Abstract
Background
Alcohol consumption is associated with oxidative stress in multiple tissues in vivo, yet the effect of chronic alcohol intake on intestinal redox state has received little attention. In this study, we investigated the redox status of 2 major intracellular redox regulating couples: glutathione (GSH)/glutathione disulfide (GSSG) and cysteine (Cys)/cystine (CySS) in a rat model of chronic alcohol ingestion.
Methods
Sprague-Dawley rats were fed the liquid Lieber-DeCarli diet consisting of 36% ethanol of total calories for 6 weeks. Control rats were pair-fed with an isocaloric, ethanol-free liquid diet. Defined mucosal samples from the jejunum, ileum, and colon were obtained and analyzed by high-performance liquid chromatography (HPLC) for GSH and Cys pool redox status. Mucosal free malondialdehyde (MDA) was measured as an indicator of lipid peroxidation.
Results
In the ethanol-fed rats, Cys and mixed disulfide (GSH-Cys) were significantly decreased in all 3 segments of intestinal mucosa. Free MDA was increased in jejunal but not in ileal or colonic mucosa. Chronic ethanol ingestion significantly increased mucosal GSH concentration in association with a more reducing GSH/GSSG redox potential in the jejunum, but these indices were unchanged in the ileum. In the colon, chronic ethanol ingestion increased oxidant stress as suggested by decreased GSH and oxidized GSH/GSSG redox potential.
Conclusions
Chronic alcohol intake differentially alters the mucosal redox status in proximal to distal intestinal segments in rats. Such changes may reflect different adaptability of these intestinal segments to the oxidative stress challenge induced by chronic ethanol ingestion.
Keywords: alcohol, intestine, redox, GSH, Cys
Animal and clinical studies on alcohol abuse, either acute or chronic, have revealed oxidative stress in tissues such as the lung,1 heart,2 pancreas,3,4 liver,5,6 plasma and brain,7 and testis.8 This is evidenced by decreased antioxidants, such as glutathione (GSH), disordered antioxidant enzyme systems, and increased lipid peroxidation products, such as malondialdehyde (MDA) and 4-hydroxynonenal. However, GSH and peroxidation products in the stomach and intestine exhibit diverse changes after alcohol abuse, depending upon the concentration and frequency of alcohol ingested. In a rat model, acute ethanol ingestion at a dose of 1 g/kg increased GSH levels in the gastric and intestinal mucosa, whereas a higher dose of 4 g/kg decreased GSH.3 Moghadasian et al observed that acute gastric gavage with 1 mL of 8% ethanol increased GSH levels in rat gastric mucosa, whereas acute lavage with absolute ethanol significantly decreased GSH concentration in gastric mucosa.9
The mechanisms for the ethanol-induced increase in GSH are unknown but have been attributed to an adaptive protective response against alcohol-induced oxidative stress. One potential mechanism is through the rate-limiting enzyme glutamate cysteine ligase, a process induced by oxidant stimulation in cells10 and the small intestine in vivo.11 Activities of GSH-related enzymes (glutathione reductase, peroxidase, and superoxide dismutase) are also modified by alcohol.11 The notion of adaptive tissue response in rats acutely treated with alcohol is further supported by the report that prior acute application of 8% ethanol to the canine stomach attenuated gastric damage in response to a second treatment with 40% ethanol.12 However, questions have been raised as to how long this adaptation lasts and whether it is continuous in the gastrointestinal tract of animals receiving chronic alcohol intake. In rats maintained on an alcohol diet for 5 weeks, Kaur el al found that ethanol-treated groups were more susceptible to iron-induced lipid peroxidation and had increased nonprotein thiol concentrations in the small intestine when compared with rats on a normal control diet.13,14 This suggested that chronic ethanol ingestion resulted in oxidant stress in the small intestine and increased the risk of injury in response to a second insult.
In addition to GSH, the low molecular thiol cysteine (Cys) provides additional cellular antioxidant defense against oxidative stress. As a precursor for GSH synthesis, cellular Cys concentrations are closely linked metabolically with changes in GSH concentrations. Additionally, it was reported that upon oxidant stimulation in human fibroblasts, cellular GSH increased through increased Cys uptake via the Xc− transporter system.15
Most ingested alcohol is absorbed in the stomach and duodenum. However, absorbed alcohol is carried by blood to the more distal intestine and may exert differential effects in proximal vs distal gut segments. Previous studies found that the ethanol metabolite acetaldehyde, a pro-oxidant, accumulated in the colonic lumen of chronic ethanol-fed rats.16 This acetaldehyde accumulation was due to the alcohol dehydrogenase activity of colonic bacteria and low endogenous activity of acetaldehyde dehydrogenase.16 The purpose of this study was to investigate the effect of chronic alcohol intake on the redox status of the GSH and Cys redox pools in jejunal, ileal, and colonic mucosa in an established rat model of chronic ethanol ingestion. Our results show that chronic ethanol ingestion caused global intestinal oxidative stress but with tissue-specific manifestations.
Materials and Methods
Animal and Ethanol Feeding
Weanling male Sprague-Dawley rats (n = 9, 175–200 g) were fed the Lieber-DeCarli liquid diet (Research Diets, New Brunswick, NJ) containing ethanol (36% of total kcal) ad libitum for 6 weeks.17 Pair-fed controls were fed an isocaloric mixture of liquid diet without ethanol (Con, n = 9, 175–200 g). Animal studies were conducted according to the Guide for the Care and Use of Laboratory Animals published by the Department of Health and Human Service. These studies were reviewed and approved by the Emory University Institutional Animal Care and Use Committee.
Sample Collection and Processing
After 6 weeks of ethanol ingestion, the rats were anesthetized and sacrificed by exsanguination at 9 AM to 12 PM. The intestine was stripped of mesenteric and vascular connections and removed from the peritoneum. The lumen was flushed with ice-cold saline to clear intestinal contents and suspended from a ring stand with a constant distal weight. The segments used for the endpoints of this study were collected sequentially at equivalent sites of each rat. The segments were longitudinally cut, and the mucosa was obtained by gentle scraping with a glass slide. The mucosa was immediately placed in liquid nitrogen for further analysis.
Thiol Determinations
The method for determination of tissue thiols has been previously described by Jones et al.18 After derivatization with iodoacetic acid and dansyl chloride, GSH and glutathione disulfide (GSSG), reduced and oxidized cysteine (Cys and CySS) and mixed disulfide (MD) were separated by high-performance liquid chromatography (HPLC). Quantitation was based on integration relative to the internal standard (γ-glutamyl-glutamate) and expressed as nmol/mg protein. In addition, the redox potential (Eh) for the GSH/GSSG and CyS/CySS couples were calculated from the respective concentrations using the Nernst equation, as previously described.18,19
Free MDA
The free MDA content in the defined intestinal mucosa samples was determined using the Bioxytech LPO-586 method (OXIS Health Products Inc, Portland, OR). Samples were thawed, homogenized in phosphate buffer (pH, 7.4), and then centrifuged at 4000g for 10 minutes. Free MDA was assayed by its reaction with N-methyl-phenylindole to generate the chromophore product according to the manufacturer’s instructions (OXIS Health Products Inc). The results were standardized relative to the protein concentration of the original homogenate.
Histological Assessment of Apoptosis in the Jejunum
Apoptotic cells were determined in colonic crypts by an examiner blinded to the study groups. Classic morphological changes including nuclear condensation, perinuclear clearing, and cell shrinkage were used as previously described.20 The apoptotic index was calculated as the number of apoptotic cells per 1000 well-oriented crypt cells counted. The number of apoptotic cells per crypt counted was also assessed.
Statistical Analysis
The Student’s t test was used to compare rats with or without ethanol ingestion. Data are presented as mean ± SEM.
Results
Chronic ethanol ingestion induced global decreases in Cys, CySS, and MD in the small and large intestine
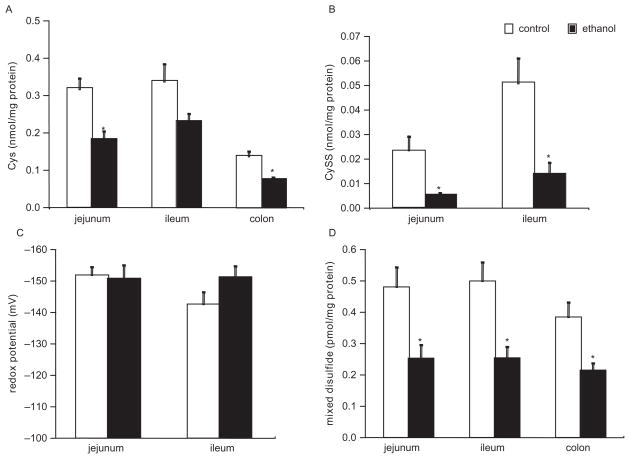
In the mucosa, Cys was decreased in each of the 3 intestinal segments but particularly in the jejunum and colon (Figure 1A). For the jejunum, ileum, and colon, Cys was decreased by −44% (P < .01), −32% (P = .06), and −43% (P = .001), respectively. In the jejunum and ileum, chronic ethanol ingestion also decreased the oxidized moiety CySS by approximately 78% and 75%, respectively, when compared to the controls (Figure 1B). For the colon, CySS concentration was undetectable in both the control and ethanol-fed rats. The redox potentials for the Cys and CySS pair were not significantly altered in the jejunum and ileum (Figure 1C). Similar to Cys concentration, MD was depleted globally in each of the intestinal segments from the ethanol-fed rats (Figure 1D). The magnitude of MD decrease was 48% (P < .01), 50% (P < .01), and 45% (P < .01) in the jejunum, ileum, and colon segments, respectively. This depletion of Cys, CySS, and MD suggested that chronic ethanol ingestion induced oxidative stress in each of the intestinal segments.
Figure 1.
Intestinal mucosal cysteine (Cys), cystine (CySS), and mixed disulfide (MD) concentration changes measured by high-performance liquid chromatography (HPLC) in ethanol-treated and control rats. (A) Mucosal Cys concentration. (B) Mucosal CySS concentration. (C) Redox potential for mucosal Cys/CySS. (D) Mucosal MD concentration. See Materials and Methods for animal diet and treatment. n = 9 for each group. *P < .05 vs control group.
Chronic ethanol ingestion induced differential changes in the GSH redox status of the small and large intestine
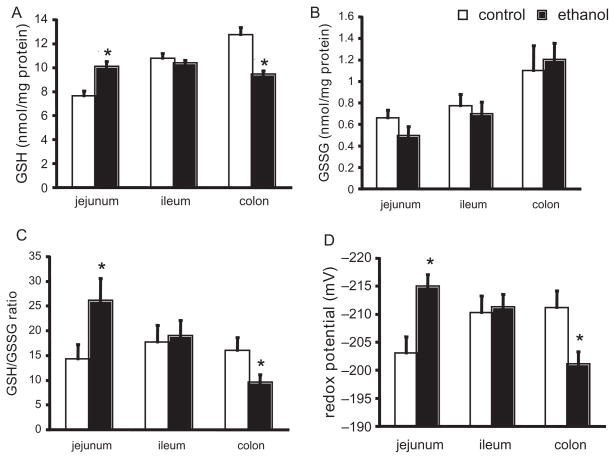
In the jejunum, chronic ethanol ingestion induced a significant increase in GSH concentrations (+33%, P = .001), but GSSG remained unchanged (Figures 2A and B). Accordingly, the ratio of GSH:GSSG was increased by 80% (P < .05; Figure 2C), and the redox potential (Eh) was markedly more reduced by 12 mV (P < .01; Figure 2D) when the samples from the ethanol-fed group were compared to those of the controls. In the colon, alterations in GSH redox status in response to chronic ethanol were opposite to that observed in the jejunum. Colonic GSH was decreased by 26% compared to controls (P < .001; Figure 2A) without a significant change in colonic GSSG (Figure 2B). This subsequently led to a 40% decrease in the GSH/GSSG ratio (P = .06; Figure 2C), and the GSH/GSSG Eh was oxidized by 11 mV (P < .05; Figure 2D) in the colonic segments of the ethanol-treated rats. In the ileum, chronic ethanol ingestion did not significantly alter gut mucosal GSH, GSSG, GSH:GSSG ratio, or Eh (Figure 2A–D). These results suggested that while chronic ethanol ingestion induced oxidative stress in the intestine, the intestinal segments were differentially affected.
Figure 2.
Intestinal mucosal glutathione/glutathione disulfide (GSH/GSSG) redox status changes measured by high-performance liquid chromatography (HPLC) in ethanol-treated and control rats. (A) Mucosal GSH concentration. (B) Mucosal GSSG concentration. (C) Mucosal GSH:GSSG ratio. (D) Mucosal GSH/GSSG redox potential. See Materials and Methods for animal diet and treatment. n = 9 for each group. *P < .05 vs control group.
Chronic ethanol ingestion induced differential changes in the oxidative stress marker MDA in the small and large intestine
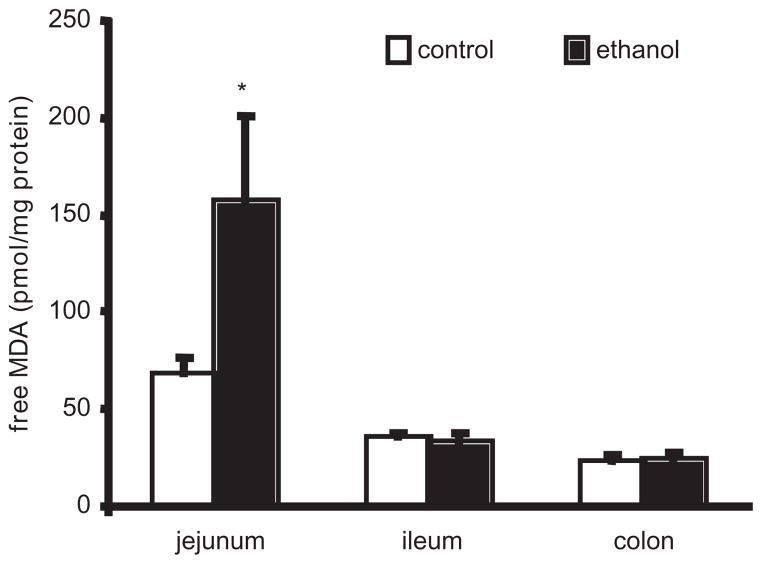
To further assess oxidative stress induced by chronic ethanol ingestion, we measured the oxidative stress marker MDA, a stable metabolite of lipid peroxidation. In the jejunum, chronic ethanol ingestion increased MDA by 154% when compared to the control group (P < .05; Figure 3). In contrast, there were no differences between the control-fed and ethanol-fed rats in the MDA pools of the ileum or the colon (Figure 3).
Figure 3.
Intestinal mucosal free malondyaldehyde (MDA) concentrations in ethanol-treated and control rats. Free MDA content in the defined intestinal mucosa samples was determined using the Bioxytech LPO-586 method. See Materials and Methods for animal diet and treatment. n = 9 for each group. *P < .05 vs control group.
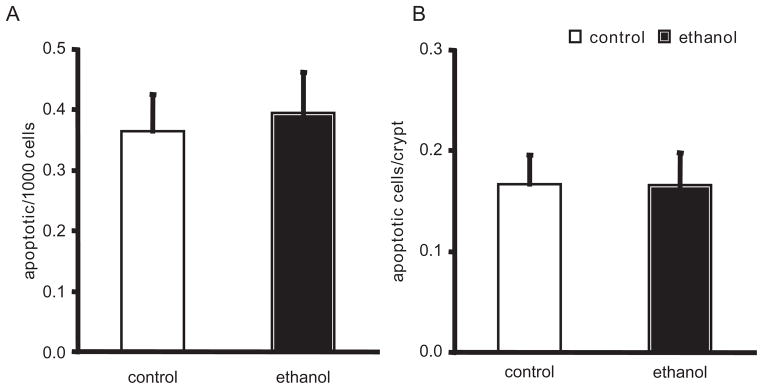
Because chronic ethanol ingestion was consistently associated with chronic oxidant stress in the jejunum, we examined whether apoptosis was upregulated in this particular intestinal segment. Using histological assessment, the number of apoptotic cells located in the jejunal crypt in the control group was not statistically different from that in the ethanol-fed group (0.36 ± 0.06 apoptotic cells/1000 cells vs 0.39 ± 0.07 apoptotic cells/1000 cells for control and ethanol, respectively; Figure 4A). Similarly, there were no statistical differences in the number of apoptotic cells per crypt (0.17 ± 0.03 apoptotic cells/crypt vs 0.17 ± 0.03 apoptotic cells/crypt; Figure 4B).
Figure 4.
Crypt cell apoptosis in the jejunum. Apoptosis was determined by classic morphological cellular features and presented as the group mean of 10 representative well-oriented crypts in each rat ± SE. (A) Apoptotic cells per 1000 cells counted. (B) Apoptotic cells per crypt. n = 9 in each experimental group. P was not significantly different between groups.
Discussion
In the small intestine, chronic ethanol ingestion is associated with morphological lesions, disrupted mucosal defenses, epithelial barrier dysfunction, and bacterial overgrowth.21–23 Given its association with oxidant stress, we examined the possibility that these effects were due to ethanol-induced oxidant stress in the different segments of the small and large intestine. When compared to pair-fed controls, Cys pools were significantly decreased in the jejunum and the colon segments derived from ethanol-fed rats. For the ileum, the ethanol-induced decrease in Cys approached but did not reach statistical significance. This decrease in the Cys pools of the jejunum and colon was due to depletion rather than simple oxidation as evidenced by no corresponding increase in the CySS or MD pools, respectively. Because the decreases in both Cys and CySS were parallel in the ethanol-fed group, there was no change in the redox potential (Eh) for the Cys/CySS thiol pair. The redox potential for the couple Cys/CySS reflects the level of reactants such as reactive oxygen species (ROS) and also affects the dynamics (net flow) of the reversible Cys-CySS reaction. The shrinkage of the Cys pool slows down the reaction, hence the loss of Cys buffering function against ROS. Cysteine has traditionally been regarded as the rate limiting amino acid for GSH synthesis. In this study, chronic alcohol ingestion increased total jejunal mucosal GSH with concurrent decreased Cys concentration, suggesting the utilization of Cys for synthesis of GSH. Differential responses of the Cys/CySS and GSH/GSSG redox pairs to oxidative stimuli have been described in various tissues and reflect the complexity of these independent nodes of cellular redox regulation.24 This differential regulation of Cys/CySS redox potential (unchanged) compared to GSH/GSSG redox potential (oxidized) by alcohol in the jeunum is consistent with these observations. Local Cys (including that converted from CySS) is available for GSH synthesis, and the fall in these levels concomitant with the increase in GSH in the jejunum with alcohol may reflect utilization to upregulate GSH synthesis.
Increases in GSH have been demonstrated in the stomach and upper small intestine after ethanol ingestion.3,9,25 In the current study, the jejunum demonstrated a significant increase in the GSH pool, the ratio of GSH to GSSG moieties, and the redox potential for the GSH/GSSG redox pair (GSH Eh). Therefore, chronic ethanol ingestion induced a shift in the jejunum to a more reduced state for the GSH/GSSG redox pair. Paradoxically, chronic ethanol ingestion increased the free lipid peroxidation product MDA in the jejunum. This suggested that oxidant stress was ongoing in the jejunum of the ethanol-fed rat. Increased tissue MDA has been shown to be associated with other indices of oxidative stress in animal models of ethanol ingestion,26 but our study is the first to associate this index with changes in GSH:GSSG redox potential. This may be a compensatory mechanism in response to the oxidant stress responsible for lipid peroxidation. Induction of enzymes involved in GSH metabolism such as GSH reductase has been reported.27,28 Similar findings to ours have been reported in a recent rat study in which chronic alcohol ingestion induces higher GSH level and lipid and protein peroxidation in the rat stomach.24 The mechanism by which the jejunum responds to chronic ethanol ingestion by independently regulating these 2 thiol redox pairs, Cys/CySS and GSH/GSSG, is unclear and requires additional investigation. The differential responses of redox pairs to redox interventions have been reported before and may reflect the complexity of independent nodes of cellular redox regulation.29 We focused on the jejunum for the measurement of apoptosis, as redox in this tissue was most dynamically affected by chronic ethanol ingestion in this gut segment as evidenced by markedly upregulated MDA levels, a decrease of Cys, CySS, and MD concentrations, and an adaptive reduction of GSH redox potential. However, the ethanol-induced increased oxidant stress did not alter apoptosis of this proximal gut segment.
Despite the changes in the CySS and the MDA pools in the ileum of the ethanol-fed rat, there were no significant changes in the Cys redox potential. Similarly, chronic ethanol ingestion did not significantly alter the GSH, GSH:GSSG ratio, or the GSH redox potential in the ileum. The absence of ethanol-induced chronic oxidant stress was further supported by no significant changes in free MDA.
In the colon, chronic ethanol ingestion resulted in decreased GSH and no corresponding increase in GSSG, resulting in a significant decrease in the GSH:GSSG ratio as well as an oxidation in the redox potential of this thiol couple. This ethanol-induced decrease in the colonic GSH pool paralleled that observed in the Cys and MD pools. This shift to a more oxidized state (GSH/GSSG and GSH Eh) suggested that chronic ethanol ingestion resulted in oxidant stress in the colon. However, the absence of increased free MDA argued that the oxidant stress was not sufficient to induce lipid peroxidation. It is unclear why the different intestinal segments would respond differently to chronic ethanol ingestion. However, both intracolonic microbes and colonic mucosal cells may contribute to ethanol metabolism in the colon to acetaldehyde, a reactive oxidant species, which, in turn, may alter mucosal redox in this distal bowel segment.29
Conclusions
Ethanol-induced oxidant stress resulted in differential regulation in the rat small bowel and colon. Furthermore, oxidant stress as reflected in the alteration of Cys/CySS, MD, and MDA was regulated independently within the colon and jejunum. In contrast, ethanol induced minimal oxidant stress in the ileum.
Footnotes
Financial disclosure: This work was supported by the National Institute of Health grants R01 DK55850 and K24 RR 023356 (TRZ), P50 AA135757, R01 HL67399, and R01 AA12197 (LASB), R01 ES011195 (DPJ), R01 DK050446 (MSL), and P30 DK 52574 (Washington University Digestive Diseases Research Core Center Grant).
References
- 1.Guidot DM, Hart CM. Alcohol abuse and acute lung injury: epidemiology and pathophysiology of a recently recognized association. J Invest Med. 2005;53:235–245. doi: 10.2310/6650.2005.53506. [DOI] [PubMed] [Google Scholar]
- 2.Vendemiale G, Grattagliano I, Altomare E, et al. Mitochondrial oxidative damage and myocardial fibrosis in rats chronically intoxicated with moderate doses of ethanol. Toxicol Let. 2001;123:209–216. doi: 10.1016/s0378-4274(01)00401-5. [DOI] [PubMed] [Google Scholar]
- 3.Altomare E, Grattagliano I, Vendemiale G, Palmieri V, Palasciano G. Acute ethanol administration induces oxidative changes in rat pancreatic tissue. Gut. 1996;38:742–746. doi: 10.1136/gut.38.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grattagliano I, Palmieri V, Vendemiale G, Portincasa P, Altomare E, Palasciano G. Chronic ethanol administration induces oxidative alterations and functional impairment of pancreatic mitochondria in the rat. Digestion. 1999;60:549–553. doi: 10.1159/000007705. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Checa JC, Kaplowitz N. The use of monochlorobimane to determine hepatic GSH levels and synthesis. Anal Biochem. 1990;190:212–219. doi: 10.1016/0003-2697(90)90183-a. [DOI] [PubMed] [Google Scholar]
- 6.Vendemiale G, Grattagliano I, Signorile A, Altomare E. Ethanol-induced changes of intracellular thiol compartmentation and protein redox status in the rat liver: effect of tauroursodeoxycholate. J Hepatol. 1998;28:46–53. doi: 10.1016/s0168-8278(98)80201-8. [DOI] [PubMed] [Google Scholar]
- 7.Aydin S, Ozaras R, Uzun H, et al. N-acetylcysteine reduced the effect of ethanol on antioxidant system in rat plasma and brain tissue. Tohoku J Exp Med. 2002;198:71–77. doi: 10.1620/tjem.198.71. [DOI] [PubMed] [Google Scholar]
- 8.Nordmann R, Ribiere C, Rouach H. Ethanol-induced lipid peroxidation and oxidative stress in extrahepatic tissues. Alcohol Alcoholism. 1990;25:231–237. doi: 10.1093/oxfordjournals.alcalc.a044996. [DOI] [PubMed] [Google Scholar]
- 9.Moghadasian MH, Godin DV. Ethanol-induced gastrointestinal damage: influence of endogenous antioxidant components and gender. Dig Dis Sci. 1996;41:791–797. doi: 10.1007/BF02213136. [DOI] [PubMed] [Google Scholar]
- 10.Shi MM, Kugelman A, Iwamoto T, Tian L, Forman HJ. Quinone-induced oxidative stress elevates glutathione and induces gamma-glutamylcysteine synthetase activity in rat lung epithelial L2 cells. J Biol Chem. 1994;269:26512–26517. [PubMed] [Google Scholar]
- 11.Diaz D, Krejsa CM, White CC, Keener CL, Farin FM, Kavanagh TJ. Tissue specific changes in the expression of glutamate-cysteine ligase mRNAs in mice exposed to methylmercury. Toxicol Let. 2001;122:119–129. doi: 10.1016/s0378-4274(01)00341-1. [DOI] [PubMed] [Google Scholar]
- 12.Victor BE, Schmidt KL, Smith GS, Miller TA. Protection against ethanol injury in the canine stomach: role of mucosal glutathione. Am J Physiol. 1991;261:G966–G973. doi: 10.1152/ajpgi.1991.261.6.G966. [DOI] [PubMed] [Google Scholar]
- 13.Kaur J. Chronic ethanol feeding affects intestinal mucus lipid composition and glycosylation in rats. Ann Nutr Metab. 2002;46:38–44. doi: 10.1159/000046751. [DOI] [PubMed] [Google Scholar]
- 14.Kaur M, Kaur J, Ojha S, Mahmood A. Ethanol effects on lipid peroxidation and glutathione-mediated defense in rat small intestine: role of dietary fats. Alcohol. 1998;15(1):65–69. doi: 10.1016/s0741-8329(97)00099-2. [DOI] [PubMed] [Google Scholar]
- 15.Bannai S. Induction of cystine and glutamate transport activity in human fibroblasts by diethyl maleate and other electrophilic agents. J Biol Chem. 1984;259(4):2435–2440. [PubMed] [Google Scholar]
- 16.Salaspuro M. Bacteriocolonic pathway for ethanol oxidation: characteristics and implications. Ann Med. 1996;28:195–200. doi: 10.3109/07853899609033120. [DOI] [PubMed] [Google Scholar]
- 17.Brown L, Harris F, Guidot D. Chronic ethanol ingestion potentiates TNF-alpha mediated oxidative stress and apoptosis in rat type II cells. Am J Physiol. 2001;281:L377–L386. doi: 10.1152/ajplung.2001.281.2.L377. [DOI] [PubMed] [Google Scholar]
- 18.Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 19.Jonas CR, Puckett AB, Jones DP, et al. Plasma antioxidant status after high-dose chemotherapy: a randomized trial of parenteral nutrition in bone marrow transplantation patients. Am J Clin Nutr. 2000;72:181–189. doi: 10.1093/ajcn/72.1.181. [DOI] [PubMed] [Google Scholar]
- 20.Inagaki-Ohara K, Takamura N, Yada S, et al. Radiation-induced crypt intestinal epithelial cell apoptosis in vivo involves both caspase-3-dependent and -independent pathways. Dig Dis Sci. 2002;47:2823–2830. doi: 10.1023/a:1021086012365. [DOI] [PubMed] [Google Scholar]
- 21.Preedy VR, Marway JS, Siddiq T, Ansari FA, Hashim IA, Peters TJ. Gastrointestinal protein turnover and alcohol misuse. Drug Alcohol Depend. 1993;34:1–10. doi: 10.1016/0376-8716(93)90040-w. [DOI] [PubMed] [Google Scholar]
- 22.Souza HS, Elia CC, Braulio VB, et al. Effects of ethanol on gut-associated lymphoid tissues in a model of bacterial translocation: a possible role of apoptosis. Alcohol. 2003;30:183–191. doi: 10.1016/s0741-8329(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 23.Fleming S, Toratani S, Shea-Donohue T, Kashiwabara Y, Vogel SN, Metcalf ES. Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcohol Clin Exp Res. 2001;25:579–589. [PubMed] [Google Scholar]
- 24.Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM, Jr, Kirlin WG. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J. 2004;18:1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- 25.Cho CH, Pfeiffer CJ, Misra HP. Ethanol and the antioxidant defense in the gastrointestinal tract. Acta Physiol Hung. 1992;80:99–105. [PubMed] [Google Scholar]
- 26.Das SK, Vasudevan DM. Alcohol-induced oxidative stress. Life Sci. 2007;81:177–187. doi: 10.1016/j.lfs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Lutnicki K, Wrobel J, Ledwozyw A, Trebas-Pietras E. The effect of ethyl alcohol on peroxidation processes and activity of anti-oxidant enzymes in rat’s gastric mucosa. Arch Vet Pol. 1992;32:117–123. [PubMed] [Google Scholar]
- 28.Amanvermez R, Tuncel OK, Demir S, Kefeli M, Bek Y, Celik C. Protective effects of cysteine, methionine and vitamin C on the stomach in chronically alcohol treated rats. J Appl Toxicol. 2008;28:591–598. doi: 10.1002/jat.1308. [DOI] [PubMed] [Google Scholar]
- 29.Visapaa JP, Tillonen J, Salaspuro M. Microbes and mucosa in the regulation of intracolonic acetaldehyde concentration during ethanol challenge. Alcohol Alcoholism. 2002;37:322–326. doi: 10.1093/alcalc/37.4.322. [DOI] [PubMed] [Google Scholar]