colony responds by adjusting the rate of outgoing foragers.
METHODS
The study was performed in August 2008 at a site near Rodeo, NM, USA, with a population of about 300 P. barbatus colonies that have been the subject of a long-term behavioral and demographic study. An annual census of the population (Gordon and Kulig 1996) makes it possible to identify the ages of all colonies on the site.
Do colonies differ in the baseline probability that an ant will leave the nest?
We measured the rate at which ants emerge in the absence of any stimulation from returning ants. We could not measure this with foragers because if all foragers are experimentally prevented from returning to the nest for more than about 20 min, foragers stop leaving the nest altogether (Gordon 2002). Instead, we measured the baseline probability that patrollers leave the nest.
When patrollers are prevented from returning to the nest, more continue to leave over the course of 20–60 min. Patrollers almost always emerge in groups or bursts of 2–6 ants. This suggests that patrollers stimulate each other to leave the nest, but we do not know how. We calculated the interval elapsed between the emergence of groups of patrollers and the number of patrollers in each group that left the nest.
Five trials, each on a different day, were performed for each of 14 mature colonies, ages 5 or older, from 8 August to 18 August 2008. Each trial began between 05:45 and 06:15 AM when patrollers were just beginning to emerge. For 1 h, the observer collected all patrollers that emerged from the nest and prevented them from returning by placing them in a plastic box. The highest mean rate at which a new burst of patrollers left the nest, when all were prevented from returning, was about 1 burst per 5 min. At 1- to 3-min intervals, the observer recorded whether a new burst of patrollers had emerged and the number of patrollers that had left the nest since the last count. At the end of the trial, the patrollers that had been collected were released, and those that were still out returned to the nest undisturbed. Once patrollers were allowed to return to the nest, foraging began as usual, and there was no apparent effect of the experiment on subsequent foraging activity.
We used Kruskal–Wallis nonparametric analysies of variances (ANOVAs) to test whether the number of patrollers in a group leaving the nest or the interval between the emergence of successive groups of patrollers differed among colonies or days.
Is variation among colonies in the baseline rate at which patrollers emerge associated with variation in foraging activity?
We tested whether a colony's baseline rate at which groups of patrollers leave the nest, independently of any returning ants, is associated with that colony's average intensity of foraging. Foraging activity was recorded, in the 14 colonies in which patroller departure rate was measured, as the numbers of foragers leaving the nest in 30 s in 1 direction. We measured foraging activity during the peak of foraging, at least 30 min after foraging began. Counts were made of foragers in the direction with the highest rate of foraging if foragers were traveling in more than 1 direction that day. Usually foraging is most active in one direction or equal in all directions (Gordon 1995), so in either case, the rate of foraging in the direction with the highest rate is representative of foraging activity. Counts were made for all 14 colonies for 11 days from 8 to 18 August 2008. Patroller removals were conducted for 5 of the 11 days on all 14 colonies; colonies differed in which 5 days those were. On each of the 11 days, 3 30-s counts of foraging rate were made over the course of about 2 min for each colony, and the average count per day was used in subsequent data analysis. Because foraging intensity changes over time during the foraging period (Gordon 1984), the sequence in which colonies were visited was changed from day to day.
We tested for an association between the baseline rate at which patrollers leave the nest and the rate at which foragers of the same colony leave the nest later on in the day. We used a Kruskal–Wallis nonparametric ANOVA to test for an effect of the number of patrollers per group to leave the nest on the foraging activity of the same colony.
Do colonies differ in their propensity to regulate foraging?
We examined differences among colonies in the foraging response to the removal of returning foragers. We performed 5 trials for each of 24 mature colonies over the course of 11 days from 7 to 19 August 2008, for a total of 120 trials. A colony was tested once on a given day. Fourteen of the 24 colonies were used in the patroller removal experiments described above, but patroller and forager removals were not done on the same day.
We designed the trials to minimize the extent to which the effects of the weather on a given day, and the age or size of the colony, would influence the detection of differences among colonies. The 24 colonies were grouped into 12 pairs. Previous work showed that colony response to the removal of returning foragers depends on the day (Gordon et al. 2008), apparently due to day-to-day variation in humidity. To reduce the effects of day on the results, both members of each pair of colonies were always tested on the same day. At least 5 pairs of colonies were tested on each day of the 11 days, with different pairs on each day.
We attempted to match the 12 pairs of colonies by size. It is not possible to measure colony size directly without destroying the colony. A colony is founded by a single queen and lives about 25 years (Gordon 1991). Excavations of colonies of known age indicate that once a colony reaches the age of 5 years, when it begins to reproduce (Gordon 1995), its size ranges from 10 000 to 12 000 ants (Gordon 1992). It appears that once a colony reaches reproductive age, its size does not change much and it maintains its characteristic foraging behavior for the rest of its life (Gordon 1991, 1992). The 24 colonies were all of reproductive age, ranging from 6 to 21 years old, and the 12 pairs were matched by age; 5 pairs differed in age by 1 year, 2 by 2 years, 4 by 3 years, and 1 by 5 years. The 12 pairs of colonies were also each matched as closely as possible in apparent numbers of foragers, although for all colonies, these numbers varied greatly among days.
In each trial, the colony was observed for 15 min. Returning foragers were removed for 3 min, using the same methods as in Gordon et al. (2008): During minutes 4–7 of the 15-min trial, beginning 240–260 s after the trial began, a third person standing at least 1 m from the nest entrance, outside the edge of the nest mound, removed all foragers returning to the nest with food. Once removals ended, returning foragers were allowed to go back to the nest undisturbed. The foragers we collected were placed in a plastic box and released near the nest entrance after the trial ended.
One observer counted the foragers leaving the nest, and a second observer counted the foragers returning to the nest with food. Observers used Nokia cell phones, programmed to record the time at which a key was pressed. Counts were made by recording the time every fifth ant was observed because the ants sometimes left the nest too fast to be counted individually; the cell phone could not record more often than about 1 item per second. Foraging rate was measured as the number of counts of 5 ants either leaving or returning to the nest during a given interval.
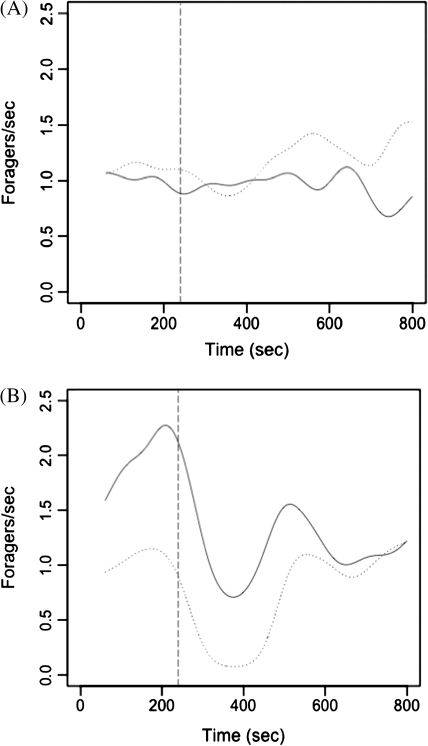
We created an index of response using the ratio of foraging rate after-to-before removals (see Figure 1). We chose the intervals over which we measured the rates for “before” and “after” to encompass the times at which a response clearly occurred in some of the trials reported here, as well as in previous work (Gordon et al. 2008). The rate of outgoing foragers decreased about 2–3 min after removals and lasted for about 5 min after removals ended. We chose 100–350 s as the interval before removals. All removals began by 260 s, but we allowed an additional 90 s for the before period to allow for the time it would have taken for removed ants to get back into the nest. We chose 350–590 s as the interval after removals.
Figure 1.
Response to change in forager return rate. Shown are smoothed results for 2 trials, each for 1 colony on 1 day. Rates of foraging were smoothed using a Gaussian kernel. Dotted line, returning foragers per second; solid line, outgoing foragers per second. Returning foragers were removed for 3 min beginning at the time indicated by the vertical dotted line. Foraging rates before removals were calculated from 100–350 s, including the time it would have taken for removed ants to return to the nest. Foraging rates after removals were calculated from 350–590 s. (A) Example of a trial with no response; foraging did not change in response to the removal of returning foragers. (B) Example of a trial in which the colony responded to the removal of returning foragers by decreasing the rate of outgoing foragers.
The index of response to removals, comparing foraging after to foraging before removals, was calculated as the base-2 log of the ratio of the rate of returning foragers after removals (350–590 s) to the rate of returning foragers before removals (100–350 s). The same ratio was calculated for outgoing foragers. Because the log after-to-before ratio was infinite when there were 0 ants foraging before removals, we omitted trials from the analysis in which foraging was very low. We omitted a total of 26 trials from the analysis, including trials with such low foraging activity that the average rate of returning foragers plus the average rate of outgoing foragers was less than 0.8 ants per second, and trials in which there were 0 ants foraging before removals. There remained a total of 94 trials.
We divided the results for all trials into 2 sets, one for trials in which the colony responded to removals with a decrease in the rate of foraging and one for trials in which the colony did not respond. Figure 1 shows an example of one trial in which the colony responded and one trial in which the colony did not respond. We chose to define colony response as a binary variable so as to use the simplest possible measure. We did this because many factors probably determine the response to removals, both the decline in the rate of outgoing foraging and its recovery after removals end, and we do not know how these factors covary. The greater the response to the decline in forager return rate due to removals, the lower the index of response, because the index is a log of the ratio. We classified each trial according to whether foraging decreased more than a threshold value. We first used the median ratio as the threshold, giving 29 trials that showed a response and 65 that did not. We then used the 30th percentile as threshold, chosen arbitrarily as a less stringent criterion, giving 52 trials that showed a response and 42 that did not.
We tested whether response to removals differed among colonies, using analysis of deviance to test for the effect of colony on response to removals (response or no response).
Does the current rate of foraging influence the response to a change in forager return rate?
We examined whether the rate of forager return before removals influences whether a colony responds to removals. For the rate of forager return before removals, we used foraging rate between 60 and 260 s when removals began. We used ANOVA to test whether the rate of returning foragers before removals differed among colonies. We used analysis of deviance to test for an effect of rate of forager return before removals on response to removals (response or no response). We also compared foraging activity before removals, both the rate of outgoing and the rate of incoming foragers between 60 and 260 s, in the 2 sets of trials (response or no response), using t-tests.
RESULTS
Baseline probability of leaving the nest and foraging activity
Colonies differ in the baseline rate at which bursts of patrollers emerge from the nest in the absence of stimulation from returning ants. There were significant effects of colony on the interval between the emergence of successive bursts of patrollers (Kruskal–Wallis, degrees of freedom [df] 13, P < 0.001) and on the number of patrollers that emerged per burst (Kruskal–Wallis, df 13, P < 0.02). The mean (standard deviation [SD]) bursts per minute ranged among colonies from 0.06 (0.006) to 0.24 (0.06). The mean (SD) number of ants per burst ranged among colonies from 1.34 (0.16) to 7.23 (3.83). Colony differences were stronger than differences among days. There was no significant day effect for the interval between the emergence of successive patrollers or the number of patrollers that emerged (Kruskal–Wallis, bursts per minute, df 10, F 1.93, P = 0.21, number of patrollers, df 10, F 0.20, P = 0.66). The mean (SD) bursts per minute ranged among days from 0.12 (0.06) to 0.18 (0.12), and the mean number of ants per burst ranged among days from 2.96 (2.84) to 5.89 (5.84).
Relation of baseline rate of patroller emergence and rate of foraging
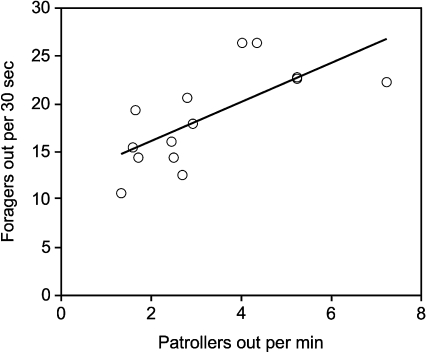
The baseline rate at which a colony’s patrollers leave the nest early in the morning predicts that colony’s average level of foraging activity (Figure 2). Small differences in the rate at which patrollers emerge early in the morning, ranging from about 2 to 7 ants per minute, were associated with large differences in the mean rate at which foragers leave the nest during the peak of foraging hours later, ranging from about 20 to 50 ants per minute. There was a significant effect of the mean number of patrollers per group that left the nest on the mean foraging activity of the same colony (Kruskal–Wallis, df 12, F 11.88, P < 0.005). There was no significant effect of the colony-specific interval between bursts of patrollers on foraging activity (Kruskal–Wallis, df 12, F 3.23, P = 0.09) and no significant interaction of the mean interval between successive groups of patrollers leaving the nest and the number of patrollers in each group (Kruskal–Wallis, df 12, F 0.03, P = 0.86).
Figure 2.
Foraging activity and patroller burst size, by colony. Each point shows the average number of patrollers that left the nest per min and the average numbers of outgoing foragers per 30 s for the same colony.
Colony differences in response to removals
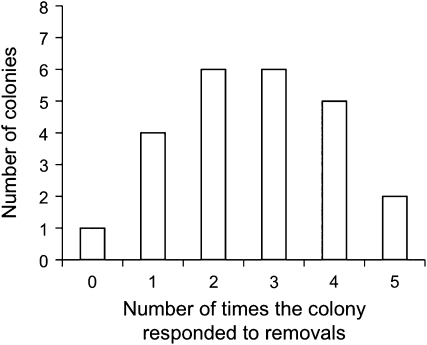
Colonies differ in their propensity to adjust the rate of outgoing foragers to changes in forager return rate (Figures 3 and 4). When trials were grouped by colony, colonies differed in whether they responded to removals (analysis of deviance, 23 df, deviance 37.2, chi square 93, P < 0.03).
Figure 3.
Frequency of colony response to decrease in forager return. Each bar shows the number of colonies that responded to a decrease in foraging return rate the indicated number of times, out of 5 trials, by changing the rate of outgoing foragers.
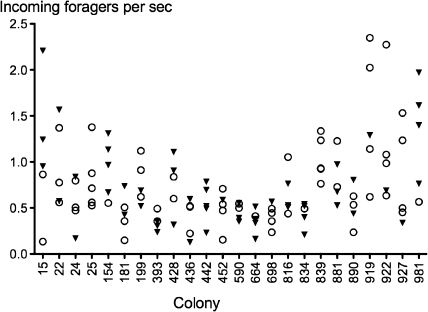
Figure 4.
Foraging activity when undisturbed and response to decrease in forager return rate. Each point shows the mean foraging activity for 3 min before forager return rate was experimentally decreased. Open circles, trials in which the colony responded by decreasing foraging. Filled triangles, trials with no response.
Effect of overall foraging activity on response to change in forager return rate
In general, when foraging activity is high, colonies were more likely to respond to a decrease in forager return rate by adjusting the rate of outgoing foragers. However, colonies varied greatly in the magnitude of foraging activity required to respond to removals (Figure 4). Colonies differed in foraging rate before removals (ANOVA, 23 df, F 2.86, P < 0.0004). Whether colonies responded to removals depended on foraging rate before removals (analysis of deviance, 1 df, deviance 6.9, chi square 122.3, P < 0.008). Using the median ratio of foraging rate after-to-before removals as the threshold for whether a colony responded in a given trial, foraging rate before removals was higher in the trials showing a response to removals (Table 1) than in the trials in which there was no response to removals. Using the less stringent criterion for response that a change in foraging rate was stronger than the 30th percentile, the results were similar: Foraging rate before removals was significantly higher in the trials showing a response to removals (Table 1) than in the trials in which there was no response to removals.
Table 1.
Foraging activity and response to decrease in forager return rate
| Variable | Median |
30th percentile |
||||||
| Response | No response | t | P | Response | No response | t | P | |
| Rate of incoming foragers | 1.19 (0.52) | 0.98 (0.58) | 1.79 | 0.001 | 1.18 (0.58) | 0.89 (0.52) | 2.59 | 0.01 |
| Rate of outgoing foragers | 1.04 (0.37) | 0.89 (0.45) | 1.64 | 0.05 | 1.02 (0.39) | 0.85 (0.47) | 1.91 | 0.01 |
Shown are comparisons of foraging rates before removals when the rate of outgoing foragers decreased in response to removals, and when the rate of outgoing foragers did not decrease. Numbers are mean number of foragers per sec (SD). Values are shown for two criteria for foraging response, median and the 30th percentile. For median criterion: responded n = 29, no response n = 65. For 30th percentile criterion, responded n = 42, no response n = 52.
DISCUSSION
Our results show that harvester ant colonies vary in the regulation of collective behavior. Colonies differ in the baseline rate at which ants leave the nest, in foraging activity, and in how closely they adjust foraging to changes in the rate of forager return. This variation among colonies produces reaction norms that may influence the evolution of collective behavior (Dingemanse et al. 2009). The regulation of foraging is ecologically important because it determines how a colony manages the trade-off between the risk of water loss when foraging and the risk of losing foraging area to a neighboring colony when not foraging (Adler and Gordon 2003). However, in some conditions, competition among neighboring colonies is intense (Sanders and Gordon 2004), and it may be worthwhile to scour the desert for any seed at all, despite the cost in desiccation.
We found that harvester ant colonies differ in the way that foraging activity responds to current conditions. First, some colonies are more likely than others to send out foragers. For example, the spread of points on the y axis of Figure 2 shows the variation among 14 colonies in average rates of outgoing foragers. Figure 4 shows differences among 24 colonies in the average rates of returning foragers. It is well known that ants within colonies differ in activity level (e.g., Jaisson et al. 1988); our results here show that colonies differ in activity level. Second, colonies also differ in the propensity to regulate foraging activity in response to a change in forager return rate (Figure 3). This propensity is related to overall foraging level.
One source of colony differences in foraging activity appears to be a colony-specific trait that sets the interval that any ant waits before it goes out, independently of stimulation from returning ants. The association between the rate at which patrollers emerge, even when no ants return, in a given colony, and the average rate at which foragers leave the nest in the same colony, suggests that both patrollers and foragers have in common a baseline rate of activity. It is unlikely that the rate at which patrollers emerge depends on colony size; young colonies and mature ones, ranging in size by a factor of 5 from 2000 to 10 000 ants, have about the same numbers of patrollers, 30–50 (Gordon 1989). Moreover, very small differences in the numbers of patrollers that emerge in each burst predict much larger differences in the numbers of ants foraging per minute (Figure 2). It is also unlikely that the rate at which patrollers emerge directly determines the rate of foraging later on. Patrollers initiate foraging when they return to the nest (Greene and Gordon 2003, 2007), and once foraging begins, the patrollers are no longer active. We do not know whether the number of patrollers active influences how many foragers first leave the nest. In any case, there is no evidence that the day’s foraging activity is determined by the initial number of foragers. Further work is needed to understand what produces colony differences in the activity of patrollers and foragers.
In many ant species, foraging is regulated by a nonlinear process that links numbers of foragers to the quality or quantity of food (Sumpter and Beekman 2003; Detrain and Deneubourg 2008). It seems that harvester ants, using encounter rate rather than pheromone trails, regulate foraging using a process that is also nonlinear. We found that a P. barbatus colony is more likely to respond to a decrease in forager return rate when the initial rate of foraging is high. The following scenario might explain why. When the rate of foraging is low, foragers leave the nest at a rate set by a baseline probability that is independent of the rate of forager return. When food is abundant and the rate of forager return is high, foragers respond to returning foragers. Removing returning foragers artificially brings the rate of forager return down to the low rate at which outgoing foraging depends only on the baseline rate. When we stop preventing the return of foragers, the rate of forager return increases (see Figure 1B) and inactive foragers begin to respond to returning foragers again, which brings the rate of outgoing foraging back up. However, on days when the foraging activity is initially very low, removals have no effect because the rate of outgoing foragers depends only on the baseline probability that is independent of the rate of foraging return.
It could be that foragers are less likely to respond to a change in the rate of forager return when rates are low because a change in a low rate is difficult to assess. The lower the rate of forager return, the fewer incoming ants each inactive forager encounters, and a small sample has a higher variance than a large one. Like the ants, it is difficult for us to detect a change in the rate of foraging when it is low (Figure 1A). The lower the rate of foraging, the higher the variation in the rate. This leads to a smaller apparent decrease in forager return rate, measured as the ratio of foraging rate after to rate before removals.
However, it is clear that the ants can respond to a change in forager return rate even when rates are low. The rate of outgoing foragers changed in response to removals at a wide range of levels of foraging activity, including some trials in which foraging rates were quite low. For example, Figure 4 shows that foraging changed in response to removals at low levels of foraging for colony 452 and foraging did not change in response to removals at high levels of foraging for colony 15. Thus, the association we found between response to removals and high foraging activity cannot be solely due either to the ants' difficulty in detecting a change in return rate or to our difficulty in detecting a relative change in the rate of outgoing foragers, when foraging activity is low.
In general, colonies are more likely to adjust to changes in forager return rate when foraging rate is high. Thus, colony differences in the level of foraging activity lead to variation in the propensity to regulate foraging. But even taking foraging rate into account, there were still differences among colonies in the probability that they responded to removals (Figure 3). It appears that in addition to overall level of foraging activity, some other factors influence how closely a colony regulates foraging. These other factors may explain why, in a previous study with a much smaller sample of colonies (Gordon et al. 2008), we did not find significant differences among colonies in response to removals. Some possibilities include the amount of stored food and the current need to feed larvae (Dussutour and Simpson 2009; Mailleux et al. 2010). Excavations show that colonies store food for many months (Gordon 1993) and differ greatly in the amount of stored food (Gordon 1992). We do not know how the amount of stored food influences the minute-to-minute regulation of foraging, and it is not possible to measure this amount directly in the field without destroying the nest.
We do not know what causes the differences among colonies in foraging behavior that we report here. Colony behavior depends on colony size in many ant species (e.g., Tschinkel 1993; Bourke 1999; Thomas and Elgar 2003; Gordon 2010). In P. barbatus, task allocation (Gordon 1987) and relations with neighbors (Gordon 1992; Gordon and Kulig 1996) change as a colony grows older and larger. However, although colony size influences colony behavior, it does not fully determine the numbers actively foraging at any moment. In P. barbatus, as in many social insect species, the moment-to-moment rate of foraging depends on factors other than colony size, such as the rate of food intake (e.g., Fernandez et al. 2003 for honey bees; O'Donnell 2001 for wasps). At most about 20% of the workers in a mature colony engage in foraging at any time (Adler and Gordon 2003), but on the year-to-year scale, the number that forage is not a simple linear function of colony size, as the numbers foraging change by a factor of 2 from ages 2 to 5 years (Gordon and Kulig 1996; Adler and Gordon 2003), while overall colony size changes by a factor of 5 during those years (Gordon 1992). On the day-to-day scale, ants from all other exterior task groups switch tasks to foraging when more foragers are needed (Gordon 1989), and foragers can be active on 1 day and revert to inactivity the next day (Gordon 1991).
Colony differences in response to changing conditions provide the variation that underlies the evolution of collective behavior. Further work is needed to determine if the differences shown here account for the long-term year-to-year differences among colonies in foraging behavior observed previously (Gordon 1991). Then, to investigate the evolution of the regulation of foraging, the next question is how variation among colonies in the regulation of foraging affects colony growth, survival, and reproductive success.
FUNDING
National Science Foundation (IBN 0718631) to D.M.G.; National Institute of Health (R01GM086884) to S.H.
Supplementary Material
Acknowledgments
We are very grateful to Daniel Fisher for helpful discussions and ideas and to LeAnn Howard, Mattias Lanas, Julie Miller, Ruth Percino, Shelby Sturgis, and Doan Lam Tran for their invaluable assistance in the field. Many thanks to Bjoern Hartmann for programming the cell phones to be data collection devices. Noa Pinter-Wollmann, Shelby Sturgis, and Katherine Fitzgerald provided valuable comments on the manuscript.
References
- Adler FR, Gordon DM. Optimization, conflict, and nonoverlapping foraging ranges in ants. Am Nat. 2003;162:529–543. doi: 10.1086/378856. [DOI] [PubMed] [Google Scholar]
- Beckers R, Deneubourg JL, Goss S. Modulation of trail laying in the ant Lasius niger (Hymenoptera:Formicidae) and its role in the collective selection of a food source. J Insect Behav. 1993;6:751–759. [Google Scholar]
- Beckers R, Deneubourg JL, Goss S, Pasteels JM. Collective decision making through food recruitment. Insectes Soc. 1990;37:258–267. [Google Scholar]
- Beshers SN, Traniello JFA. The adaptiveness of worker demography in the attine ant Trachymyrmex septentrionalis. Ecology. 1994;75:763–775. [Google Scholar]
- Beverly B, McLendon H, Nacu S, Holmes S, Gordon DM. How site fidelity leads to individual differences in the foraging activity of harvester ants. Behav Ecol. 2009;20:633–638. [Google Scholar]
- Bourke AFG. Colony size, social complexity and reproductive conflict in social insects. J Evol Biol. 1999;12:245–257. [Google Scholar]
- Burd M. Foraging behaviour of Atta cephalotes (leaf-cutting ants): an examination of two predictions for load selection. Anim Behav. 2000;60:781–788. doi: 10.1006/anbe.2000.1537. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Smit AA, Huber ZJ, Wiernasz D. The structure of foraging activity in colonies of the harvester ant Pogonomyrmex occidentalis. Behav Ecol. 2010;21:337–342. [Google Scholar]
- Collignon B, Detrain C. Distributed leadership and adaptive decision-making in the ant Tetramorium caespitum. Proc R Soc B Biol Sci. 2010;277:1267–1273. doi: 10.1098/rspb.2009.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt L, Krause J, Couzin ID, Roper TJ. “Leading according to need” in self-organizing groups. Am Nat. 2009;173:304–312. doi: 10.1086/596532. [DOI] [PubMed] [Google Scholar]
- Crozier RH, Pamilo P. Evolution of social insect colonies. Oxford: Oxford University Press; 1996. [Google Scholar]
- DeHeer CJ, Herbers JM. Population genetics of the socially polymorphic ant Formica podzolica. Insectes Soc. 2004;51:309–316. [Google Scholar]
- Detrain C, Deneubourg JL. Collective decision-making and foraging patterns in ants and honeybees. In: Simpson SJ, editor. Advances in insect physiology, vol. 3. New York: Academic Press; 2008. pp. 123–173. [Google Scholar]
- Dingemanse NJ, Kazem AJN, Reale D, Wright J. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol Evol. 2009;25:81–89. doi: 10.1016/j.tree.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Dussutour A, Simpson SJ. Communal nutrition in ants. Curr Biol. 2009;19:740–744. doi: 10.1016/j.cub.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Farji-Brener AG, Amador-Vargas S, Chinchilla F, Escobar S, Cabrera S, Herrera MI, Sandoval C. Information transfer in head-on encounters between leaf-cutting ant workers: food, trail condition or orientation cues? Anim Behav. 2010;79:343–349. [Google Scholar]
- Fernandez PC, Gil M, Farina WM. Reward rate and forager activation in honeybees: recruiting mechanisms and temporal distribution of arrivals. Behav Ecol Sociobiol. 2003;54:80–87. [Google Scholar]
- Gordon DM. Specific patterns in the social activities of harvester ant colonies. Insectes Soc. 1984;31:74–86. [Google Scholar]
- Gordon DM. Group-level dynamics in harvester ants: young colonies and the role of patrolling. Anim Behav. 1987;35:833–843. [Google Scholar]
- Gordon DM. Dynamics of task switching in harvester ants. Anim Behav. 1989;38:194–204. [Google Scholar]
- Gordon DM. Behavioral flexibility and the foraging ecology of seed-eating ants. Am Nat. 1991;138:379–411. [Google Scholar]
- Gordon DM. How colony growth affects forager intrusion in neighboring harvester ant colonies. Behav Ecol Sociobiol. 1992;31:417–427. [Google Scholar]
- Gordon DM. The spatial scale of seed collection by harvester ants. Oecologia. 1993;95:479–487. doi: 10.1007/BF00317431. [DOI] [PubMed] [Google Scholar]
- Gordon DM. The development of an ant colony's foraging range. Anim Behav. 1995;49:649–659. [Google Scholar]
- Gordon DM. The regulation of foraging activity in red harvester ant colonies. Am Nat. 2002;159:509–518. doi: 10.1086/339461. [DOI] [PubMed] [Google Scholar]
- Gordon DM. Ant encounters: interaction networks and colony behavior. Primers in complex systems. Princeton (NJ): Princeton University Press; 2010. [Google Scholar]
- Gordon DM, Holldobler B. Worker longevity in harvester ants. Psyche. 1987;94:341–346. [Google Scholar]
- Gordon DM, Holmes S, Nacu S. The short-term regulation of foraging in harvester ants. Behav Ecol. 2008;19:217–222. doi: 10.1093/beheco/arq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DM, Kulig A. Founding, foraging and fighting: colony size and the spatial distribution of harvester ant nests. Ecology. 1996;77:2393–2409. [Google Scholar]
- Greene MJ, Gordon DM. Cuticular hydrocarbons inform task decisions. Nature. 2003;423:32. doi: 10.1038/423032a. [DOI] [PubMed] [Google Scholar]
- Greene MJ, Gordon DM. Interaction rate informs harvester ant task decisions. Behav Ecol. 2007;18:451–455. [Google Scholar]
- Jaisson P, Fresneau D, Lachaud JP. Individual traits of social behavior in ants. In: Jeanne RL, editor. Interindividual behavioral variability in social insects. Boulder (CO): Westview Press; 1988. pp. 1–51. [Google Scholar]
- Lighton JRB, Bartholomew GA. Standard energy metabolism of a desert harvester ant Pogonomyrmex rugosus: effects of temperature, body mass, group size and humidity. Proc Natl Acad Sci U S A. 1988;85:4765–4769. doi: 10.1073/pnas.85.13.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighton JRB, Feener DH., Jr Water-loss rate and cuticular permeability in foragers of the desert ant Pogonomyrmex rugosus. Physiol Zool. 1989;62(6):1232–1256. [Google Scholar]
- Mailleux A-C, Buffin A, Detrain C, Deneubourg J-L. Recruiter or recruit: who boosts the recruitment in starved nests in mass foraging ants? Anim Behav. 2010;79:31–35. [Google Scholar]
- O'Donnell S. Worker biting interactions and task performance in a swarm-founding eusocial wasp (Polybia occidentalis, Hymenoptera: Vespidae) Behav Ecol. 2001;12:353–359. [Google Scholar]
- Powell S. Ecological specialization and the evolution of a specialized caste in Cephalotes ants. Funct Ecol. 2008;22:902–911. [Google Scholar]
- Pratt SC, Sumpter DJT. A tunable algorithm for collective decision-making. Proc Natl Acad Sci U S A. 2006;103(43):15906–15910. doi: 10.1073/pnas.0604801103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KG, Keller L. Ecology and evolution of social organization: insights from fire ants and other highly eusocial insects. Annu Rev Ecol Syst. 1995;26:631–656. [Google Scholar]
- Sanders NJ, Gordon DM. The interactive effects of climate and interspecific neighbours on mortality of red harvester ants. Ecol Entomol. 2004;29:632–637. [Google Scholar]
- Saverschek N, Herz H, Wagner M, Roces F. Avoiding plants unsuitable for the symbiotic fungus: learning and long-term memory in leaf-cutting ants. Anim Behav. 2010;79:689–698. [Google Scholar]
- Schafer RJ, Holmes S, Gordon DM. Forager activation and food availability in harvester ants. Anim Behav. 2006;71:815–822. doi: 10.1016/j.anbehav.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A, Bell AM, Johnson JC. Behavioral syndromes: an integrative overview. Q Rev Biol. 2004;79:241–277. doi: 10.1086/422893. [DOI] [PubMed] [Google Scholar]
- Snyder LE. Nonrandom behavioral interactions among genetic subgroups in a polygynous ant. Anim Behav. 1993;46:431–439. [Google Scholar]
- Sumpter D, Beekman M. From nonlinearity to optimality: pheromone trail foraging by ants. Anim Behav. 2003;66:273–280. [Google Scholar]
- Sundstrom L. Genetic population structure and sociogenetic organization in Formica truncorum (Hymenoptera, Formicidae) Behav Ecol Sociobiol. 1993;33:345–354. [Google Scholar]
- Thomas M, Elgar MA. Colony size affects division of labour in the ponerine ant Rhytidoponera metallica. Naturwissenschaften. 2003;90:88–92. doi: 10.1007/s00114-002-0396-x. [DOI] [PubMed] [Google Scholar]
- Tschinkel W. Sociometry and sociogenesis of colonies of the fire ant Solenopsis invicta during one annual cycle. Ecol Monogr. 1993;63:425–457. [Google Scholar]
- Wiernasz DC, Hines J, Parker D, Cole BJ. Mating for variety increases colony activity in the harvester ant, Pogonomyrmex occidentalis. Mol Ecol. 2008;17:1137–1144. doi: 10.1111/j.1365-294X.2007.03646.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.