Abstract
Brassica campestris Male Fertility 2 (BcMF2) is a putative polygalacturonase (PG) gene previously isolated from the flower bud of Chinese cabbage (Brassica campestris L. ssp. chinensis Makino, syn. B. rapa ssp. chinensis). This gene was found to be expressed specifically in tapetum and pollen after the tetrad stage of anther development. Antisense RNA technology was used to study the function of BcMF2 in Chinese cabbage. Scanning and transmission electron microscopy revealed that there were deformities in the transgenic mature pollen grains such as abnormal location of germinal furrows. In addition, the homogeneous pectic exintine layer facing the exterior seemed to be overdeveloped and predominantly occupied the intine, thus reversing the normal proportional distribution of the internal endintine layer and the external exintine layer. Since it is a continuation of the intine layer, the pollen tube wall could not grow normally. This resulted in the formation of a balloon-like swelling structure in the pollen tube tip in nearly 80% of the transgenic pollen grains. Premature degradation of tapetum was also found in these transgenic plants, which displayed decreased expression of the BcMF2 gene. BcMF2 might therefore encode a new PG with an important role in pollen wall development, possibly via regulation of pectin's dynamic metabolism.
Keywords: Brassica campestris, Brassica rapa, Chinese cabbage, intine, PG, polygalacturonase, pollen wall
Introduction
An important feature of the Arabidopsis pollen transcriptome is the existence of a large number of genes related to cell wall synthesis and regulation (Becker et al., 2003; Pina et al., 2005). Analysis of Chinese cabbage (Brassica campestris L. ssp. chinensis Makino, syn. B. rapa ssp. chinensis) pollen gene expression also reveals that genes related to cell wall synthesis and regulation occupy a large proportion of the pollen transcriptome (Huang et al., 2008). A unique characteristic of the microspore is the pollen wall. In trinucleate pollen species, although there is species-specific variation, the pollen wall is generally composed of the outer exine and inner intine. The exine, facing the exterior, comprises two layers: the innermost and featureless nexine; and the outer sculpted sexine, which presents multiple species-specific pores and furrows (Shukla et al., 1998). The intine is the innermost layer and is located adjacent to the pollen plasma membrane, which is a highly ordered complex of polysaccharides and structural proteins similar to other plant cell walls (Owen and Makaroff, 1995). The intine also consists of two layers: a granular exintine with pectin and protein inclusions facing the exterior, and a microfibrillar cellulosic endintine facing the interior. The exine is formed from the material released by tapetum (sporophytic origin), while the intine is secreted by the microspore (gametophytic origin) during the ring-vacuolated microspore stage (Owen and Makaroff, 1995). Both layers of the pollen wall are important for pollen hydration, activation, germination, and pollen tube growth (Blackmore and Barnes, 1990; Owen and Makaroff, 1995). Thus, abnormalities of the pollen wall often render the pollen grain unable to fulfil its biological function.
As an indispensable part of pollen development, pollen wall formation is a complicated process involving the activity of numerous genes. Several mutants aberrant in exine formation have been characterized (Aarts et al., 1997; Paxson-Sowders et al., 1997, 2001; Ariizumi et al., 2003), but relatively few mutants defective in intine formation have been found. Aside from genetic screening, isolating genes or proteins specific to cell wall metabolism has provided a feasible way to elucidate the molecular mechanism underlying pollen wall formation. Such experiments were further facilitated by the release of the Arabidopsis genome. A large number of genes, representing many gene families, are considered to be involved in cell wall synthesis and regulation, such as a number of cDNA clones encoding pectin-degrading enzymes including polygalacturonase (PG), pectic lyases, pectin methylesterase (PME), and polymethylgalacturonases. The PG family of enzymes dominates plant cell function, functioning as hydrolases and as loosening enzymes in the degradation of pectin and the disintegration of the cell wall. A high level of exo-PG activity has been detected in the pollen of many species (Hadfield et al., 1998). Many pollen-specific PG genes have also been cloned, and it appears that a number of PG family members might be present in pollen (Allen et al., 1993; Honys and Twell, 2003). It is speculated that PG in a pollen tube disintegrates the style cell wall, allowing pollen tubes to pass through. Alternatively, PG could act on a pollen tube's own cell wall, thus accelerating its growth (Brown et al., 1990). However, most of the current research on pollen PG genes remains at the level of cloning and expression pattern identification. There is negligible direct evidence regarding the role of PG genes in the development of pollen or pollen tubes.
A previous study identified a male-sterile mutant which lacks mature pollen, Brassica campestris male sterile (bcms), among B. campestris ssp. chinensis (Huang et al., 2008). Genome-wide transcriptional profiling was performed on the flower buds of bcms and the wild type using cDNA amplified fragment length polymorphism (cDNA-AFLP) technology together with genome array analysis. This methodology detected five putative PG genes expressed exclusively in wild-type flower buds (Wang et al., 2005; Huang et al., 2008; genome array data not shown). All of these genes [BcMF2 (Brassica campestris Male Fertility 2), BcMF6, BcMF9, BcMF16, and BcMF17] display typical exo-PG structure in the deduced amino acid sequences, but possess distinct nucleotide sequences. These putative PG genes were previously considered important in pollen development, but the exact roles were not fully delineated. Here, a novel investigation of one such gene, BcMF2, is presented. Sequence analysis revealed that BcMF2 shares 83% and 82% similarity with the nucleotide and amino acid sequences, respectively, of the Arabidopsis exo-PG gene PGA4 (At1g02790). The PGA4 gene was detected early in the Arabidopsis pollen transcriptome and was considered pollen specific; however, its exact role in pollen development is not yet clear (Honys and Twell, 2003). In this study, the function of BcMF2 in pollen development was investigated with antisense RNA technology. Possible effects of BcMF2 on pollen and the pollen wall are discussed; the results obtained in this study may provide valuable insight regarding PG genes and pollen.
Materials and methods
Plant materials
The bcms mutant of B. campestris ssp. chinensis Makino was found in the field in the 1980s. It was maintained by crossing it with its wild-type ancestor. The plants were then cultivated in the experimental farm of Zhejiang University.
RNA extraction, cDNA synthesis, and reverse transcription-PCR analysis
Total RNA was extracted from different tissues using Trizol reagent (Gibco, Berlin, Germany), while the poly(A)+ RNA was isolated from the total RNA with an Oligotex mRNA Mini Kit (Qiagen, Hilden, Germany). The first-strand cDNA and double-stranded cDNA were then synthesized using a SMART™ cDNA Library Construction Kit (Clontech, Mountain View, USA) according to the manufacturer's instructions.
Two rounds of reverse transcription-PCR (RT-PCR) were conducted with two independently isolated total RNA samples as templates. The RT-PCR was performed for 18, 23, 28, 33, and 38 cycles to determine the linearity of the PCR, and ultimately 28 cycles was determined as the optimal number. A 350 bp Actin-1 fragment was amplified under the same conditions to serve as a positive control. The gene specificity of the RT-PCR products was then confirmed by sequencing.
In situ hybridization
Flower buds at different developmental stages were fixed in 4% formaldehyde phosphate-buffered saline (PBS) solution with 0.1% Triton X-100 and 0.1% Tween-20, serially dehydrated, cleared with dimethylbenzene, and embedded in paraffin. Sections (8 μm) of flower buds were hybridized to specific digoxigenin-labelled RNA probes (Roche). Templates for the BcMF2-specific probes were obtained from amplification with specific primers. The sense and antisense probes were synthesized using an SP6/ T7 transcription kit (Roche).
Antisense RNA construction and plant transformation
Partial cDNA sequences (nucleotides 232–680) of BcMF2 were amplified by PCR and cloned in the antisense orientation into the binary vector pBI121. Restriction digestion, PCR, and DNA sequencing confirmed the correct orientation of the fragment. The antisense BcMF2 RNA construct was transferred into the Chinese cabbage mediated by Agrobacterium tumefaciens as described by Yu et al. (2004). As a control, an empty vector pBI121was also transferred into the Chinese cabbage.
PCR analysis, Southern hybridization, and northern blotting
For PCR and Southern hybridization, genomic DNA was extracted from ∼0.5–1 g of fresh young leaves of putative transformed and non-transgenic plants according to the procedures described by Cao et al. (1995). PCR was performed to detect a 680 bp fragment of the NPTII gene in the pBI121 vector. Genomic DNA was digested with EcoRI for Southern hybridization to confirm the integration of the antisense RNA fragment of BcMF2 into the plant genome. The digested genomic DNAs were fractionated on 0.8% agarose gels, transferred onto a nylon membrane, and hybridized to α-32P labelling probes. The DNA probes were prepared by labelling the NPTII gene using a Random Primer DNA Labeling Kit Ver.2 (Dalian, China) according to the manufacturer's instructions.
Northern blotting of total RNA from flower buds and flowers was performed to detect the expression of BcMF2 in the transgenic or control plants. Total RNA (20 μg) was separated by electrophoresis on a 1.2% formaldehyde agarose gel, followed by blotting onto a nylon membrane (Hybond-N+, Amersham Pharmacia), and probed with α-32P labelling, as for the procedure in Southern hybridization. Hybridization was performed as previously described (Cho et al., 1997). The Southern and Northern hybridized membranes were scanned with a Typhoon 9210 scanner (Amersham Bioscience, Uppsala, Sweden).
Pollen analysis, microscopy, and pollen germination observation
For pollen staining, anthers were dissected to release pollen grains followed by incubation in different histochemical stains. To stain nuclei and cellulose, 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes) was used. Solutions of DAPI were prepared and used as previously described (Regan and Moffatt, 1990). Aniline blue (0.1%) was used to stain the callose in pollen and observed with epifluorescence optics in a Leica microscope.
For in vitro pollen germination, pollen grains were collected and cultured in the culture medium [15% sucrose (w/v); 0.4 mmol l−1 HBO3; 0.4 mmol l−1 Ca(NO3)2; 0.1% agar (w/v)]. The pollen grains spread on the agar plates were cultured immediately at 20–25 °C, 100% relative humidity. The germinating pollen grains were counted under a microscope. Three independent BcMF2 transgenic lines and the corresponding control lines were examined and more than five flowers from each plant were tested. From each culture, at least 300 pollen grains were examined to calculate an average germination rate, and 20 pollen tubes were measured for the average pollen tube length.
For environmental scanning electron microscopy (SEM), individual pollen grains from either control or BcMF2 transgenic plants were mounted on SEM (Philips XL-40) stubs and coated with palladium–gold using standard techniques and vacuum desiccation. Digital images were then taken.
For transmission electron microscopy (TEM), anthers were fixed with 2.5% glutaraldehyde (containing 0.01% Tween-20) overnight, and rinsed in 0.1 M phosphate buffer, then transferred to 1% osmic acid for 1 h. Specimens were washed again in phosphate buffer as above, and dehydrated through an ethanol series to 80% ethanol. Samples were then embedded in Spurr's resin, with the ultrathin sections stained with uranyl acetate and lead citrate before being viewed in a JEM-1230 electron microscope operating at 80 kV.
Results
BcMF2 is a PG gene expressed in pollen
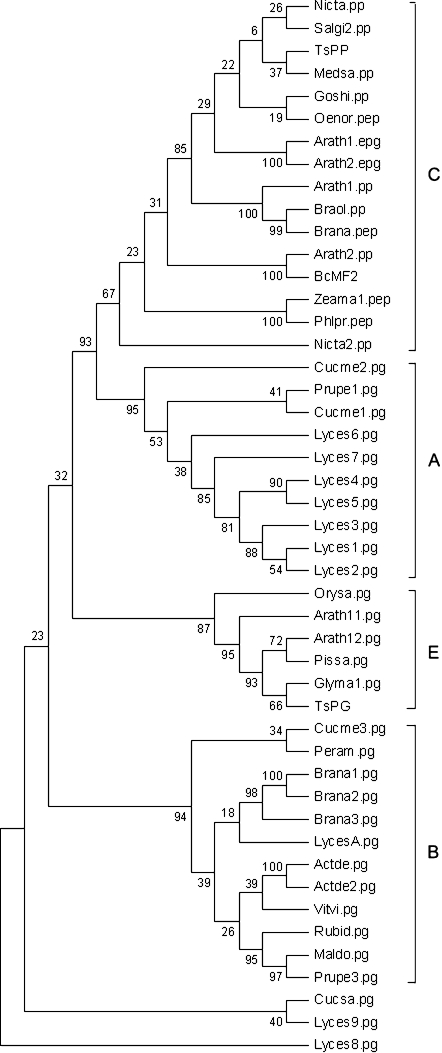
To investigate the BcMF2 gene further, its deduced amino acid sequence was compared with those of other published plant PGs. In addition to the sequences formerly used to classify the plant PGs by Markovič and Janeček (2001), other known PGs were also downloaded from the database (Supplementary Table 1 available at JXB online) and then the PG phylogenetic tree was reconstructed using the maximum parsimony (MP) method. This resulted in a tree with four main clades, similar to the one previously reconstructed (Torki et al., 2000; Athanasiou et al., 2003) (Fig. 1). BcMF2 belongs to clade C, in which it was expected to observe pollen expression of the corresponding PG genes, encoding exo-PG (Torki et al., 2000). If the hypothesis that clade C is comprised entirely of genes expressed in pollen (Torki et al., 2000) is true, the separation of BcMF2 to clade C may suggest a pollen-expressed pattern.
Fig. 1.
BcMF2 protein in the molecular phylogenetic trees based on the amino acid sequence of plant PGs using the maximum parsimony (MP) method. Numbers on the tree represented confidence values from bootstrap test (1000 replicates).
To test this hypothesis, the expression pattern of BcMF2 was analysed. Its expression was first investigated in different tissues using RT-PCR and Northern blotting. Flower buds of five different sizes, open flowers, germinal siliques, scapes, and leaves were selected as materials. The developmental stage of the various sized flower buds was determined by cytological observation. In wild-type Chinese cabbage, flower buds with a diameter ≤1.0 mm (stage I flower buds) corresponded to the onset of microspore mother cell formation or the period leading up to it. Flower buds with a diameter of ∼1.6 mm (stage II flower buds) represented the meiosis period. Flower buds with a diameter of ∼2.2 mm (stage III flower buds) corresponded to the tetrad period. Flower buds with a diameter of 2.8 mm (stage IV flower buds) represented the uninucleate pollen period. Finally, flower buds with the largest diameter were the opening flower buds (stage V flower buds). At this point, opened anthers and a pollen-spreading appearance could be observed (Huang et al., 2008). Materials from the same tissue obtained from the bcms mutant were used as negative controls.
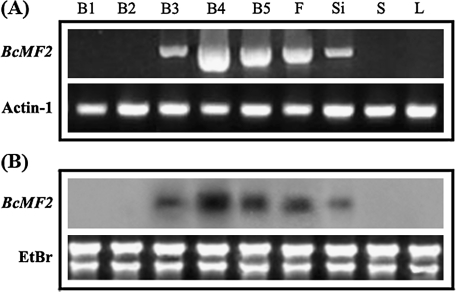
Both RT-PCR and Northern blotting results showed that transcription of BcMF2 first occurred in the stage III flower buds. The expression of BcMF2 reached its maximum in the stage IV flower buds and decreased gradually thereafter as development proceeded, though it was comparatively high in the stage V flower buds and the open flowers. Expression continued until the formation of siliques. Notably, no BcMF2 expression was detected in the leaves or scapes of stage II or stage I flower buds prior to meiosis (Fig. 2). Meanwhile, BcMF2 expression was not detected in any tissue of the bcms mutant (data not shown).
Fig. 2.
Spatial and temporal expression pattern of BcMF2 from Chinese cabbage. (A) RT-PCR analysis of the expression pattern of BcMF2. (B) Northern blotting analysis of the expression pattern of BcMF2. (B1–B5) The flower buds of Chinese cabbage at stages I–V, respectively. F, Si, S, and L indicate the flowers, germinal siliques, scape, and leaves, respectively. Transcripts for the ubiquitously expressed Actin-1 gene were used as controls.
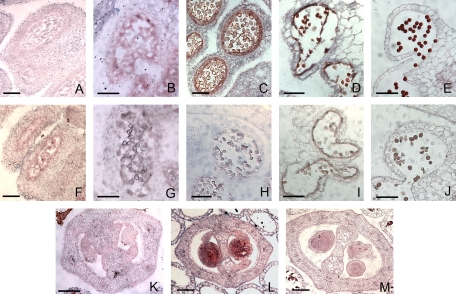
To investigate further the cellular location of BcMF2 mRNA, in situ hybridization analysis was performed on transverse sections of flower buds at different developmental stages. Hybridization signals were first detected in the tapetal cells and in the microspore tetrads of the anther (Fig. 3B), though the signals were comparatively weak. Stronger signals were found in anthers at the uninucleate microspore stage (Fig. 3C). Strong signals were also shown in the tapetum at early degradation stage and in the binucleate microspore; at this time, the signal in microspores was stronger than that in previous stages (Fig. 3D). In anthers at the mature pollen stage, the tapetum was completely degraded; during this period, the BcMF2 signal in the mature pollen grains reached its peak (Fig. 3). There was also strong expression in the ovules of old flower buds at the anther dehiscence stage (Fig. 3L). No expression signal was observed either in the anthers prior to meiosis (Fig. 3A) or in the sense control (Fig. 3F–J, M).
Fig. 3.
Analysis of the expression pattern of BcMF2 in Chinese cabbage using in situ hybridization. (A−E) Sections of anthers at pollen mother, tetrads, uninucleate, binucleate, and mature pollen stages, respectively, hybridized with a BcMF2 antisense probe. (F−J) Section of anthers at pollen mother, tetrad, uninucleate, binucleate, and mature pollen stages, respectively, hybridized with a BcMF2 sense probe as a negative control. (K) Section of anthers at the uninucleate pollen stage hybridized with a BcMF2 antisense probe showing no expression in ovules. (L) Section of anthers at the mature pollen stage hybridized with a BcMF2 antisense probe showing strong expression in ovules. (M) Section of anthers at the mature pollen stage hybridized with a BcMF2 sense probe as a control. Scale bars = 20 μm.
Inhibition of BcMF2 expression results in abnormal pollen tube growth and consequent reduction in male fertility
To investigate the exact role of BcMF2 in the development of pollen or anther, antisense RNA was used specifically to inhibit BcMF2 expression in Chinese cabbage. Antisense RNA expression vectors under the constitutive cauliflower mosaic virus (CaMV) 35S promoter were introduced to a normal fertile plant by the Agrobacterium-mediated method. After kanamycin screening, PCR, and Southern blotting analysis, a total of seven transformed lines (named bcmf2) were obtained. At the same time, control transformed plants were created with a pBI121 empty vector.
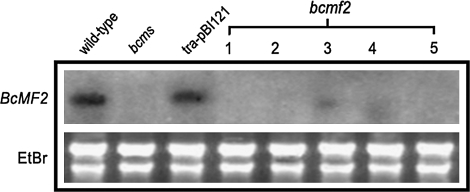
Northern blotting of five randomly selected transgenic plants from each transgenic line revealed that BcMF2 expression was fairly inhibited in the flower buds of transgenic plants. Three of the five transgenic lines had no expression signals in the flower buds; in the other two, a weak hybridization signal was detected, but it was significantly lower than expression levels in normal plants (Fig. 4). In contrast, a high expression level of BcMF2 was detected in the flower buds of the wild-type Chinese cabbage from which the gene was originally isolated. High levels were also observed in the empty vector-transformed plant, selected as control plants in this experiment. As expected, no hybridization signal was detected in the flower buds of the bcms mutant. These results verified that the antisense gene effectively inhibited gene expression.
Fig. 4.
Detection of the expression of BcMF2 in flower buds of antisense transgenic Chinese cabbage by northern blotting. bcms, bcms mutant of Chinese cabbage; tra-pBI121, transformed plants with an empty pBI121 vector; 1–5 refers to five different transgenic lines of bcmf2.
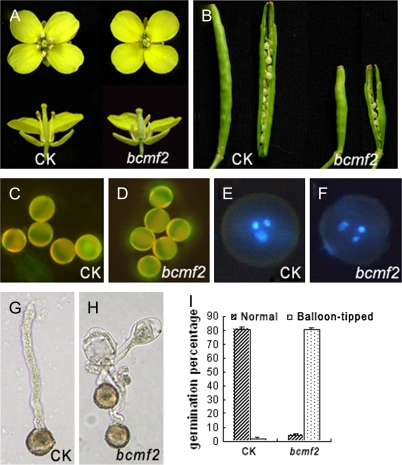
It was found that all the transgenic plants had normal vegetative growth; they flowered normally and had normal sepal, petal, gynoecium, nectary, and other floral organs. Seven bcmf2 plants from three transgenic lines were also compared with empty vector-transformed plants. The plants were shown by Northern blotting analysis to lack BcMF2 expression in flower buds and had shorter filaments and shrunken anthers, with a relatively small amount of pollen (Fig. 5A). Three bcmf2 plants from three different lines, all displaying this phenotype, were chosen for further investigation. To test the fertility of the transgenic plants, self-pollination tests were performed during the budding period. The transgenic plants were able to bear fruit pods when pollinated with enough pollen grains. However, comparing the transgenic self-pollinated fruit pods with those of the empty vector-transformed plants revealed size reductions of ∼50% in the transgenic plants. Each empty vector-transformed plant could produce a fruit pod with ∼15 seeds, whereas the fruit pods of transgenic plants could only produce between one and five seeds (Fig. 5B). A test-cross was then performed, pollinating transgenic plants with the normal pollen of an empty vector-transformed plant. The resulting fruit pod showed no difference in fruit size or seed number in comparison with the self-pollinated fruit pods of empty vector-transformed plants. These facts demonstrate that the female fertility and ovule development of these transgenic plants are normal, such that the reduction of fruit set rate should be attributed to the abnormality in male organs. Aniline blue staining showed that the callose in transgenic pollen was normal (Fig. 5C, D), while DAPI staining showed that transgenic pollen underwent normal karyokinesis (Fig. 5E, F). Furthermore, when enough transgenic pollen grains were collected and germinated in vitro, it was found that the germination rate reached 84%. This was similar to the 83% germination rate of the empty vector-transformed plant pollen (calculated after a 4 h incubation). It seemed that the transgenic pollen lacked any abnormality. At 6 h after incubation, the germination rate of transgenic pollen grains was still maintained at 84%. However, ∼80% of the pollen tubes formed abnormally; these pollen tubes exhibited a balloon-swelling structure in the tip after the tube had grown to a certain length. Only ∼4% of the transgenic pollen grains grew normal pollen tubes. Even for this segment of the population, the pollen tubes eventually stopped growing and exhibited inclusions of disorderly distribution (Fig. 5H, I). For the empty vector-transformed plants, the proportion of pollen germinated with normal tube growth reached 81%; balloon-tipped structures appeared at a rate of only ∼2% (Fig. 5G, I). To test further whether the pollen tube growth was normal in vivo, an attempt was made to observe pollen tube growth in the pistil after pollination. However, no high-quality images are available because the bulk of vascular bundles in the large, thick pistils of Chinese cabbages also stain with aniline blue, and this interfered with discrimination of pollen tubes. Nonetheless, the much lower fruit set rate for transgenic plants as compared with empty vector-transformed plants after self-fertilization may provide indirect but persuasive evidence for abnormality in the in vivo pollen tube elongation of these transgenic plants. Furthermore, the seeds harvested from transgenic plants were pollinated by pollen grains with normal pollen tube growth. In contrast, pollen tubes with a swelling at the tip could not reach the ovules.
Fig. 5.
Characterization of bcmf2 transgenic Chinese cabbage. (A) Top and side view of the flowers from empty vector-transformed plants (CK) and bcmf2 transgenic plants. (B) Siliques with seeds of empty vector-transformed plants (CK) and bcmf2 transgenic plants. (C and D) Aniline blue staining of the callose of pollen from empty vector-transformed plants (CK) and bcmf2 transgenic plants. (E and F) Staining of the nuclei of the pollen from empty vector-transformed plants (CK) and bcmf2 transgenic plants. (G and H) In vitro germination showed normal pollen tube growth of the empty vector-transformed plant (CK) and the balloon-tipped pollen tube of the bcmf2 transgenic plants. (I) Comparison of the percentage growth of the pollen tube between the empty vector-transformed plant (CK) and bcmf2 transgenic plants.
BcMF2 inhibition leads to pollen deformities with abnormal intine development
In order to determine the mechanism underlying abnormal pollen tube growth in bcmf2 transgenic plants, pollen morphology was investigated further with SEM. The pollen development process was examined using TEM. SEM showed that nearly 100% of the pollen from bcmf2 plants had deformities (Fig. 6A, B). Compared with the normal ovular shape of pollen grains, with three evenly distributed germinal furrows (Fig. 6C, D), the pollen grains of transgenic plants were irregular in shape with a disordered distribution of germinal furrows.
Fig. 6.
Scanning electron microscope of pollen from bcmf2 transgenic Chinese cabbage. (A and B) Deformities in pollen grains of the bcmf2 transgenic plants. (C and D) Normal pollen grains of empty vector-transformed plants (CK).
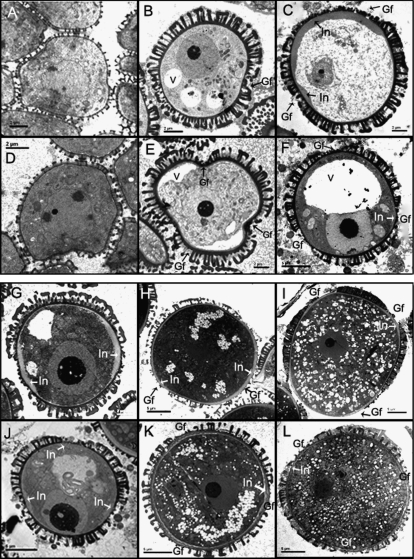
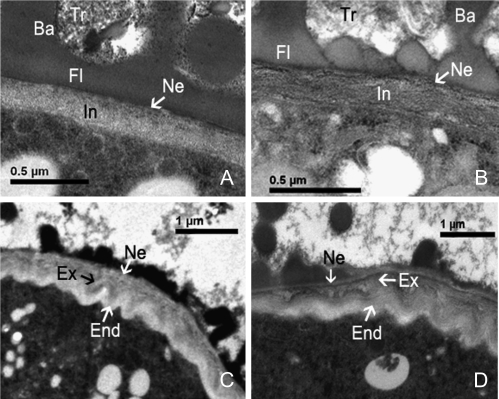
Pollen development initially seemed to be normal in the transgenic plants. The cell nuclei exhibited a normal division and differentiation process; the cell organelles formed normally; all of the distinct developmental stages including uninucleate, binucleate, and trinucleate could be observed (Fig. 7B, G, H). These observations supported previous results obtained by aniline blue staining and DAPI staining, showing normal callose metabolism and normal karyokinesis. However, close scrutiny revealed that the pollen shape was somewhat different from that of control pollen. This difference, probably due to abnormal germinal furrow formation, could be detected at the early uninucleate microspore stage. In normal control pollen, germinal furrow formation followed pollen wall formation. At the free microspore stage, the exine began to take shape, exhibiting clear tectum and baculum layers (Fig. 7D). At the uninucleate stage, the exine layers underwent further thickening; however, in the regions of the three prospective germinal furrows, no exine deposition occurred. Moreover, these regions were depressed towards the interior, leading to a cloverleaf pattern on the microspore (Fig. 7E). In the case of transgenic pollen, germinal furrows were indicated by a regional lack of exine, but the cloverleaf pattern was replaced by an irregular shape caused by the disordered distribution of germinal furrows (Fig. 7B). In late-stage uninucleate pollen, the outer and inner layers of intine began to form in control pollen, where the intine within the germinal furrows was much thicker than that in the other regions (Fig. 7F). At this stage, the intine of the transgenic pollen also began to form, but the germinal furrow's intine layer was thickened to a greater extent than in the control plants. Moreover, coverage was significantly increased in most pollen grains, creating the appearance of only one or two germination furrows. The germinal furrow frequently accounted for almost half of the pollen circumference in transgenic pollen (Fig. 7C). At the early binucleate pollen stage, intine within the germinal furrow of both the control (Fig. 7J) and transgenic pollen underwent further thickening. However, thickening was clearly becoming more extreme in the transgenic pollen (Fig. 7G). When pollen developed to the trinucleate and maturation stages, the intine grew further in both the control (Fig. 7K, L) and transgenic pollen (Fig. 7H, I). However, the relative proportion of exintine and endintine in the mature intine differed between the transgenic and control pollen, potentially misinterpreted as varying intine composition. Outside the germinal furrow region, the transgenic pollen had no obvious demarcation between the exintine and endintine layers. Notably, the homonymous granular exintine layer facing the exterior predominantly occupied the intine (Fig. 8A). In the same region of the control pollen, although the demarcation of exintine and endintine layers also was not clearly delineated, the intine appeared more microfibrillar, mainly occupied by the endintine layer (Fig. 8B). The demarcation was clear, however, in the region inside of the germinal furrow. A multilamellar intine within the germinal furrow was visible in both transgenic and control pollen: a granular exintine towards the exterior and a microfibrillar endintine towards the interior, beneath the nexine. However, in the transgenic pollen, the granular exintine overdeveloped to a thicker layer and occupied predominantly the intine layer. The internal microfibrillar layer occupied only a relatively small proportion of the total (Fig. 8C). In contrast, the internal microfibrillar endintine occupied a large proportion in control pollen, where the external granular exintine occupied a small proportion (Fig. 8D). Therefore, the abnormal thickening of the intine in transgenic plants was due to overaccumulation of the granular exintine. Exine of the transgenic pollen had relatively normal development, including the formation of tryphine at late pollen maturity stages. From these results, it is concluded that the deformity of the pollen grains detected by SEM might result from abnormal pollen wall development. The abnormal thickening of intine results in an imbalanced force upon the pollen wall, causing the twisted shape observed in transgenic pollen grains.
Fig. 7.
Transmission electron micrographs of pollen development of bcmf2 transgenic Chinese cabbage. (A) The free microspore of bcmf2 transgenic plants was normal. (B) Loss of the cloverleaf pattern of the microspore at the early uninucleate pollen stage in bcmf2 transgenic plants. (C) The great extent of the thickening in intine within the germinal furrow of bcmf2 transgenic plants at the late uninucleate pollen stage. (D–F) The free microspore, early-stage uninucleate microspores, and late-stage uninucleate pollen of empty vector-transformed plants. (G−I) The uneven distribution of the germinal furrow and an abnormal thickening of the intine observed in bcmf2 transgenic plants from binucleate to mature pollen stage. (J−L) Development of the microspore of empty vector-transformed plants from the binucleate to mature pollen stage showing three evenly distributed germinal furrows. Gf, germinal furrow; In, intine; V, vacuole.
Fig. 8.
Transmission electron micrographs of late intine formation in bcmf2 transgenic Chinese cabbage. (A) Mature intine outside of the germinal furrow region of a bcmf2 transgenic plant showing a homonymous granular exintine layer facing the exterior predominantly occupying the intine. (B) Mature intine outside of the germinal furrow region of empty vector-transformed plants showing more microfibrillar endintine facing the interior predominantly occupting the intine. (C) Mature intine inside of the germinal furrow region of a bcmf2 transgenic plant had a larger granular exintine layer and a small microfibrillar endintine layer. (D) Mature intine inside of the germinal furrow region of empty vector-transformed plants showed an apparent large proportion of the internal microfibrillar endintine and a small proportion of the external granular exintine. Ba, bacula; End, endintine; Ex, exintine; Fl, foot layer; In, intine; Ne, nexine; Tr, trythine.
Inhibition of BcMF2 expression results in early degradation of the anther tapetum
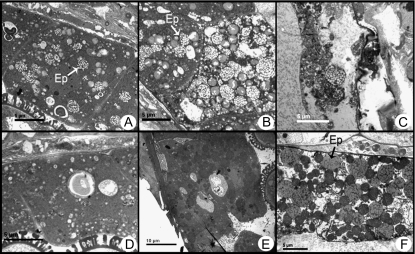
The anther tapetum development process in bcmf2 transgenic plants was also observed with TEM. No obvious defect in morphology or organellar distribution was detected, but the degradation process of tapetum seemed to be accelerated. Prior to the late uninucleate pollen stage, the tapetum development of the transgenic plants was not significantly different from that of the empty vector-transformed plant. However, for late-stage uninucleate pollen, it was observed that a large number of elaioplasts emerged in the anther tapetum of the transgenic plants, which forecast late degradation of tapetum (Fig. 9A). At the early binucleate pollen stage, the elaioplasts increased in number and were further enlarged (Fig. 9B). In late-stage binucleate and trinucleate pollen, the tapetum underwent serious degradation and fell off. The anther locule was full of material being shed at the last stage (Fig. 9C). However, in empty vector-transformed plants, a large amount of elaioplasts began to appear in the late-stage binucleate pollen. The tapetum degradation process was noticeably slower than in the transgenic plants (Fig. 9D, E). At the trinucleate pollen stage, the tapetum was still relatively complete (Fig. 9F).
Fig. 9.
Transmission electron microscopy of tapetum development of bcmf2 transgenic Chinese cabbage. (A) A large number of elaioplasts emerged in the bcmf2 tapetum at the late uninucleate stage. (B) At the early binucleate stage, the elaioplasts increased and enlarged further in the bcmf2 tapetum. (C) Tapetum in the bcmf2 transgenic plant begins to degenerate during the late binucleate stage. (D and E) A few elaioplast appeared in tapetum at the late uninucleate stage and the early binucleate stage of the empty vector-transformed plants. (F) At the trinucleate pollen stage, tapetum of the empty vector-transformed plants was still relatively complete. Ep, elaioplast.
Consistent with the morphological observations, as well as the self-pollination and test-cross tests which showed normal ovule function in the bcmf2 transgenic plants, there was no apparent phenotype at the ultrastructural level associated with inhibited expression of BcMF2 in the ovule.
Discussion
BcMF2 is a new PG gene that modulates intine development
The BcMF2 gene, previously isolated from Chinese cabbage, includes the typical PG sequence in its predicted protein sequence (Wang et al., 2005). The gene also shares high similarity with Arabidopsis PGA4 mRNA (At1g02790). PG is a hydrolase and a loosening enzyme that disintegrates the cell wall structure through the degradation of pectin. Most of the PGs are expressed in the late stages of pollen development (Robert et al., 1993; John et al., 1994; Tebbutt et al., 1994) and are considered to be necessary for pollen maturation and pollen tube growth (Allen et al., 1993; Honys and Twell, 2003; Pina et al., 2005). During pollen germination, hydrated pollen grains release many proteins anchored in the pollen wall, including PG. Thus, most previous studies concluded that PG in the pollen tube disintegrated the style cell wall, allowing the pollen tube to pass through the style. Alternatively, some studies concluded that PG acted on the pollen tube's own cell wall, and accelerated its growth amidst style tissue (Brown et al., 1990). However, to date, there is little direct evidence regarding the role of PG genes in the development of pollen or of pollen tubes, though many pollen-specific PG genes have been identified (Allen et al., 1993; Honys and Twell, 2003). One of the pollen-associated PG genes that has been well described is QRT3 in Arabidopsis. QRT3 is specifically and transiently expressed in the tapetum during the phase when microspores separate from their meiotic siblings. Mutation of QRT3 results in failed degradation of the mother pollen cell wall and subsequent tetrad separation. It is presumed that the QRT3 protein plays a direct and specific role in degradation of the pectic polysaccharides comprising the mother pollen cell wall (Rhee et al., 2003). Notably, molecular evolution analysis attributed BcMF2 protein to clade C, a clade comprised entirely of PG genes expressed in pollen, flower buds, or flowers. Expression pattern analysis showed that BcMF2 was expressed preferentially in the developing tapetum and microspores from the tetrad stage onward. These results suggest that BcMF2 may encode a new PG with a modulatory role in pollen development.
In order to study BcMF2’s function, antisense RNA expression vectors under the constitutive CaMV 35S promoter were introduced to a normal fertile plant. The activity of the CaMV 35S promoter in pollen and anthers has always been controversial. Great variability in CaMV 35S promoter activity is observed in comparisons between the pollen and anthers of tobacco and Arabidopsis (Wilkinson et al., 1997). However, this promoter is highly active in floral organs and in the pollen of transgenic strawberry plants (de Mesa et al., 2004). Furthermore, the CaMV 35S promoter has already been used as a positive control when analysing the activity of a tapetum-expressed promoter in B. napus (Robert and Hong, 1999). In the present study, placing the antisense RNA vector under the CaMV 35S promoter resulted in substantial gene inhibition in Chinese cabbage (B. campestris) flower buds. The construct did, however, show small differences in the level of target gene expression among various transgenic plants. This result was consistent with that of previous studies showing normal CaMV 35S promoter activity in the pollen and anthers of Chinese cabbage (Cao et al., 2006; Li et al., 2007; Zhang et al., 2008). Therefore, it is feasible to use the CaMV 35S promoter as a substitute when the gene's own promoter is not available in investigations of Chinese cabbage pollen or anther genes.
Self-fertilization and test-cross revealed that the transgenic bcmf2 plant with inhibited BcMF2 expression displayed substantially reduced male fertility, but normal female fertility. The most dramatic aberrations in bcmf2 transgenic plants (detected by SEM) were pollen deformities characterized by the disordered location of germinal furrows. TEM revealed that pollen grains underwent an abnormal thickening in the intine region due to the overdevelopment of the granular exintine. The external exintine layer occupied most of the intine, thus reversing the typical proportions of internal endintine and external exintine layers. Disordered intine distribution is also found in Arabidopsis ms33 mutants, although the pattern is reversed. In ms33 pollen, the endintine is much thicker than the exintine at the bicellular stage. Intine formation occurs prematurely, affecting pollen grain desiccation and therefore pollen viability (Fei and Sawhney, 2001). These differences in the phenotypes observed for the ms33 mutant and the bcmf2 plant show that BcMF2 and MS33 have different activity patterns in the context of intine formation. In general, the granular exintine in intine is composed of pectin and various proteins, while the microfibrillar endintine consists of cellulose. The BcMF2-encoded PG, a hydrolase enzyme active in the degradation of pectin, may be necessary for pectin's dynamic metabolism. The abnormal thickening of the exintine, filled with pectin, that follows inhibition of BcMF2 expression may result from a decline in PG activity and subsequent abnormalities in pectin's dynamic metabolism in the intine.
It has been demonstrated that germinal furrow formation is accompanied by development of intine. Within the germinal furrow, there is a layer of tissue connecting the aperture foramen and intine, called the zwischenkorpe layer (El-Ghazaly and Jelsen, 1987; Marquez et al., 1997). This term actually denotes the outer intine. Thus, the disorder in bcmf2 germinal furrows may be a consequence of abnormal intine formation. Abnormal thickening of intine might not adequately balance the force in the pollen wall and consequently result in pollen grains with a twisted shape.
Pollen tube arrest in antisense BcMF2 transgenic plants may result from confused pectin metabolism
During in vitro pollen germination, most of the pollen tubes in bcmf2 transgenic plants formed a balloon-shaped swelling structure at the tip after growing to a certain length, then halted growth. Experiments have been conducted to try to determine if there was any connection between intine formation and pollen tube elongation. Significantly, the pollen tube cell wall is a continuation of the intine layer. Not long after pollen grains contact water, the exine (outer layer of the pollen wall) abruptly breaks after pressure accumulation due to expansion of the intine. The intine swells after being released from the exine; in optimal conditions, the germinating pollen cell will emerge from one end of the pollen grain and the extending pollen tube then travels through the appropriate hole (the germinal furrow) of the intine. As the pollen tube emerges, the intine layer becomes continuous with the cell wall of the tube (Stone et al., 2004). It is widely accepted that pollen tubes grow by tip extension, with highly active cytoplasmic streaming transporting vesicles and organelles towards the tip; these vesicles and organelles then deposit cell wall components. Recent research has also revealed the highly anisotropic growth behaviour of the cell wall of angiosperm pollen tubes (Taylor and Hepler, 1997; Geitmann, 1999). At least two regions exist in the axial direction, the approximately hemispherical growing tip and the cylindrical shank. The tip region is generally characterized by a single wall layer (Lancelle and Hepler, 1992) and is known to be composed mainly of newly synthesized methyl esterified pectins (Geitmann et al., 1995). This pectin layer can continue along the entire length of the pollen tube and forms the outer layer of the cell wall in the pollen tube shank, where a secondary wall layer is generally formed adjacent to the plasma membrane. Turgor is believed to be the primary motive force behind tip growth, and the pectin layer has also been demonstrated to play an important role in the process (Geitmann and Steer, 2006).
Pollen tube growth is a dynamic complex system, and a balloon-swelling structure in the tip can exert various influences, for example the transition from anisotropic to isotropic growth. In bcmf2 plants, pollen tube elongation was interrupted after the tube grew to a certain length. Therefore, it seems that the initial segment of the pollen tube remains relatively normal. However, elongation did not proceed normally and ultimately resulted in halted growth. The balloon-shaped swelling tip in bcmf2 did not continue to expand indefinitely at the apex after it was shaped. This highlights the significant departure of this transgenic phenotype from the typical trajectory of pollen tube growth. Here, a possible explanation for this phenotype is proposed. The primary inner turgor behind tip growth may encounter the suddenly immobilized growth wall, forcing formation of a balloon-shaped swelling structure in the tip, similar to the blowing of a bubble. The bubble does not burst, possibly due to the thickened intine in the bcmf2 pollen tube tip. In this hypothetical model, BcMF2 encodes a PG necessary for pectin's dynamic metabolism in the pollen tube. Inhibiting protein expression may cause disordered pectin metabolism, beginning at an early stage of intine development and extending to pollen tube wall formation. As the initial pollen tube wall is continuous with the pollen grain intine, the enzymatic activity localized within the intine is incorporated into the lateral tube walls (Edlund et al., 2004). Alternatively, it has been widely shown that genes encoding proteins related to cell wall biosynthesis and regulation are highly expressed in pollen (Becker et al., 2003; Honys and Twell, 2003). It is therefore not surprising that the BcMF2 gene was expressed early in pollen development. However, at present, the mechanism underlying BcMF2 gene involvement in pectin metabolism cannot be explained. To resolve this puzzle, more experimental evidence is needed.
The bcmf2 phenotype may suggest a link between tapetum and intine development
Another impressive phenotype resulting from the inhibition of BcMF2 expression was premature tapetum degradation. The exine formed from material released by the tapetum demonstrates a direct correlation of tapetum development with exine formation. Currently, there are a number of tapetum-expressed genes that are known to affect exine development. For example, mutating MS2, which codes for fatty acid reductase, prevents exine formation (Aarts et al., 1997). There are relatively few reports on an association between tapetum and intine development. In contrast to exine, intine is secreted from the microspore at the vacuolation stage and is produced by gamete differentiation. However, two mutants defective in intine formation, ms33 and ms1, were also found to be defective in tapetum degeneration. As mentioned above, ms33 mutants displayed early intine formation as well as early tapetum degeneration (Fei and Sawhney, 2001). In the ms1 mutant, reduced intine was accompanied by abnormal tapetum development (Vizcay-Barrena and Wilson, 2006). It should be noted that, in these mutants, both intine formation and exine development were affected, leading the authors of both studies to speculate that abnormal exine development may lead to disordered intine. When the pollen grain undergoes dehydration, intine must adapt to the particular stage of exine development, with consequent pollen grain volume changes, despite independent exine and intine development (Shukla et al., 1998). Moreover, exine development depends on the accumulation of materials secreted by the tapetum. Therefore, tapetum development affected the development of intine by influencing the formation of the outer wall (Fei et al., 2001). However, exine abnormalities have not been observed in bcmf2 pollen. Thus, the relationship between tapetum development and intine cannot be entirely explained by abnormal exine development that leads to disordered intine. A distinct link between the development of tapetum and intine is therefore proposed. However, further experiments need to be conducted to elucidate the underlying mechanism.
Pollen-associated genes such as BcMF2 may also be expressed in the embryo
In situ hybridization analysis showed expression of BcMF2 in the ovules of flower buds in later stages of development. However, inhibited expression of BcMF2 did not affect ovule development or ovule function. Interestingly, it was reported that QRT3, the PG gene in Arabidopsis influencing degradation of pectic polysaccharides in the pollen mother cell wall, also showed strong expression in the ovules of open flowers. However, there is no apparent phenotype associated with the qrt3 mutation in the ovules either at the histological level or in terms of ovule function (Rhee et al., 2003). In addition, both RT-PCR and Northern blotting results showed that the expression of BcMF2 continued in siliques after fertilization. Interestingly, no expression signal was detected in siliques from the bcms mutant. It is noteworthy, however, that this expression pattern was not unique for BcMF2. When the spatial and temporal expression patterns of 20 specifically or predominantly accumulated transcripts in wild-type flower buds compared with bcms mutants were investigated, it was found that 12 of them showed expression in wild-type siliques but not in those of bcms mutants (Huang et al., 2008). As a matter of fact, siliques harvested from wild-type and bcms mutant plants were not identical. Following pollen abortion, no fertilization of ovules in bcms siliques occurred. However, siliques from wild-type plants were filled with fertilized ovules after self-pollination. Otherwise, the ovules in the bcms mutants were normal, demonstrated by crossing the bcms mutant with its wild-type ancestor. Based on all of these results, it is presumed that BcMF2 may not be expressed in the ovule but rather in the embryo.
The old Chinese cabbage flower buds which were collected in this research corresponded to the anther dehiscence and pollen dispersal stage. Hence, it is likely that the gynoecium had been pollinated. If this were true, it would be expected that BcMF2 inhibition would not affect ovule function in the transgenic plants. Similarly, it is proposed that QRT3 is expressed in the embryo but not in the ovule. The characteristic self-pollination in Arabidopsis may lead to fertilization in the open flower. Furthermore, this pattern of expression exists not only in the PG gene family but also in many other genes associated with pollen development. Two possible explanations for why these male reproductive organ-expressed genes appeared again in the embryo were proposed. First, these genes may be essential for development after the zygotic stage. Secondly, the phenomenon may be due to paternal genomic imprinting in the plant. Further investigation is required to address the question conclusively.
Supplementary data
Supplementary data are available at JXB online.
Acknowledgments
This work was supported by the Chinese Natural Science Foundation (no. 30671426) and the Chinese National Project of Research and Development for High Technology (no. 2006AA100108).
Glossary
Abbreviations
- BcMF2
Brassica campestris Male Fertility 2
- bcms
Brassica campestris male sterile
- cDNA-AFLP
cDNA-amplified fragment length polymorphism
- DAPI, 4′
6-diamidino-2-phenylindole
- PG
polygalacturonase
- RT-PCR
reverse transcription-PCR, SEM, scanning electron microscopy
- TEM
transmission electron microscopy
References
- Aarts MG, Hodge R, Kalantidis K, Florack D, Wilson ZA, Mulligan BJ, Stiekema WJ, Scott R, Pereira A. The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. The Plant Journal. 1997;12:615–623. doi: 10.1046/j.1365-313x.1997.00615.x. [DOI] [PubMed] [Google Scholar]
- Allen RL, Lonsdale DM. Molecular characterization of one of the maize polygalacturonase gene family members which are expressed during late pollen development. The Plant Journal. 1993;3:261–271. doi: 10.1111/j.1365-313x.1993.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Hatakeyama K, Hinata K, Sato S, Kato T, Tabata S, Toriyama K. A novel male-sterile mutant of Arabidopsis thaliana, faceless pollen-1, produces pollen with a smooth surface and an acetolysis-sensitive exine. Plant Molecular Biology. 2003;53:107–116. doi: 10.1023/B:PLAN.0000009269.97773.70. [DOI] [PubMed] [Google Scholar]
- Athanasiou A, Khosravi D, Tamari F, Shore JS. Characterization and localization of short-specific polygalacturonase in distylous Turnera subulata (Turneraceae) American Journal of Botany. 2003;90:675–682. doi: 10.3732/ajb.90.5.675. [DOI] [PubMed] [Google Scholar]
- Becker J, Boavida LC, Carneiro J, Haury M, Feijó JA. Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiology. 2003;133:713–725. doi: 10.1104/pp.103.028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore S, Barnes SH. Pollen wall development in angiosperms. In: Blackmore S, Knox RB, editors. Microspores evolution and ontogeny. San Diego: Academic Press; 1990. pp. 173–192. [Google Scholar]
- Brown SM, Crouch ML. Characterization of a gene family abundantly expressed in Oenothera organensis pollen that shows sequence similarity to polygalacturonase. The Plant Cell. 1990;2:263–274. doi: 10.1105/tpc.2.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J-S, Cao S-C, Yi Q-M. RAPD analysis on genomic DNA of Chinese cabbage and the other groups of Brassica. Acta Horticulture Sinica. 1995;22:47–52. [Google Scholar]
- Cao J, Yu X, Ye W, Lu G, Xiang X. Functional analysis of a novel male fertility CYP86MF gene in Chinese cabbage (Brassica campestris L. ssp. chinensis Makino) Plant Cell Reports. 2006;24:715–723. doi: 10.1007/s00299-005-0020-6. [DOI] [PubMed] [Google Scholar]
- Cho HT, Kende H. Expression of expansin genes is correlated with growth in deepwater rice. The Plant Cell. 1997;9:1661–1671. doi: 10.1105/tpc.9.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mesa MC, Santiago-Doménech N, Pliego-Alfaro F, Quesada MA, Mercado JA. The CaMV 35S promoter is highly active on floral organs and pollen of transgenic strawberry plants. Plant Cell Reports. 2004;23:32–38. doi: 10.1007/s00299-004-0776-0. [DOI] [PubMed] [Google Scholar]
- Edlund AF, Swanson R, Preuss D. Pollen and stigma structure and function: the role of diversity in pollination. The Plant Cell. 2004;16:S84–S97. doi: 10.1105/tpc.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghazaly G, Jensen WA. Development of wheat (Triticum aestivum) pollen. II. Histochemical differentiation of wall and ubisch bodies during development. American Journal of Botany. 1987;74:1396–1418. [Google Scholar]
- Fei HM, Sawhney VK. Ultrastructural characterization of male sterile33 (ms33) mutant in Arabidopsis affected in pollen desiccation and maturation. Canadian Journal of Botany. 2001;79:118–129. [Google Scholar]
- Geitmann A. The rheological properties of the pollen tube cell wall. In: Cresti M, Cai G, Moscatelli A, editors. Fertilization in higher plants: molecular and cytological aspects. Berlin: Springer-Verlag; 1999. pp. 283–302. [Google Scholar]
- Geitmann A, Li YQ, Cresti M. Ultrastructural immunolocalization of periodic pectin depositions in the cell wall of Nicotiana tabacum pollen tubes. Protoplasma. 1995;187:168–171. [Google Scholar]
- Geitmann A, Steer M. The architecture and properties of the pollen tube cell wall. In: Malho R, editor. The pollen tube, plant cell monographs. Berlin: Springer-Verlag; 2006. pp. 177–200. [Google Scholar]
- Hadfield KA, Rose JKC, Yaver DS, Berka RM, Bennett AB. Polygalacturonase gene expression in ripe melon fruit supports a role for polygalacturonase in ripening-associated pectin disassembly. Plant Physiology. 1998;117:363–373. doi: 10.1104/pp.117.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D. Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiology. 2003;132:640–652. doi: 10.1104/pp.103.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Cao J, Ye W, Liu T, Jiang L, Ye Y. Transcriptional differences between the male-sterile mutant bcms and wild-type Brassica campestris ssp.chinensis reveal genes related to pollen development. Plant Biology. 2008;10:342–355. doi: 10.1111/j.1438-8677.2008.00039.x. [DOI] [PubMed] [Google Scholar]
- John ME, Petersen MW. Cotton (Gossypium hirsutum L.) pollen-specific polygalacturonase mRNA: tissue and temporal specificity of its promoter in transgenic tobacco. Plant Molecular Biology. 1994;26:1989–1993. doi: 10.1007/BF00019509. [DOI] [PubMed] [Google Scholar]
- Lancelle SA, Hepler PK. Ultrastructure of freeze-substituted pollen tubes of Lilium longiflorum. Protoplasma. 1992;167:215–230. [Google Scholar]
- Li Y, Cao J, Huang L, Yu X, Xiang X. BcMF13, a new reproductive organ-specific gene from Brassica campestris ssp. chinensis, affects pollen development. Molecular Biology Reports. 2008;35:207–214. doi: 10.1007/s11033-007-9072-8. [DOI] [PubMed] [Google Scholar]
- Markovič O, Janeček S. Pectin degrading glycoside hydrolases of family 28: sequence-structural features, specificities and evolution. Protein Engineering Design and Selection. 2001;14:615–631. doi: 10.1093/protein/14.9.615. [DOI] [PubMed] [Google Scholar]
- Marquez J, Seoane-Camba JA, Suarez-Cervera M. Allergenic and antigenic proteins released in the apertural sporoderm during the activation process in grass pollen grains. Sexual Plant Reproduction. 1997;10:269–278. [Google Scholar]
- Owen HA, Makaroff CA. Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. ecotype Wassilewskija (Brassicaceae) Protoplasma. 1995;185:7–21. [Google Scholar]
- Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA. DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiology. 2001;127:1739–1749. [PMC free article] [PubMed] [Google Scholar]
- Paxson-Sowders DM, Owen HA, Makaroff CA. A comparative ultrastructural analysis of exine pattern development in wild type Arabidopsis and a mutant defective in pattern formation. Protoplasma. 1997;198:53–65. [Google Scholar]
- Pina C, Pinto F, Feijo J, Becker J. Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiology. 2005;138:744–756. doi: 10.1104/pp.104.057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan SM, Moffatt BA. Cytochemical analysis of pollen development in wild-type Arabidopsis and a male sterile mutant. The Plant Cell. 1990;2:877–889. doi: 10.1105/tpc.2.9.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SY, Osborne E, Poindexter PD, Somerville CR. Microspore separation in the quartet 3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother cell wall degradation. Plant Physiology. 2003;133:1170–1180. doi: 10.1104/pp.103.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert L, Hong HP. Brassica sp. gene promoter highly expressed during tapetum development. 1999 United States Patent 5919919. [Google Scholar]
- Robert LS, Allard S, Gerster JL, Cass L, Simmonds J. Isolation and characterization of a polygalacturonase gene highly expressed in Brassica napus pollen. Plant Molecular Biology. 1993;23:1273–1278. doi: 10.1007/BF00042360. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Vijayaraghavan MR, Chaudhry B. Biology of pollen. New Delhi, India: APH Publishing Corporation; 1998. [Google Scholar]
- Stone LM, Seaton KA, Kuo J, McComb JA. Fast pollen tube growth in Conospermum species. Annals of Botany. 2004;93:369–378. doi: 10.1093/aob/mch050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LP, Hepler PK. Pollen germination and tube growth. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:461–491. doi: 10.1146/annurev.arplant.48.1.461. [DOI] [PubMed] [Google Scholar]
- Tebbutt SJ, Rogers HJ, Lonsdale DM. Characterization of a tobacco gene encoding a pollen-specific polygalacturonase. Plant Molecular Biology. 1994;25:283–297. doi: 10.1007/BF00023244. [DOI] [PubMed] [Google Scholar]
- Torki M, Mandaron P, Mache R, Falconet D. Characterization of a ubiquitous expressed gene family encoding polygalacturonase in Arabidopsis thaliana. Gene. 2000;242:427–436. doi: 10.1016/s0378-1119(99)00497-7. [DOI] [PubMed] [Google Scholar]
- Vizcay-Barrena G, Wilson ZA. Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. Journal of Experimental Botany. 2006;57:2709–2717. doi: 10.1093/jxb/erl032. [DOI] [PubMed] [Google Scholar]
- Wang Y-Q, Ye W-Z, Cao J-S, Yu X-L, Xiang X, Lu G. Cloning and characterization of the microspore development related gene BcMF2 in Chinese cabbage pak-choi (Brassica campestris L. ssp. chinensis Makino) Journal of Integrative Plant Biology. 2005;47:863–872. [Google Scholar]
- Wilkinson JE, Twell D, Lindsey K. Activities of CaMV 35S and nos promoters in pollen: implications for field release of transgenic plants. Journal of Experimental Botany. 1997;48:265–275. [Google Scholar]
- Yu X, Cao J, Ye W, Wang Y. Construction of an antisense CYP86MF gene plasmid vector and production of a male-sterile Chinese cabbage transformant by the pollen-tube method. Journal of Horticulture and Biotechnology. 2004;79:833–839. [Google Scholar]
- Zhang Q, Cao J, Huang L, Xiang X, Yu X. Characterization and functional analysis of a novel PCP gene BcMF5 from Chinese cabbage (Brassica campestris L. ssp. chinensis Makino) Journal of Plant Physiology. 2008;165:445–455. doi: 10.1016/j.jplph.2007.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.