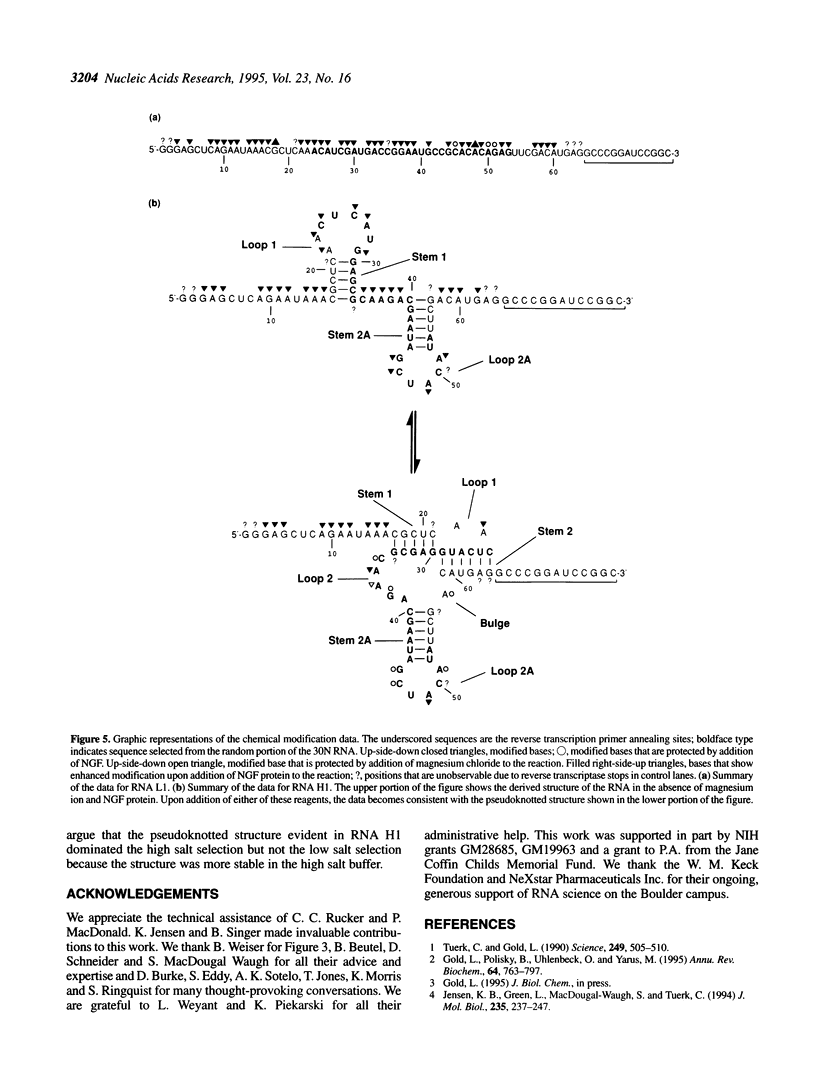
Abstract
High affinity RNA ligands to human nerve growth factor (NGF) were selected from pools of random RNA using SELEX [Tuerk, C. and Gold, L. (1990) Science, 249, 505-510]. Nerve growth factor, which is a protein required for the development of neurons, is not known to bind nucleic acids as part of its natural function. We describe two of the selected RNA molecules in detail. One of them is highly structured, folding into a pseudoknot with an additional hairpin-loop; this structure provides salt-resistant binding to NGF. The other is unstructured and elevated salt concentrations inhibit its binding. These molecules compete with each other for NGF binding. Our RNAs may furnish useful diagnostic tools for the study of an important neurotrophic protein; additionally, they illustrate another example of the potential for nucleic acids to take part in novel binding interactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. N., Noller H. F. Mutations in ribosomal proteins S4 and S12 influence the higher order structure of 16 S ribosomal RNA. J Mol Biol. 1989 Aug 5;208(3):457–468. doi: 10.1016/0022-2836(89)90509-3. [DOI] [PubMed] [Google Scholar]
- Allen P., Worland S., Gold L. Isolation of high-affinity RNA ligands to HIV-1 integrase from a random pool. Virology. 1995 Jun 1;209(2):327–336. doi: 10.1006/viro.1995.1264. [DOI] [PubMed] [Google Scholar]
- Bear D. G., Schleich T., Noller H. F., Garrett R. A. Alteration of 5S RNA conformation by ribosomal proteins L18 and L25. Nucleic Acids Res. 1977 Jul;4(7):2511–2526. doi: 10.1093/nar/4.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock L. C., Griffin L. C., Latham J. A., Vermaas E. H., Toole J. J. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature. 1992 Feb 6;355(6360):564–566. doi: 10.1038/355564a0. [DOI] [PubMed] [Google Scholar]
- Chen H., Gold L. Selection of high-affinity RNA ligands to reverse transcriptase: inhibition of cDNA synthesis and RNase H activity. Biochemistry. 1994 Jul 26;33(29):8746–8756. doi: 10.1021/bi00195a016. [DOI] [PubMed] [Google Scholar]
- Connell G. J., Yarus M. RNAs with dual specificity and dual RNAs with similar specificity. Science. 1994 May 20;264(5162):1137–1141. doi: 10.1126/science.7513905. [DOI] [PubMed] [Google Scholar]
- Ellington A. D., Szostak J. W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990 Aug 30;346(6287):818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- Gold L., Polisky B., Uhlenbeck O., Yarus M. Diversity of oligonucleotide functions. Annu Rev Biochem. 1995;64:763–797. doi: 10.1146/annurev.bi.64.070195.003555. [DOI] [PubMed] [Google Scholar]
- Hertz G. Z., Hartzell G. W., 3rd, Stormo G. D. Identification of consensus patterns in unaligned DNA sequences known to be functionally related. Comput Appl Biosci. 1990 Apr;6(2):81–92. doi: 10.1093/bioinformatics/6.2.81. [DOI] [PubMed] [Google Scholar]
- Illangasekare M., Sanchez G., Nickles T., Yarus M. Aminoacyl-RNA synthesis catalyzed by an RNA. Science. 1995 Feb 3;267(5198):643–647. doi: 10.1126/science.7530860. [DOI] [PubMed] [Google Scholar]
- Irvine D., Tuerk C., Gold L. SELEXION. Systematic evolution of ligands by exponential enrichment with integrated optimization by non-linear analysis. J Mol Biol. 1991 Dec 5;222(3):739–761. doi: 10.1016/0022-2836(91)90509-5. [DOI] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Improved predictions of secondary structures for RNA. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7706–7710. doi: 10.1073/pnas.86.20.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Predicting optimal and suboptimal secondary structure for RNA. Methods Enzymol. 1990;183:281–306. doi: 10.1016/0076-6879(90)83019-6. [DOI] [PubMed] [Google Scholar]
- Jenison R. D., Gill S. C., Pardi A., Polisky B. High-resolution molecular discrimination by RNA. Science. 1994 Mar 11;263(5152):1425–1429. doi: 10.1126/science.7510417. [DOI] [PubMed] [Google Scholar]
- Jensen K. B., Green L., MacDougal-Waugh S., Tuerk C. Characterization of an in vitro-selected RNA ligand to the HIV-1 Rev protein. J Mol Biol. 1994 Jan 7;235(1):237–247. doi: 10.1016/s0022-2836(05)80030-0. [DOI] [PubMed] [Google Scholar]
- Kubik M. F., Stephens A. W., Schneider D., Marlar R. A., Tasset D. High-affinity RNA ligands to human alpha-thrombin. Nucleic Acids Res. 1994 Jul 11;22(13):2619–2626. doi: 10.1093/nar/22.13.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauhon C. T., Szostak J. W. RNA aptamers that bind flavin and nicotinamide redox cofactors. J Am Chem Soc. 1995 Feb 1;117(4):1246–1257. doi: 10.1021/ja00109a008. [DOI] [PubMed] [Google Scholar]
- Lehman N., Joyce G. F. Evolution in vitro of an RNA enzyme with altered metal dependence. Nature. 1993 Jan 14;361(6408):182–185. doi: 10.1038/361182a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987 Sep 4;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Levi A., Alemà S. The mechanism of action of nerve growth factor. Annu Rev Pharmacol Toxicol. 1991;31:205–228. doi: 10.1146/annurev.pa.31.040191.001225. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac repressor binding to non-operator DNA: detailed studies and a comparison of eequilibrium and rate competition methods. J Mol Biol. 1972 Dec 30;72(3):671–690. doi: 10.1016/0022-2836(72)90184-2. [DOI] [PubMed] [Google Scholar]
- Lin Y., Qiu Q., Gill S. C., Jayasena S. D. Modified RNA sequence pools for in vitro selection. Nucleic Acids Res. 1994 Dec 11;22(24):5229–5234. doi: 10.1093/nar/22.24.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsch J. R., Szostak J. W. In vitro evolution of new ribozymes with polynucleotide kinase activity. Nature. 1994 Sep 1;371(6492):31–36. doi: 10.1038/371031a0. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Nieuwlandt D., Wecker M., Gold L. In vitro selection of RNA ligands to substance P. Biochemistry. 1995 Apr 25;34(16):5651–5659. doi: 10.1021/bi00016a041. [DOI] [PubMed] [Google Scholar]
- Pleij C. W., Rietveld K., Bosch L. A new principle of RNA folding based on pseudoknotting. Nucleic Acids Res. 1985 Mar 11;13(5):1717–1731. doi: 10.1093/nar/13.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi J. D., Wyatt J. R., Tinoco I., Jr Conformation of an RNA pseudoknot. J Mol Biol. 1990 Jul 20;214(2):437–453. doi: 10.1016/0022-2836(90)90192-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Schneider D., Gold L., Platt T. Selective enrichment of RNA species for tight binding to Escherichia coli rho factor. FASEB J. 1993 Jan;7(1):201–207. doi: 10.1096/fasebj.7.1.7678562. [DOI] [PubMed] [Google Scholar]
- Schneider D., Tuerk C., Gold L. Selection of high affinity RNA ligands to the bacteriophage R17 coat protein. J Mol Biol. 1992 Dec 5;228(3):862–869. doi: 10.1016/0022-2836(92)90870-p. [DOI] [PubMed] [Google Scholar]
- Stein A., Crothers D. M. Conformational changes of transfer RNA. The role of magnesium(II). Biochemistry. 1976 Jan 13;15(1):160–168. doi: 10.1021/bi00646a025. [DOI] [PubMed] [Google Scholar]
- Stern S., Moazed D., Noller H. F. Structural analysis of RNA using chemical and enzymatic probing monitored by primer extension. Methods Enzymol. 1988;164:481–489. doi: 10.1016/s0076-6879(88)64064-x. [DOI] [PubMed] [Google Scholar]
- Stern S., Powers T., Changchien L. M., Noller H. F. RNA-protein interactions in 30S ribosomal subunits: folding and function of 16S rRNA. Science. 1989 May 19;244(4906):783–790. doi: 10.1126/science.2658053. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Hartzell G. W., 3rd Identifying protein-binding sites from unaligned DNA fragments. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1183–1187. doi: 10.1073/pnas.86.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk C., Eddy S., Parma D., Gold L. Autogenous translational operator recognized by bacteriophage T4 DNA polymerase. J Mol Biol. 1990 Jun 20;213(4):749–761. doi: 10.1016/S0022-2836(05)80261-X. [DOI] [PubMed] [Google Scholar]
- Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990 Aug 3;249(4968):505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- Tuerk C., MacDougal-Waugh S. In vitro evolution of functional nucleic acids: high-affinity RNA ligands of HIV-1 proteins. Gene. 1993 Dec 27;137(1):33–39. doi: 10.1016/0378-1119(93)90248-2. [DOI] [PubMed] [Google Scholar]
- Tuerk C., MacDougal S., Gold L. RNA pseudoknots that inhibit human immunodeficiency virus type 1 reverse transcriptase. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6988–6992. doi: 10.1073/pnas.89.15.6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Berman C., Dull T. J. Human beta-nerve growth factor gene sequence highly homologous to that of mouse. Nature. 1983 Jun 30;303(5920):821–825. doi: 10.1038/303821a0. [DOI] [PubMed] [Google Scholar]
- Unnithan S., Green L., Morrissey L., Binkley J., Singer B., Karam J., Gold L. Binding of the bacteriophage T4 regA protein to mRNA targets: an initiator AUG is required. Nucleic Acids Res. 1990 Dec 11;18(23):7083–7092. doi: 10.1093/nar/18.23.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J. R., Puglisi J. D., Tinoco I., Jr RNA pseudoknots. Stability and loop size requirements. J Mol Biol. 1990 Jul 20;214(2):455–470. doi: 10.1016/0022-2836(90)90193-P. [DOI] [PubMed] [Google Scholar]
- Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989 Apr 7;244(4900):48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]
- van Belkum A., Verlaan P., Kun J. B., Pleij C., Bosch L. Temperature dependent chemical and enzymatic probing of the tRNA-like structure of TYMV RNA. Nucleic Acids Res. 1988 Mar 25;16(5):1931–1950. doi: 10.1093/nar/16.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]