Abstract
To study the role of metallothioneins (MTs) in Zn accumulation, the expression of TcMT2a, TcMT2b, and TcMT3 was analysed in three accessions and 15 F3 families of two inter-accession crosses of the Cd/Zn hyperaccumulator Thlaspi caerulescens, with different degrees of Zn accumulation. The highest expression levels were found in the shoots of a superior metal-accumulating calamine accession from St Laurent le Minier, with >10-fold TcMT3 expression compared with another calamine accession and a non-metallicolous accession. Moreover, F3 sibling lines from the inter-accession crosses that harboured the MT2a or MT3 allele from St Laurent le Minier had higher expression levels. However, there was no co-segregation of TcMT2a or TcMT3 expression and Zn accumulation. To examine the functions of TcMTs in plants, TcMT2a and TcMT3 were ectopically expressed in Arabidopsis. The transformant lines had reduced root length in control medium but not at high metal concentrations, suggesting that the ectopically expressed proteins interfered with the physiological availability of essential metals under limited supply. The Arabidopsis transformant lines did not show increased tolerance to Cd, Cu, or Zn, nor increased Cd or Zn accumulation. Immunohistochemical analysis indicated that in roots, MT2 protein is localized in the epidermis and root hairs of both T. caerulescens and Arabidopsis thaliana. The results suggest that TcMT2a, TcMT2b, and TcMT3 are not primarily involved in Zn accumulation as such. However, the elevated expression levels in the metallicolous accessions suggests that they do contribute to the metal-adapted phenotype, possibly through improving Cu homeostasis at high Zn and Cd body burdens. Alternatively, they might function as hypostatic enhancers of Zn or Cd tolerance.
Keywords: Cd, crosses, metallothionein, protein, quantitative real-time PCR, Thlaspi caerulescens, Zn
Introduction
The molecular basis of metal hyperaccumulation in plants has gained increasing interest during the past few years. One of the best studied metal hyperaccumulator species is Thlaspi caerulescens, having accessions that differ in metal tolerance, root to shoot transport, and uptake. Metal accumulation in T. caerulescens is suggested to be a consequence of increased root to shoot transport and internal sequestration to storage organelles (Assunção et al., 2003b). Besides metal transporters, metallothioneins (MTs) are often strongly expressed in hyperaccumulators (Roosens et al., 2004; van de Mortel et al., 2006), suggesting that they also play some role in the hyperaccumulation phenotype.
In Arabidopsis thaliana, MTs form a diverse family, with seven translated genes belonging to four types (Cobbett and Goldsbrough, 2002). At least three metallothionein genes, MT1, MT2, and MT3, have been isolated from T. caerulescens (Roosens et al., 2004, 2005; Hassinen et al., 2007). The T. caerulescens MT2 gene isolated from a superior Cd-accumulating calamine accession from St Félix de Pallières (FP), South-France, shows characteristics of both MT2a- and MT2b-type genes (Roosens et al., 2005), whereas the MT2 gene isolated from another calamine accession from La Calamine (LC), Belgium, is a close homologue of AtMT2a (Hassinen et al., 2007). Compared with Arabidopsis, a T. caerulescens MT3, isolated from the FP accession, shows a modified cysteine arrangement in the C-terminal metal-binding domain, which has been suggested to result in altered metal-binding characteristics, including a higher affinity for Cu (Roosens et al., 2004). Moreover, another isoform or allele of MT3 has been isolated from the T. caerulescens accession LC, in which the cysteine arrangement is the same as in Arabidopsis MT3 (Hassinen et al., 2007). These differences in the amino acid sequence may reflect divergent selection resulting from different soil metal compositions at the sites of origin.
Even though plant MTs have been the subject of many studies, their functions are still not fully understood, partly because it is difficult to isolate the intact proteins from plant tissues. Evidence suggests that MTs may play a role in metal buffering or homeostasis by providing a pool of available micronutrients for the cells, or in the protection of cells from oxidative damage caused by free radicals (Mir et al., 2004).
The expression of plant MT genes is primarily inducible by copper, suggesting that they are predominantly involved in Cu homeostasis or Cu tolerance (Zhou and Goldsbrough, 1994; Guo et al., 2003). MT2a expression in roots appeared to be correlated with Cu tolerance among Arabidopsis ecotypes (Murphy and Taiz, 1995). Based on a detailed co-segregation analysis, Van Hoof et al. (2001) suggested that enhanced MT2b expression in roots may be essential, though not sufficient for high-level Cu tolerance in cupricolous Silene vulgaris. However, enhanced MT2b expression was also consistently found in accessions from calamine soils with normal Cu concentrations (Jack et al., 2007). Therefore, MTs may also be involved in the detoxification or homeostasis of metals other than Cu. When the MT1 family was knocked-down in Arabidopsis by an RNA interference (RNAi) construct, the knock-down lines were all hypersensitive to Cd and accumulated several-fold lower levels of As, Cd, and Zn than did the wild type, while Cu and Fe levels were unaffected. The authors concluded that MT1 confers tolerance to Cd and can assist in Zn homeostasis (Zimeri et al., 2005). Arabidopsis MT2a and MT3 have been shown to increase Cd tolerance when expressed in Vicia faba guard cells, also suggesting that the MTs may play a role in Cd detoxification (Lee et al., 2004). Ectopic expression of Brassica juncea BjMT2 in A. thaliana resulted in increased tolerance to both Cu and Cd (Zhigang et al., 2006).
Plant MTs have been shown to increase tolerance to metals in yeast, and are thus functional metal-binding proteins (Zhou and Goldsbrough, 1994; van Hoof et al., 2001; Guo et al., 2008). Thlaspi caerulescens MT2 and MT3 are capable of conferring Cd, Cu, or Zn tolerance (Roosens et al., 2005) or Cd accumulation (Hassinen et al., 2007) in yeast, although to levels not exceeding those of Arabidopsis MT genes, suggesting that the proteins from Thlaspi are not superior in their metal-binding capability compared with those of Arabidopsis. An exception is TcMT3 from the FP accession, which confers increased Cu tolerance compared with Arabidopsis MT3 in yeast (Roosens et al., 2004).
The function of MTs in Zn accumulation has not been addressed previously. Two MT genes have been isolated from T. caerulescens (TcMT2-LC and TcMT3-LC) that showed higher expression in a Zn-adapted, calamine accession (LC) compared with an accession originating from uncontaminated soil [Lellingen (LE)] (Hassinen et al., 2007). In the present study, another MT2-type gene was isolated and the expression of TcMT2a, TcMT2b, and TcMT3 was analysed in T. caerulescens accessions and in lines from inter-accession crosses to test for possible co-segregation of MT expression and Zn accumulation capacity. Metal accumulation was analysed in transgenic Arabidopsis expressing TcMT2a or TcMT3. Moreover, MT2 protein localization in roots was analysed in T. caerulescens as well as in transgenic Arabidopsis using immunohistochemistry.
Materials and methods
Plant material
Three T. caerulescens accessions, differing in metal uptake, translocation from root to shoot, and tolerance, were used. The accession LC originates from soil contaminated with calamine ore waste (Zn, Cd, and Pb) near La Calamine, Belgium, and accession St Laurent le Minier (LM), formerly named ‘Ganges’, from a similar site in France (Zhao et al., 2002). Accession LE is from a non-metalliferous soil near Lellingen, Luxembourg (Meerts and Van Isacker, 1997). The LC×LE cross is described in Assunção et al. (2003b), and the LC×LM ( = GA) cross in Deniau et al. (2006). In this study, three low- and two high-accumulating F3 lines of the LC×LE cross and five low- and five high-accumulating F3 lines of the LC×LM cross were used. When grown at 10 μM Zn in the nutrient solution, the high-accumulating lines accumulated at least 10 times more Zn than the low-accumulating ones (data not given). The plants were grown as described by Assunção et al. (2003a). They were exposed to 0, 10, 100, and 1000 μM ZnSO4 for 1 week, after which the shoots and roots were separately collected, frozen in liquid nitrogen, and stored at –80 °C until use.
Isolation of MT sequences
TcMT2a-LC and TcMT3-LC were previously isolated from a cDNA library prepared from T. caerulescens accession LC (Hassinen et al., 2007). The TcMT2a-LM and TcMT3-LM alleles from the accession LM were amplified in the present study using primers designed from LC cDNA. The TcMT2b was isolated from the accessions LC and LM, using primers designed from a Thlaspi expressed sequence tag (EST; DN923775) homologous to Arabidopsis MT2b. The primer sequences for MT2b were: 5′ ATGTCTTGCTGTGGAGGAAACT 3′ and 5′ TCATTTGCAGGTACAAGGGTT 3′.
Quantitative real-time PCR
Each sample consisted of shoots or roots pooled from three plants. Total RNA was isolated with an RNeasy extraction kit using DNase I on-column digestion (Qiagen). The cDNA was synthesized using an oligo(dT) primer from 1 μg of total RNA with a DyNAmo 2 step SYBR Green qPCR kit (Finnzymes). Quantitative real-time PCRs were done using a Dynamo HS SYBR Green kit (Finnzymes) in a 20 μl reaction volume with 0.5 μM gene-specific primers and 2 μl of diluted cDNA, derived from 2.5 ng of total RNA, as a template. Thlaspi caerulescens tubulin, a homologue to Arabidopsis α2 tubulin (At1g04820), was used as a reference gene. The amplicon lengths were 297 bp for tubulin, 115 bp for MT2a, 194 bp for MT2b, and 121 bp for MT3. The TcMT2a amplicon was from the 3′-untranslated region (UTR), while TcMT2b and TcMT3 primers spanned an intron. All primers had full homology to both LM and LC alleles. The primer sequences were 5′ CCTACGCACCAGTCATCTCT 3′ and 5′ CGAGATCACCTCCTGGAACA 3′ for tubulin; 5′ GCAATAATGGCTGTAGCCTTGT 3′ and 5′ GAAGTACAAACGGGACCATCAA 3′ for TcMT2a; 5′ GTCTTGCTGTGGAGGAAACTGT 3′ and 5′ CATCATTCTCGGCAACGAAGGT 3′ for TcMT2b; and 5′ CCAGCTACACCTTGGACATGGT 3′ and 5′ TTGACGCAGCTGCAGGTAGA 3′ for TcMT3. The reactions were performed in an iCycler iQ Real-time PCR (Bio-Rad) in triplicate. The PCR was: 95 °C for 15 min, followed by 35 cycles of 95 °C for 15 s, 58 °C for 20 s, 72 °C for 20 s. After final annealing (72 °C, 5 min), and redenaturation (95 °C, 1 min), a melt curve analysis was done by decreasing the temperature from 95 °C to 60 °C at 0.5 °C intervals. The fold-change in gene expression was calculated using the comparative Ct method (2–ΔΔCt) (Livak and Schmittgen, 2001). The correlation coefficient of amplification, determined from serial dilutions, was 0.996 for TcMT2a, 0.998 for TcMT2b and TcMT3, and 0.999 for tubulin.
Determination of MT alleles in the crosses
The allelic distribution of MT genes was analysed from shoots of 12 plants of the parental accessions LM and LC, and the five low- and high-accumulating F3 sibling lines from the corresponding inter-accession cross. TcMT2a was amplified from the 3′ UTR using a 5′ fluorescently labelled reverse primer (5′ GCAATAATGGCTGTAGCCTTGT 3′ and 5′ FAM-GAAGTACAAACGGGACCATCAA 3′). The length of the TcMT2a-LM amplicon was 118 bp, which is 3 bp longer than the TcMT2a-LC amplicon. TcMT3 was amplified from genomic DNA with a 5′ FAM-labelled forward primer (5′ FAM-ACAAGACCCAGTGCGTGTAAGT 3′; 5′ TACTCTTCTTGCTGCGGTCACA 3′). The primers span the first intron which is 16 bp longer in LM, resulting to amplicon lengths of 386 bp for the LM allele and 370 bp for the LC allele. The TcMT2b gene was amplified from genomic DNA (primers 5′ TAATCTGTTCACGTCCTCGGTT 3′ and 5′ FAM-CATCATTCTCGGCAACGAAGGT 3′), with amplicon lengths of 313 bp and 302 bp for the LC and the LM allele, respectively, due to an 11 bp longer intron in the LC allele. After purification of the PCR products, the samples were applied to an automated sequencer (MegaBACE 750 DNA Analysis System, Amersham). The data were analysed using fragment analysis software (Fragment Profiler, Amersham).
Antibody production and immunofluorescence staining
Antibodies were developed against two synthetic biotinylated peptides (P1, GGCKRNPDLGYSGE; and P2, VLGVAPAMKNQYEASGE), designed from the spacer region between the T. caerulescens TcMT2a-LC cysteine-rich domains (Fig. 1). A rabbit was immunized with 100 μg of both P1 and P2 three times at 4-week intervals, using avidin-conjugated P1 and P2 in the first and second immunizations. Raising of antibodies was monitored using an enzyme-linked immunosorbent assay (ELISA) with serum taken before injection as a reference. Serum was collected 6 weeks after the last injection. The antibodies were purified by affinity chromatography, first with streptavidin–Sepharose followed by P1 and P2 coupled to streptavidin–Sepharose (Amersham Biosciences). Antibodies were tested with ELISA using streptavidin–P1- and –P2-coated plates.
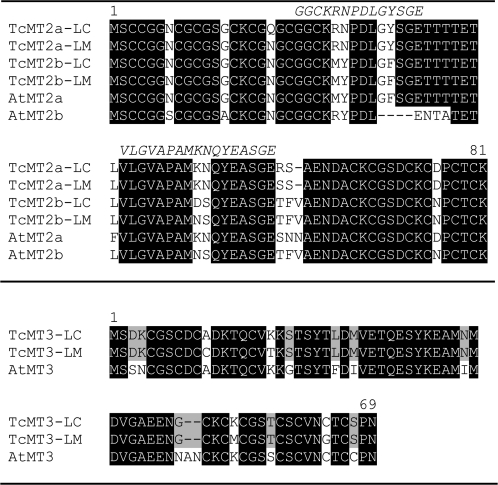
Fig. 1.
Deduced TcMT2a, TcMT2b, and TcMT3 amino acid sequences from T. caerulescens accessions LC (La Calamine, Hassinen et al., 2007) and LM (St Laurent le Minier). Arabidopsis thaliana AtMT2a (At3g09390), AtMT2b (At5g02380), and AtMT3 (At3g15353) are included for comparison. Peptides used for antibody production are shown in italics and cover the amino acids 21–34 and 42–58 of T. caerulescens MT2a-LC.
For whole-mount immunofluorescence staining, T. caerulescens accessions LC, LE, and LM, as well as A. thaliana were germinated in Petri dishes on filter paper soaked in 0.5 MS (Murashige–Skoog) medium. The 4-d-old Arabidopsis and 7-d-old T. caerulescens seedlings were fixed in 4% paraformaldehyde at 4 °C for 2 h and washed with phosphate-buffered saline (PBS; three times 5 min). The cell walls were digested with 4% cellulase ‘Onuzuka R-10’ (Duchefa) and 3% macerozyme R-10 (Duchefa) at 25 °C for 2 h, and washed with PBS. Before incubating with the primary antibody, the samples were blocked with Image-iT Signal Enhancer (Invitrogen) for 30 min and with blocking solution (2% milk powder, 0.5% bovine serum albumin) for 1.5 h. The plants were incubated with the affinity-purified MT2 antibody (1.7 μg ml−1) at 4 °C overnight. After washing, the samples were incubated in 1:200 diluted secondary antibody (goat anti-rabbit IgG Alexa Fluor 488, Invitrogen) for 1.5 h. The samples were counterstained with 10 μg ml−1 propidium iodide (Invitrogen). After washing, the seedlings were transferred on microscope slides, mounted with PVA-DABCO antifading mounting medium (Sigma-Aldrich), and inspected with a confocal laser scanning microscope Eclipse-TE300 (Nikon Corporation, Japan)/Ultra VIEW (Perkin-Elmer, UK). As a negative control, purified MT2 antibody pre-adsorbed on the peptides used for immunization was included in each experiment. The images were processed using ImageJ (Abramoff et al., 2004) and Adobe Photoshop softwares.
Arabidopsis transformation
The TcMT2a-LC and TcMT3-LC cDNAs were cloned into the pCAMBIA2301 vector under the cauliflower mosaic virus (CaMV) 35S promoter. Recombinant Agrobacterium tumefaciens (LBA4404) clones were selected in a medium containing kanamycin, rifampicin, and streptomycin. Arabidopsis thaliana (Col-0) was transformed using the floral dip method (Clough and Bent, 1998) and T0 seeds were germinated on 0.5 MS agar plates under kanamycin selection. The presence of the transgene was confirmed by PCR with pCAMBIA2301-specific primers. Homozygous T3 lines were further selected by segregation for kanamycin resistance.
Analysis of growth and metal content
Seeds of transgenic (T3) and wild-type Arabidopsis plants were sown on 0.5 MS agar plates (control), or on plates supplemented with 400 μM ZnSO4, 40 μM CuSO4, or 15 μM CdSO4. The plates were incubated at 22 °C with 16 h light/8 h darkness horizontally for 3 d, and then turned to the vertical position. The root length was determined using ImageJ software (Abramoff et al., 2004) after 7 d of growth.
For the analysis of metal uptake, the seeds of wild-type and transgenic (T3) Arabidopsis were sown in a peat/soil/perlite/sand mixture (20/20/30/30; v/v/v/v). Three-week-old plants were transferred to modified half-strength Hoagland solution (Schat et al., 1996): 3 mM KNO3, 2 mM Ca(NO3)2, 1 mM NH4H2PO4, 0.5 mM MgSO4, 1 μM KCl, 25 μM H3BO3, 2 μM ZnSO4, 2 μM MnSO4, 0.1 μM CuSO4, 0.1 μM (NH4)6Mo7O24, 20 μM Fe(Na)EDTA, 2 mM MES. The pH was set to 5.5 using KOH. After 2 weeks, the plants were transferred to the same nutrient solution (control) or to a similar solution supplemented with 10, 25, or 100 μM ZnSO4 or 1 μM or 10 μM CdSO4. The solutions were aerated continuously and changed twice a week. The hydroponic culture was done in a climate chamber with 20/15 °C (day/night), 65% relative humidity, 150 μmol m−2 s−1 light 12 h/day.
After 1 week of exposure, the roots were desorbed with ice-cold 5 mM PbNO3 for 1 h. After washing with water, the shoot and root samples were dried at 65 °C for 40 h. The samples were decomposed with suprapur HNO3 by microwave digestion, and the Cd and Zn contents of the samples were analysed using a flame atomic absorption spectrophotometer (Perkin Elmer AAS 5100).
Results
MT2a, MT2b, and MT3 genes of Thlaspi caerulescens
MT2a and MT3 cDNAs which had been isolated from T. caerulescens accession LC (Hassinen et al., 2007) differ from the TcMT2 and TcMT3 isolated from accession FP from the Ganges region (Roosens et al., 2004, 2005) at the amino acid sequence level. These different MT alleles or isoforms in different Thlaspi accessions prompted the further amplification and sequencing of TcMT2a, TcMT2b, and TcMT3 genes from a non-metallicolous and from the two calaminous accessions. The presence of both MT2 types, i.e. MT2a and MT2b, in T. caerulescens was confirmed.
The MT genes from Thlaspi have generally longer introns than those of their Arabidopsis homologues. For example, the size of MT2a intron is 376 bp in Thlaspi and 206 bp in Arabidopsis, and those for MT2b are 363 bp and 176 bp, respectively. For MT3 the lengths of the first intron are 343 bp and 208 bp in Thlaspi and Arabidopsis, respectively, whereas the second intron is about of equal size (78/84 bp).
The isolation of MT2a and MT2b cDNAs from the same plants indicates that there are two translated MT2 isoforms also in T. caerulescens (Fig. 1). As TcMT2b-LC has ∼88% and 83% identity with Arabidopsis AtMT2b and AtMT2a, respectively, it is considered to be an MT2b. Thlaspi caerulescens MT2a and MT2b show higher sequence identity to each other than do the Arabidopsis type 2a and 2b genes.
The MTs of accession LM show some differences from the ones in the other Thlaspi accessions. The MT2a genes from LE, LC, or the serpentine accession Monte Prinzera are identical to each other but different from MT2a-LM. MT2a-LM also differs from the TcMT2-FP which originates from the accession FP from the Ganges region (Roosens et al., 2005), the latter being identical to TcMT2b-LC. On the other hand, the two accessions from the Ganges region have identical MT3 proteins (Fig. 1) that differ from the other accessions analysed in the present study.
Expression of MT2a, MT2b, and MT3 in T. caerulescens accessions and F3 sibling lines
The relative expression of the MT genes, calculated as fold-change normalized to the endogenous reference gene α-tubulin, was analysed in the roots and shoots of three T. caerulescens parental accessions and in low and high Zn-accumulating F3 sibling lines from two inter-accession crosses at deficient, control, and excess Zn supply using quantitative real-time PCR.
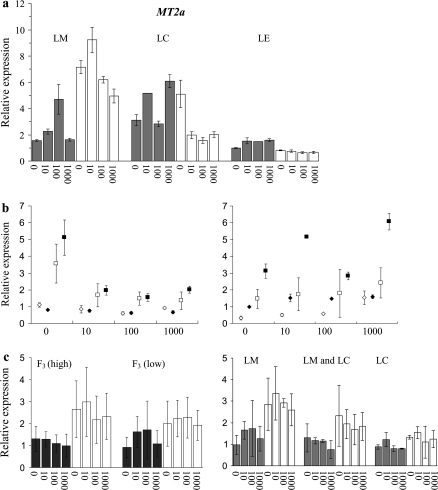
The TcMT2a expression was higher in the roots and shoots of both calamine accessions (LC and LM), compared with the non-metallicolous accession LE (Fig. 2a). The highest expression was found in Zn-exposed LM shoots. In a cross between the calamine accession LC and the non-metallicolous accession LE, the expression of TcMT2a was higher in the low-accumulating F3 lines than in the high-accumulating lines (Fig. 2b). However, among the F3 lines of the cross between the calamine accessions LC and LM, there was no correlation between TcMT2a expression and Zn accumulation capacity (Fig. 2c).
Fig. 2.
TcMT2a expression in T. caerulescens at 0, 10, 100, and 1000 μM ZnSO4 in (a) roots (grey) and shoots (white) of the calaminous accessions St Laurent le Minier (LM; high accumulation, high tolerance) and La Calamine (LC; low accumulation, high tolerance), and in the non-metallicolous accession Lellingen (LE; high accumulation, low tolerance); (b) in F3 sibling lines of an LC×LE cross, two high or three low Zn-accumulating lines (open diamonds and squares, respectively), and in LC (filled squares) and LE (filled diamonds) shoots (left) and roots (right); (c) in the roots (dark grey) and in the shoots (white) of five low- and five high-accumulating F3 sibling lines of an LC×LM cross, grouped according to high or low Zn accumulation (left) or according to the distribution of LM and LC alleles: five lines with exclusively the MT2a-LM allele, two lines with exclusively the MT2a-LC allele, and three lines containing both alleles (right). The data indicate the relative expression compared with the expression in LE roots at Zn deficiency (value 1), and represent means of three pooled roots or shoots. Error bars, ±SD.
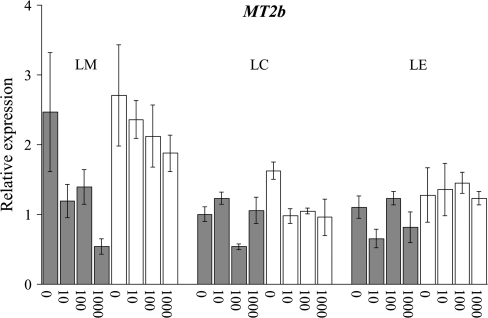
The expression of MT2b was rather constant in the different accessions. As for MT2a, the highest expression of MT2b was found in LM shoots, but the differences were small (Fig. 3). There was no apparent correlation between the expression of MT2b and the Zn uptake capacity among the LC×LM F3 lines (data not shown).
Fig. 3.
TcMT2b expression in roots (grey) and shoots (white) of accessions St Laurent le Minier (LM), La Calamine (LC), and Lellingen (LE) at 0, 10, 100, and 1000 μM ZnSO4. The data are the relative expression of samples consisting of three pooled roots or shoots compared with the expression in LC roots at Zn deficiency (value 1). Error bars, ±SD.
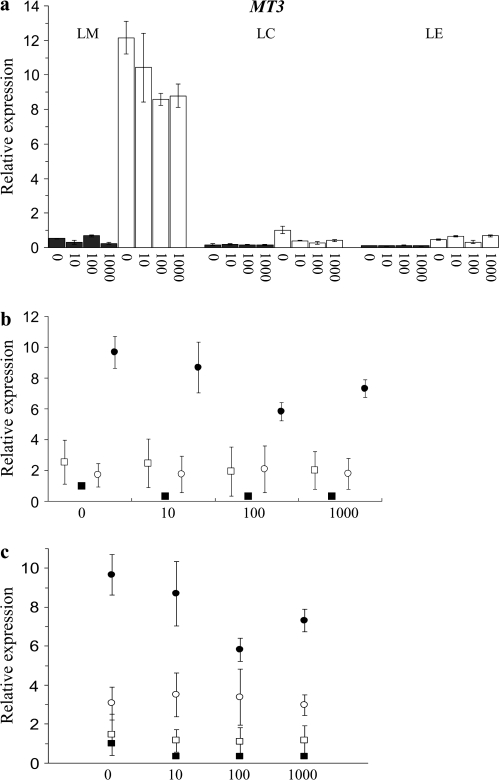
The greatest differences in expression between the accessions were found in TcMT3. The expression of TcMT3 was >10-fold in the shoots of LM than in those of the other accessions (Fig. 4). In the roots of LM grown at Zn deficiency, the expression of TcMT3 was ∼3-fold compared with LC and 4-fold compared with LE. However, there was no correlation of Zn accumulation capacity and MT3 expression among the F3 lines.
Fig. 4.
Relative TcMT3 expression in 0, 10, 100, and 1000 μM ZnSO4 (a) in shoots (white) and in roots (black) of the parent calaminous accessions LM (high tolerance and accumulation) and LC (high tolerance, low accumulation), and in non-metallicolous LE (low tolerance, high accumulation); in shoots of five high and low Zn-accumulating F3 lines from an LC×LM cross; (b) selected for low or high accumulation (open squares and circles, respectively) and in parent accessions LC (filled squares) and LM (filled circles); (c) grouped according to allelic distribution of the F3 lines. Means and SDs of four lines containing exclusively the TcMT3-LM allele (open circles) and six lines containing both the TcMT3-LM and TcMT3-LC alleles (open squares) are shown. The data are the mean relative expression in samples consisting of three roots or shoots compared with the expression in LC shoots at Zn deficiency (value 1). Error bars, ±SD.
Allele origins of MT2 and MT3 in the F3 lines
As the MT2a and MT3 alleles of LM and LC differ in their sequence, it was possible to establish the allele origins of the LC×LM F3 lines. In addition to the sequence differences in the coding region, the MT2a alleles from LC and LM differ in the 3′-UTR, the LM allele being 3 bp longer than the LC allele, which was used to differentiate between the alleles. Among the LC×LM lines, five contained exclusively the MT2a-LM allele (three high- and two low-accumulating lines), two lines contained exclusively the MT2a-LC allele (both low-accumulating lines), and three lines contained both alleles (one low- and two high-accumulating lines). Among the F3 families, high expression in the shoot was clearly associated with the MT2a-LM allele (Fig. 2c).
MT3-LM and MT3-LC alleles were identified from genomic DNA, in which the first intron of the LM allele is 16 bp longer than that in the LC allele. None of the 10 F3 lines analysed contained exclusively the MT3-LC allele, and four contained exclusively the MT3-LM allele, of which two were high-accumulating and two were low-accumulating lines (data not shown). High expression in the shoot was clearly associated with the MT3-LM allele (Fig. 4c).
Localization of the MT2 protein
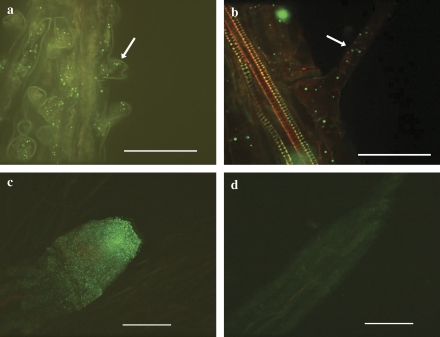
The TcMT2 protein was localized using whole-mount immunohistochemistry from the roots of T. caerulescens and from wild-type Arabidopsis as well as transgenic Arabidopsis lines expressing TcMT2 by using a TcMT2a-targeted anti-peptide antibody coupled to fluorescently labelled secondary antibody. In the roots, MT2 protein is localized mainly in the epidermal cells and in root hairs, both in T. caerulescens and in A. thaliana (Fig. 5), with relatively high abundance at the root tip. In transgenic Arabidopsis expressing TcMT2a, a strong fluorescence signal was observed especially at the root tip compared with the wild-type plants, indicating that the transformants produce TcMT2 protein.
Fig. 5.
Localization of MT2 protein (arrows) in root epidermal cells and root hairs in (a) 7-d-old T. caerulescens; (b) 4-d-old Arabidopsis. The root tip of 4-d-old Arabidopsis (c) transformed with TcMT2 under the 35S promoter shows a bright fluorescence compared with (d) the wild type (Col-0). Immunocytochemical analysis was performed using primary antibodies raised against TcMT2. The secondary antibody was conjugated with Alexa Fluor 488, and fluorescent staining was imaged by laser-scanning confocal microscopy. The samples were stained with propidium iodide. Bar = 100 μM (c, d) or 50 μM (a, b). (This figure is available in colour at JXB online.)
The TcMT2a antibody was made against the spacer region between the cysteine-rich domains, with two and three amino acid mismatches between the two peptides and TcMT2b protein. As two MT2 isoforms are present in Thlaspi, it cannot be ruled out that the antibody binds to both MT2b and MT2a. However, the localization observed here is in line with MT2a, with no indication of localization in the phloem which has been observed for MT2b (Guo et al., 2003). The antibody is also able to detect Arabidopsis MT2, as the first peptide has a three amino acid mismatch with AtMT2a while the second peptide is identical to AtMT2a.
Expression of TcMT2a and TcMT3 in Arabidopsis
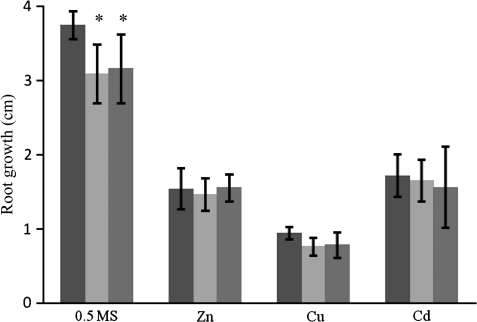
Arabidopsis seedlings were exposed to Cu, Cd, or Zn, and the root lengths were analysed from the vertically grown plants. In transgenic Arabidopsis lines expressing TcMT2a or TcMT3, the root length was reduced compared with wild-type Arabidopsis in control medium (Fig. 6). However, at 15 μM Cd, 40 μM Cu, or 400 μM Zn, the root lengths of the TcMT2a and TcMT3 transformant lines were not significantly different from those in wild-type Arabidopsis, on average, and none of the individual transformant lines performed significantly better than the wild type. The same results were found in replicate experiments with other metal concentrations, i.e. 300 μM or 500 μM ZnSO4, 20, 30, or 35 μM CuSO4, or 5 μM or 10 μM CdSO4 (data not shown). There were no differences in germination or in shoot growth in the transformant lines compared with the wild-type plants.
Fig. 6.
Root length of wild-type (black), TcMT2a (light grey), or TcMT3 (dark grey) transformed Arabidopsis seedlings grown in 0.5 MS (control) or in 0.5 MS spiked with 400 μM ZnSO4, 40 μM CuSO4, or 15 μM CdSO4 for 11 d. The data are means of three plates (each containing 10 plants) of three independent TcMT2a or TcMT3 transformants. Error bars indicate ±SD. *Means significantly different from wild-type (two-way ANOVA, followed by a posteriori testing using the minimum significant range, MSR).
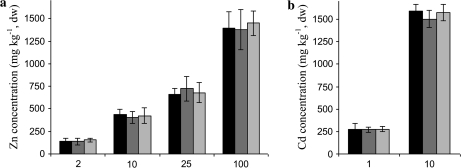
The MT2 and MT3 transformant lines did not show any differences in the concentrations of Cd and Zn in the roots or shoots compared with the wild-type Arabidopsis (Fig. 7). Also the root Cu concentration in the control solution was similar in the wild-type and the transformant lines (data not shown).
Fig. 7.
Shoot (a) Zn concentration in control solution (2 μM Zn) or in solution spiked with 10, 25, or 100 μM ZnSO4 and (b) Cd concentration in 1 μM or 10 μM CdSO4 exposure in wild-type (black), TcMT2 (dark grey), or TcMT3 (light grey) transformed Arabidopsis seedlings. The data are means of three independent TcMT2 or TcMT3 transformants, with two samples (containing three pooled roots or shoots) each. Error bars, ±SD.
Discussion
The simultaneous isolation of both MT2a- and MT2b-type genes in this study shows that T. caerulescens has two MT2 isoforms just like Arabidopsis does. The previously isolated MT2 sequences are, in fact, examples of the two isoforms rather than of different alleles of the same locus. The MT2 isolated from St Félix de Pallières (Roosens et al., 2005) can now be classified as a 2b-type gene, whereas the isoform previously isolated from La Calamine (Hassinen et al., 2007) is a 2a-type gene. The TcMT3s isolated have sequence differences in the N- and C-terminal domains, with the sequence from LC resembling the Arabidopsis sequence (Ala11 and Cys62) and that from LM being similar to a previously isolated sequence (Cys11 and Gly62) from St Félix de Pallières also originating from the Ganges region (Roosens et al., 2004).
Differences in MT expression levels between the Thlaspi accessions under study were obvious. In general, the lowest expression was observed in the non-metallicolous accession LE, whereas the highest expression was observed in the accession LM, which is capable of accumulating high concentrations of Cd and Zn in the shoots. Especially striking was the high MT3 expression in LM compared with the calaminous LC or non-metallicolous LE, which is in line with the higher expression in the FP from the Ganges region compared with another calamine and a serpentine accession (Roosens et al., 2004). However, there is no correlation among the accessions in MT expression and Zn accumulation capacity (Assunção et al., 2003a): LM has high MT expression and accumulation, LE has low MT expression but high accumulation, whereas LC has generally higher MT expression but much lower accumulation capacity than does LE.
Zn accumulation capacity does not co-segregate with high MT expression in the F3 sibling lines either. Even though there were some positive indications for TcMT2a expression with Zn accumulation in the LC×LE crosses, this was not evident in the F3 lines from LC×LM crosses. However, the results show a correlation between high MT expression and the LM allele among the progeny of the cross. Among the F3 lines, the expression of MT2a and MT3 was higher in the lines having the LM allele exclusively. The variation in MT expression among F3 lines is thus determined by allele origin, which could be due to differential cis-regulation of the MT genes.
When Arabidopsis plants were transformed with TcMT2a and TcMT3, no change in Zn content was evident compared with the wild-type plants, implying that overexpression of TcMTs in Arabidopsis does not enhance Zn accumulation. Moreover, the expression of MT2a, MT2b, or MT3 was not induced upon Zn exposure. Taken together, the data imply that the differential MT expression between the accessions is not a direct cause or consequence of the variation in Zn accumulation capacity among the accessions, and the properties are clearly under independent genetic control. This has also been shown in yeast, where no direct involvement in Zn immobilization of yeast CUP-1 Cu-thionein or Zn-Cu thionein Crs5 is evident either (Pagani et al., 2007).
The reason for the variation in MT expression between the accessions remains open. Since the homeostatic networks of different metals are strongly interconnected in yeast (Eide et al., 2005), as well as in plants (van de Mortel et al., 2006), MTs could be involved in the homeostasis or accumulation of metals other than Zn. Moreover, it has been shown that plant MT type 2 from Quercus suber can form complexes with either Cd, Cu, or Zn in vivo (Domenech et al., 2006, 2007).
One hypothesis would be that MTs are related to Cd accumulation. In the LC×LM cross, Cd and Zn accumulation capacities were correlated (Deniau et al., 2006), suggesting that the F3 lines that are high accumulating for Zn are most probably also high accumulating for Cd, possibly due to Zn uptake via a highly Cd-preferential uptake system present in LM (Zha et al., 2004). However, overexpression of MTs in Arabidopsis did not enhance Cd accumulation either. Additionally, MT expression and Cd accumulation are not correlated among accessions, since LE has higher Cd accumulation capacity, but lower expression than does LC (Assuncao et al., 2003a). Moreover, MTs of type 2 and 3 are usually not significantly induced by Cd (Zhou and Goldsbrough, 1994; Roosens et al., 2005). Therefore, it is unlikely that differential MT expression would be a direct cause or consequence of differential Cd accumulation capacity either.
Another hypothesis is that MTs have a role in Cd or Zn tolerance, rather than in the accumulation of these metals. The expression levels of MTs, i.e. LM>LC>LE, follow the order of Cd and Zn tolerance (Assuncao et al., 2003a; Zha et al., 2004). Tolerance-correlated enhanced MT2b expression has also been found in calamine populations of non-hyperaccumulating metallophytes, e.g Silene vulgaris (Jack et al., 2007). MTs seem to act as hypostatic factors in metal tolerance, enhancing the tolerance level when some other epistatic tolerance genes are also more highly expressed (van Hoof et al., 2001). The TcMT proteins themselves bind and potentially provide tolerance to metals, as they can complement Zn and Cd tolerance in yeast (Hassinen et al., 2007). Moreover, expression of Brassica juncea BjMT2 increased tolerance against Cd when expressed in A. thaliana, as evidenced by increased chlorophyll content and shoot growth (Zhigang et al., 2006). However, in Arabidopsis, the TcMT overexpression did not enhance tolerance to Zn, Cd, or Cu, as determined from root growth. No change in shoot growth was evident either. This might be due to the absence of some epistatic factor from the Arabidopsis genetic background, comparable with the situation in non-metallicolous S. vulgaris (Van Hoof et al., 2001). Alternatively, these results might be explained by incorrect tissue specificity, due to the 35S promoter. These differences may also reflect the differential metal-binding capacities of MT proteins from different plant species, as shown for differential Cu tolerance in yeast mutants provided by MT3 from Thlaspi and Arabidopsis (Roosens et al., 2004), due to differences in amino acid sequence. Moreover, it has been demonstrated that the sequences between the cysteine-rich domains, so-called spacer regions, also have a role in the functioning of MT proteins (Domènech et al., 2006, 2007).
Another possible role for MTs could be in metal, especially Cu, homeostasis. When expressed in yeast, the MT3-FP from Ganges conferred higher Cu tolerance than did Arabidopsis MT3 (Roosens et al., 2004), whereas MT3-LC was not effective (Hassinen et al., 2007). Also the MT3-FP expression is constitutive, not inducible by Cu excess, as was the case in a calamine and a serpentine accession (Roosens et al., 2004), suggesting that the very high MT3 expression in FP or LM serves to guarantee Cu homeostasis in a cellular environment with high Cd content. Moreover, A. thaliana expressing BjMT2 had reduced root growth in the absence of Cu exposure, which was proposed to be due to disturbances in metal homeostasis (Zhigang et al., 2006). The reduced root growth at control conditions was also seen in Arabidopsis expressing TcMTs. At higher metal concentrations, the root growth was similar to that in the wild-type plants, perhaps reflecting the attainment of a balance between the overexpressed MT protein and metal availability. If the MTs are involved in Cu homeostasis, the addition of excess metals might displace Cu from TcMT and restore the homeostasis.
The localization of the MT2 protein also supports the role of MTs in metal buffering rather than in accumulation. In the roots of T. caerulescens and A. thaliana, the MT2 protein localized in the epidermal cells and root hairs, with a strong fluorescence at the root tip. The localization is in line with the previous findings where Arabidopsis MT2 mRNA co-localized with the MT2 protein in the root tip (Murphy et al., 1997). Localization at the root tip has been reported for the Arabidopsis MT2a; it was concluded that MT2a acts as a chaperone protecting the root apex, the first tissue to absorb excess metals (Guo et al., 2003).
The results imply that MT2 and MT3 are not the primary determinants of Zn accumulation, as Zn accumulation and MT expression did not co-segregate. Moreover, ectopic expression of TcMT2a and TcMT3 did not result in increased metal accumulation or metal tolerance. However, the higher expression of MTs in the superior metal-accumulating accession suggests that the MTs do contribute to the metal-adapted phenotype, possibly through enhancing either Cd or Zn tolerance, albeit as hypostatic factors, or Cu homeostasis in a high Cd/Zn environment.
Acknowledgments
This project was funded by the EC FP5 project ‘PHYTAC’ (QLRT-2001-00429). VH was partly funded by The Finnish Cultural Foundation of Northern Savo and Alfred Kordelin fund.
Glossary
Abbreviations
- FP
accession St Félix de Pallières
- LC
accession La Calamine
- LE
accession Lellingen
- LM
accession St Laurent le Minier
- MT
metallothionein
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Assunção AGL, Ten Bookum WM, Nelissen HJM, Vooijs R, Schat H, Ernst WHO. Differential metal-specific tolerance and accumulation patterns among Thlaspi caerulescens populations originating from different soil types. New Phytologist. 2003a;159:411–419. doi: 10.1046/j.1469-8137.2003.00819.x. [DOI] [PubMed] [Google Scholar]
- Assunção AGL, Ten Bookum WM, Nelissen HJM, Vooijs R, Schat H, Ernst WHO. A cosegregation analysis of zinc (Zn) accumulation and Zn tolerance in the Zn hyperaccumulator Thlaspi caerulescens. New Phytologist. 2003b;159:383–390. doi: 10.1046/j.1469-8137.2003.00758.x. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annual Review of Plant Biology. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- Deniau AX, Pieper B, Ten Bookum WM, Lindhout P, Aarts MG, Schat H. QTL analysis of cadmium and zinc accumulation in the heavy metal hyperaccumulator Thlaspi caerulescens. Theoretical and Applied Genetics. 2006;113:907–920. doi: 10.1007/s00122-006-0350-y. [DOI] [PubMed] [Google Scholar]
- Domènech J, Mir G, Huguet G, Capdevila M, Molinas M, Atrian S. Plant metallothionein domains: functional insight into physiological metal binding and protein folding. Biochimie. 2006;88:583–593. doi: 10.1016/j.biochi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Domènech J, Orihuela R, Mir G, Molinas M, Atrian S, Capdevila M. The CdII -binding abilities of recombinant Quercus suber metallothionein: bridging the gap between phytochelatins and metallothioneins. Journal of Biological Inorganic Chemistry. 2007;12:867–882. doi: 10.1007/s00775-007-0241-y. [DOI] [PubMed] [Google Scholar]
- Eide DJ, Clark S, Nair TM, Gehl M, Gribskov M, Guerinot ML, Harper JF. Characterization of the yeast ionome: a genome-wide analysis of nutrient mineral and trace element homeostasis in Saccharomyces cerevisiae. Genome Biology. 2005;6:R77. doi: 10.1186/gb-2005-6-9-r77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Bundithya W, Goldsbrough PB. Characterization of the Arabidopsis metallothionein gene family: tissue-specific expression and induction during senescence and in response to copper. New Phytologist. 2003;159:369–381. doi: 10.1046/j.1469-8137.2003.00813.x. [DOI] [PubMed] [Google Scholar]
- Guo W, Meetam M, Goldsbrough PB. Examining the specific contributions of individual arabidopsis metallothioneins to copper distribution and metal tolerance. Plant Physiology. 2008;146:1697–1706. doi: 10.1104/pp.108.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinen VH, Tervahauta AI, Halimaa P, Plessl M, Aarts MGM, Peräniemi S, Servomaa K, Schat H, Kärenlampi SO. Isolation of Zn-responsive genes from two accessions of the hyperaccumulator plant Thlaspi caerulescens. Planta. 2007;225:977–989. doi: 10.1007/s00425-006-0403-0. [DOI] [PubMed] [Google Scholar]
- Jack E, Hakvoort HWJ, Reumer A, Verkleij JAC, Schat H, Ernst WHO. Real-time PCR analysis of metallothionein-2b expression in metallicolous and non-metallicolous populations of Silene vulgaris (Moench) Garcke. Environmental and Experimental Botany. 2007;59:84–91. [Google Scholar]
- Lee J, Shim D, Song WY, Hwang I, Lee Y. Arabidopsis metallothioneins 2a and 3 enhance resistance to cadmium when expressed in Vicia faba guard cells. Plant Molecular Biology. 2004;54:805–815. doi: 10.1007/s11103-004-0190-6. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2 –ΔΔC T method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Meerts P, Van Isacker N. Heavy metal tolerance and accumulation in metallicolous and non-metallicolous populations of Thlaspi caerulescens from continental Europe. Plant Ecology. 1997;133:221–231. [Google Scholar]
- Mir G, Domènech J, Huguet G, Guo W-J, Goldsbrough P, Atrian S, Molinas M. A plant type 2 metallothionein (MT) from cork tissue responds to oxidative stress. Journal of Experimental Botany. 2004;55:2483–2493. doi: 10.1093/jxb/erh254. [DOI] [PubMed] [Google Scholar]
- Murphy A, Taiz L. Comparison of metallothionein gene expression and nonprotein thiols in ten Arabidopsis ecotypes. Correlation with copper tolerance. Plant Physiology. 1995;109:945–954. doi: 10.1104/pp.109.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A, Zhou J, Goldsbrough PB, Taiz L. Purification and immunological identification of metallothioneins 1 and 2 from Arabidopsis thaliana. Plant Physiology. 1997;113:1293–1301. doi: 10.1104/pp.113.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani A, Villarreal L, Capdevila M, Atrian S. The Saccharomyces cerevisiae Crs5 metallothionein metal-binding abilities and its role in the response to zinc overload. Molecular Microbiology. 2007;63:256–269. doi: 10.1111/j.1365-2958.2006.05510.x. [DOI] [PubMed] [Google Scholar]
- Roosens NH, Bernard C, Leplae R, Verbruggen N. Evidence for copper homeostasis function of metallothionein (MT3) in the hyperaccumulator Thlaspi caerulescens. FEBS Letters. 2004;577:9–16. doi: 10.1016/j.febslet.2004.08.084. [DOI] [PubMed] [Google Scholar]
- Roosens NH, Leplae R, Bernard C, Verbruggen N. Variations in plant metallothioneins: the heavy metal hyperaccumulator Thlaspi caerulescens as a study case. Planta. 2005;222:716–729. doi: 10.1007/s00425-005-0006-1. [DOI] [PubMed] [Google Scholar]
- Schat H, Vooijs R, Kuiper E. Identical major gene loci for heavy metal tolerances that have independently evolved in different local populations and subspecies of Silene vulgaris. Evolution. 1996;50:1888–1895. doi: 10.1111/j.1558-5646.1996.tb03576.x. [DOI] [PubMed] [Google Scholar]
- van de Mortel JE, Villanueva LA, Schat H, Kwekkeboom J, Coughlan S, Moerland PD, Ver Loren van Themaat E, Koornneef M, Aarts MGM. Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiology. 2006;142:1127–1147. doi: 10.1104/pp.106.082073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoof NA, Hassinen VH, Hakvoort HW, Ballintijn KF, Schat H, Verkleij JA, Ernst WH, Kärenlampi SO, Tervahauta AI. Enhanced copper tolerance in Silene vulgaris (Moench) Garcke populations from copper mines is associated with increased transcript levels of a 2b-type metallothionein gene. Plant Physiology. 2001;126:1519–1526. doi: 10.1104/pp.126.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha HG, Jiang RF, Zhao FJ, Vooijs R, Schat H, Barker JHA, McGrath SP. Co-segregation analysis of cadmium and zinc accumulation in Thlaspi caerulescens interecotypic crosses. New Phytologist. 2004;163:299–312. doi: 10.1111/j.1469-8137.2004.01113.x. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Hamon RE, Lombi E, McLaughlin MJ, McGraph SP. Characteristics of cadmium uptake in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. Journal of Experimental Botany. 2002;53:535–543. doi: 10.1093/jexbot/53.368.535. [DOI] [PubMed] [Google Scholar]
- Zhigang A, Cuijie L, Yuangang Z, Yejie D, Wachter A, Gromes R, Rausch T. Expression of BjMT2, a metallothionein 2 from Brassica juncea, increases copper and cadmium tolerance in Escherichia coli and Arabidopsis thaliana, but inhibits root elongation in Arabidopsis thaliana seedlings. Journal of Experimental Botany. 2006;57:3575–3582. doi: 10.1093/jxb/erl102. [DOI] [PubMed] [Google Scholar]
- Zhou J, Goldsbrough PB. Functional homologues of fungal metallothionein genes from Arabidopsis. The Plant Cell. 1994;6:875–884. doi: 10.1105/tpc.6.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimeri AM, Dhankher OP, McCaig B, Meagher RB. The plant MT1 metallothioneins are stabilized by binding cadmium and are required for cadmium tolerance and accumulation. Plant Molecular Biology. 2005;58:839–855. doi: 10.1007/s11103-005-8268-3. [DOI] [PubMed] [Google Scholar]