Abstract
Drosophila Wingless (Wg) acts as a morphogen to control pattern formation in a concentration dependent manner. Previous studies demonstrated important roles of heparan sulfate proteoglycans (HSPGs) in controlling Wg signaling and distribution. Here we examined the role of Sulfated (Sulf1), a Drosophila homolog of vertebrate heparan sulfate 6-O endosulfatase, in Wg signaling and distribution. We show that sulf1 is specifically up-regulated by Wg signaling in the wing disc. We found that expression of Wg target gene senseless(sens) was elevated in the sulf1 mutant wing discs. Sulf1 also negatively regulate extracellular levels of Wg. Genetic interaction experiments indicate that Wg antagonist Notum may work synergistically with Sulf1 to restrict Wg signaling, and Dally, a member of Drosophila HSPGs, is a potential target of Sulf1. Our results demonstrate that sulf1 is a novel Wg target gene and by a feedback mechanism, it negatively regulated Wg signaling and distribution in vivo.
Keywords: Sulfated, 6-O endosulfatase, proteoglycan, Wingless, Morphogen
Introduction
During development, a group of secreted signaling molecules called morphogens play essential roles in the formation of proper tissue patterns. Morphogens are secreted from a few localized cells, spread across developing tissues, and form concentration gradients. According to this gradient, responding cells receive the positional information and determine specific cell fates (Lawrence and Struhl, 1996; Gurdon and Bourillot, 2001; Tabata and Takei, 2004). One of the well-characterized morphogen molecules is Wingless (Wg), a Drosophila homologue of the Wnt1 in mammals. Wg plays essential roles in various patterning events (Nusslein-Volhard and Wieschaus, 1980; Campbell et al., 1993; Couso et al., 1993; Bienz, 1994). The function of Wg as a morphogen is well established in Drosophila wing disc (Zecca et al., 1996; Neumann and Cohen, 1997; Strigini and Cohen, 2000). However, the molecular mechanism(s) of how Wg forms its activity gradient is still not fully understood.
During wing development, Wg is required for the early development of wing blade structures (Williams et al., 1993), while in the later stage Wg is involved in forming bristles along wing margin (Phillips and Whittle, 1993). In the third instar wing discs, Wg is expressed in two to three rows of cells along dorsoventral (DV) compartment boundary, and forms a concentration gradient. Wg acts as a morphogen to activate the expression of its target genes including achaete-scute (ac), distalless (dll) and senseless (sens) in a concentration dependent manner (Zecca et al., 1996; Neumann and Cohen, 1997; Strigini and Cohen, 2000). Therefore modulation of Wg gradient is essential in regulating the expression of target genes and the developmental processes.
Heparan sulfate proteoglycan (HSPG) is one of the key regulators for Wg gradient formation (Lin, 2004). Previous studies demonstrated that HSPGs play essential roles in regulating Wg signaling and its gradient formation. HSPGs are macromolecules on cell surface and within extracellular matrix. They are composed of a core protein, to which heparan sulfate (HS) glycosaminoglycan (GAG) chains are attached (Bernfield et al., 1999; Perrimon and Bernfield, 2000; Esko and Selleck, 2002). Based on the core protein structure, HSPGs are classified into three families: syndecan, perlecan and glypican (Lin, 2004). Two genes encoding for glypicans, division abnormally delayed (dally) and dally-like protein (dlp) are involved in Wg distribution and signaling activities (Lin and Perrimon, 1999; Tsuda et al., 1999; Baeg et al., 2001). dally mutants show the defects of wing margin, and exhibit genetic interaction with Wg receptor Dfz2 (Lin and Perrimon, 1999), indicating that Dally is required for Wg signaling activity. However, Dlp exhibits biphasic activities in Wg signaling (Baeg et al., 2004; Kirkpatrick et al., 2004; Kreuger et al., 2004; Franch-Marro et al., 2005; Han et al., 2005; Hufnagel et al., 2006). Our recent data demonstrate that Dlp protein core is involved in its biphasic activities in Wg signaling (Yan et al., 2009).
The biosynthesis of HS chains on Dally and Dlp are carried out by three Drosophila EXT genes tout-velu (ttv), sister of ttv (sotv) and brother of ttv (botv) (Bornemann et al., 2004; Han et al., 2004a; Perrimon and Hacker, 2004; Takei et al., 2004). Disruption of any of these genes leads to reduced HS levels, as well as reduced extracellular Wg levels and expression of Wg target genes (Wodarz and Nusse, 1998; Bornemann et al., 2004; Han et al., 2004a; Perrimon and Hacker, 2004; Takei et al., 2004). Subsequent sulfations of HS chains are carried out by sulfateless (sfl), a gene encoding for N-deacetylase/N-sulfotransferase (Lin, 2004). Genetic experiments demonstrated that Sfl is required for Wg signaling and distribution (Lin and Perrimon, 1999; Baeg et al., 2001). Sfl mutant embryos showed defects in several Wg-dependent developmental processes and extracellular Wg levels are also reduced in sfl mutant clones in wing discs (Lin and Perrimon, 1999; Baeg et al., 2001). These data suggest that sulfation levels on HS are critical for Wg signaling and distribution. However, currently, it is unclear whether other genes can modulate the sulfation levels and patterns of HS to regulate Wg signaling activities.
In addition, notum, a gene encoding for alpha/beta-hydrolase, has been shown to restrict Wg signaling activity, possibly by modulating Dally and Dlp activities (Gerlitz and Basler, 2002; Giraldez et al., 2002; Han et al., 2004b). Studies also showed that expression of notum itself is induced by high level of Wg signaling, indicating that Wg signaling is regulated by a feedback mechanism. However, little is known whether other genes can regulate Wg signaling distribution by similar mechanism(s).
Sulf1 encodes for 6-O endosulfatase, a secreted protein distributed on the cell surface and extracellular matrix (Dhoot et al., 2001). The first characterized endosulfatase is Qsulf1 in quail embryos (Dhoot et al., 2001). QSulf1 has effect on cell differentiation by modulating Wnt signaling. Later, Qsulf2, another gene in avian, and two mammal orthologues in mouse and human were identified and characterized (Morimoto-Tomita et al., 2002; Ai et al., 2006). Early studies showed that the cell surface endosulfatase modulates HSPGs by specifically removing 6-O sulfates from mature HS chains (Ai et al., 2003). In msulf1 mutant fibroblasts, the levels of 6-O sulfation on HS chains are elevated, and this increase was significantly enhanced in msulf1 msulf2 double mutant fibroblasts (Lamanna et al., 2006). Since sulfation levels on HS chains are required in Wg signaling, 6-O endosulfatase may restrict Wg signaling. Surprisingly, studies in cell culture showed that QSulf1 can promote Wnt signaling activity (Ai et al., 2003). Ai et al. proposed that QSulf1 reduces sulfation level on HS chains and thus releases the Wnt ligand proteins to promote Wnt signaling transduction (Ai et al., 2003). However, no evidence so far confirms this hypothesis in vivo. Neither msulf1 or msulf2 knockout mice showed obvious defects in Wnt signaling (Lamanna et al., 2006). Thus, the molecular mechanisms of how 6-O endosulfatase regulates Wnt signaling are remained to be determined.
Here, we attempt to analyze the roles of sulf1 during development in Drosophila. Drosophila sulf1 is the only gene encoding for 6-O endosulfatase. We show that sulf1 is specifically up-regulated along DV compartment boundary and anteroposterior (AP) compartment boundary in late third instar larvae. Our studies demonstrate that Wg signaling is required to activate expression of sulf1. Both loss-of-function and gain-of-function experiments demonstrate that Sulf1 is a negative regulator for Wg signaling. Genetic interaction experiments indicate that Notum may work synergistically with Sulf1 to restrict Wg signaling, and Dally is a potential target of Sulf1. Together, these data suggest that Sulf1 may provide another level of fine regulation in Wg gradient formation.
Results
Dynamic expression patterns of Sulf1 in wing disc
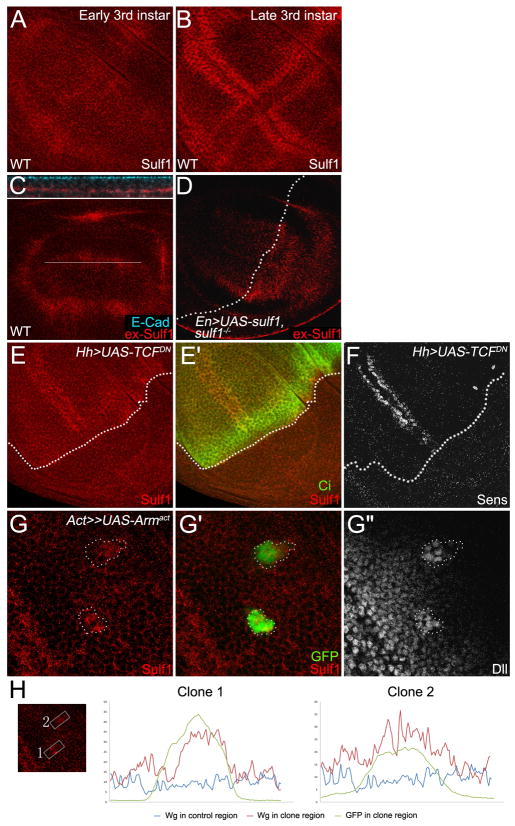
To determine the function of Sulf1, we generated the Sulf1 antibody and examined the expression patterns of sulf1 in wing disc. Sulf1 protein was detected ubiquitously at low levels in early third instar wing discs (Fig. 1A). However, at late third instar stage, Sulf1 level was up-regulated along DV and AP compartment boundaries (Fig. 1B). Interestingly, along the DV boundary, sulf1 was preferentially expressed on each side of the Wg expressing cells. This pattern is similar to sens, one of Wg short-range target genes (Zecca et al., 1996), raising a possibility that expression of sulf1 is regulated by Wg.
Fig. 1. sulf1 is dynamically expressed and its expression is up-regulated by Wg signaling.
A,B: Immuno-staining of 6-O endosulfase in wild-type wing discs. A: In early 3rd instar discs, sulf1 is ubiquitously expressed in entire wing disc. B: In late 3rd instar discs, expression of sulf1 is highly up-regulated in each side of DV compartment boundary and AP compartment boundary. C,D: Extracellular Sulf1 staining in wild-type wing disc (C) and in wing discs overexpressing sulf1 in the posterior compartment driven by En-Gal4 on sulf1 mutant background (D). The upper panel in C shows z-section of the line in lower panel. Staining of E-cadherin marks the apical side of the cells. High levels of extracellular Sulf1 are detected at basal-lateral regions. Dotted line in D shows AP compartment boundary. E: Sulf1 staining is eliminated in TCFDN expression domain marked by the absence of Ci (E′). F: Sens staining shows the loss of Wg signaling in TCFDN expression domain. AP boundary is outlined by broken lines. G: A gain-of-function Armadillo (Arm) Armact was ectopically expressed by using FLP-OUT system. Sulf1 staining is elevated in the clones (G) that are marked by GFP (G′). Dll staining shows enhanced Wg signaling activity in the clones (G″). The clones are outlined by broken lines. H: Signal profiles of Sulf1 (red line) and GFP (green line) are plotted from selected areas in the box and non-clone control region (blue line). Wing discs in C, D, E, F, G, H were all obtained from late 3rd instar larva. In all images, anterior compartment faces left, and dorsal compartment faces up.
Previously studies in vertebrates have shown that Sulf1 and Sulf2 are secreted enzymes (Dhoot et al., 2001; Morimoto-Tomita et al., 2002; Ai et al., 2003). Consistent with this view, high levels of extracellular Sulf1 were detected in the wing discs by an extracellular staining protocol (Strigini and Cohen, 2000; Baeg et al., 2001) (Fig. 1C). Extracellular Sulf1 staining is mainly at basal-lateral side of the wing disc (Fig. 1C upper panel). Interestingly, extracellular Sulf1 was broadly distributed in all of the wing pouch cells, suggesting that secreted Sulf1 mainly from DV and AP boundary can be diffused into other surrounding cells. Ectopic expression of Sulf1 induced by en-GAL4 on sulf1 mutant background showed enhanced the levels of extracellular Sulf1 in P compartment (Fig. 1D). However, the level of extracellular Sulf1 is also higher in A compartment next to P compartment compared with that in more distal region, suggesting that ectopically expressed Sulf1 in P compartment can diffuse from P to A compartment.
Wg signaling is necessary and sufficient to activate sulf1 expression
To determine whether sulf1 expression is up-regulated by Wg signaling, we first examined the expression level of sulf1 when Wg signaling activity is inhibited. We expressed dominant-negative TCF (TCFDN) by using hh-Gal4-gal80ts. Flies were shifted to 29 °C to suppress gal80 and to allow expression of TCFDN. 16 hours after shifting to 29°C, late third instar larvae were dissected and stained for Sulf1 antibody. In a control experiment, Sens levels are eliminated in the P compartment expressing TCFDN (Fig. 1F), indicating that Wg signaling is blocked. As expected, Sulf1 staining is strikingly reduced at the whole P compartment where Wg signaling is inhibited (Fig. 1E–E′). This result suggests that Wg signaling activity is required for high level expression of sulf1 at both DV boundary and AP boundary.
Next we tested whether Wg signaling is sufficient to activate sulf1 expression. We ectopically expressed a gain-of-function Armadillo (Armact) using FLP-OUT system (Struhl and Basler, 1993), and examined Sulf1 levels by immuno-staining in wing discs. Larvae were heat shocked in 37°C for half an hour in first instar stage, and dissected in late third instar larvae. As shown in Fig. 1G″, ectopic expression of Armact caused over-activated Wg signaling determined by Dll levels. Consistent with our view, Sulf1 levels were also enhanced in the clones expressing Armact (Fig. 1G–G′), indicating that Wg signaling is sufficient to increase Sulf1 protein level in wing discs. The intensity plot files (Fig. 1H) also confirmed that the Sulf1 staining increased in the Armact expressing clones comparing to that in the non clone area. Collectively, these data suggest that the expression of sulf1 in the late third instar wing discs is activated by Wg signaling.
Sulf1 regulates Wg signaling and distribution
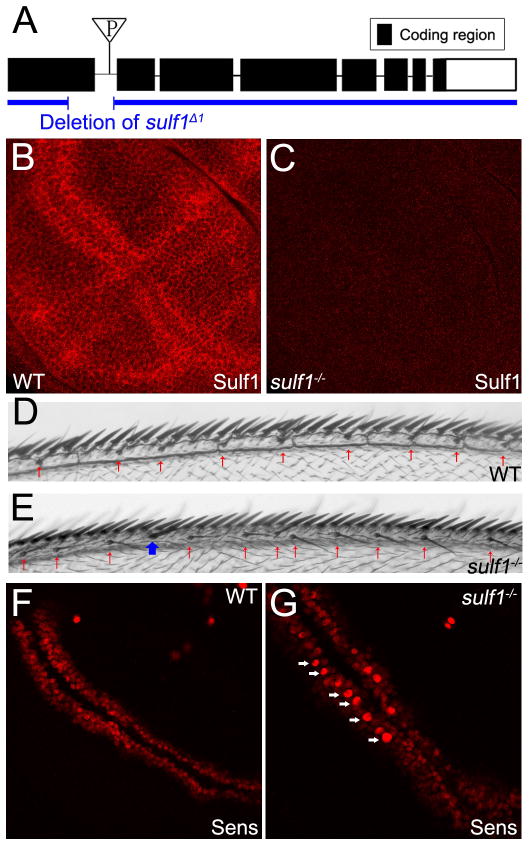
To examine further the function of Sulf1, we generated sulf1 mutant by imprecise excision of P-element inserted in the first intron of sulf1 gene (P{GT1}GT000656). Allele sulf1Δ1 was identified by PCR and confirmed by sequencing. This allele contains a 287 base pairs (bps) deletion starting from the 480th bp in the first exon, causing a frame shift (Fig. 2A). Reverse transcriptase PCR was performed and the sequence of the transcripts confirmed the frame shift (data not shown). Antibody staining showed that Sulf1 protein is absent in homozygous mutant larvae (Fig. 2B, C). These data argue that sulf1Δ1 is a null allele.
Fig. 2. sulf1 mutant showed enhanced Wg activities.
A: Schematic map of Drosophila sulf1 (CG6725). The triangle shows the location of the P-element (P{GT1}GT000656) used to generate the sulf1 mutant. B,C: Immuno-staining of Sulf1 in late 3rd instar wing discs of wild-type (B) and discs of sulf1 homozygous mutant (C). Sulf1 protein is absent in sulf1−/− discs. Images B and C are processed identically. D,E: Dorsal-anterior view of adult fly wings of wild-type (D) and sulf1−/− (E). Homozygous mutant flies are viable, and show increased number of chemosensory bristles (CSBs) (red arrows) and ectopic mechanosensory bristle (MSB) on the wing margin (blue arrow). F,G: Immuno-staining of Sens in the wild-type (F) and sulf1−/− (G) wing discs. White arrows show the stronger Sens staining in the sulf1−/− disc. Images F and G are processed identically. H,I: Immuno-staining of Wg doesn’t show significant difference between the wild-type (H) and sulf1−/−(I) wing discs. Images H and I are processed identically. In all images, wing discs were obtained from late 3rd instar larva. Anterior compartment faces left, and dorsal compartment faces up.
Homozygous sulf1 mutant flies are viable and fertile. However, adult homozygous mutant flies exhibit some specific phenotypes, including eye roughness (data not shown) and an increased number of bristles in adult wings. During wing development, Wg is involved in forming bristles along wing margin (Phillips and Whittle, 1993). Excess Wg signaling activities can lead to increase of bristles on wing margin. In the wide-type anterior wing margin (Fig. 2D), two rows of bristles can be observed in the dorsal side. The row of thick mechanosensory bristles (MSBs) is more anterior and adjacent to the compartment boundary. The row of thin chemosensory bristles (CSBs) locates at a more posterior position. In sulf1 mutant, the number of CSBs increased (showed by red arrows in Fig. 2E), and ectopic MSBs were also found between normal row of MSBs and CSBs (blue arrow in Fig. 2E). These data suggest that Wg signaling activity is enhanced in sulf1 mutant flies. To confirm this, we examined the levels of Wg short-range target gene sens. In the sulf1 mutant wing discs, the Sens staining is enhanced compare to that in wide-type (Fig. 2F, G). However, only around 40% (n>50) sulf1 mutant wing discs showed enhanced Sens staining. The enhanced Sens staining is mainly in the anterior compartment of the wing discs, which is consistent with the increased number of bristles in the anterior adult wing margin.
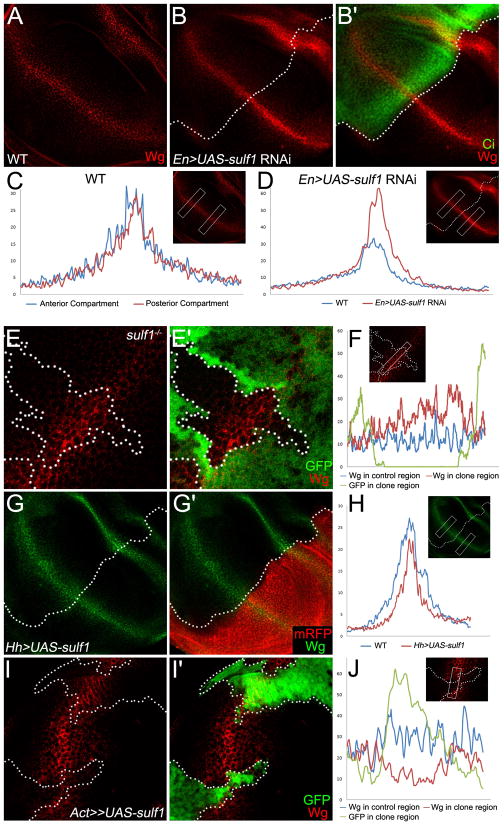
To further analyze whether Sulf1 is required to restrict Wg distribution, we performed following two lines of experiments. First, we examined extracellular Wg staining in the wing disc in which sulf1 activities are depleted by expressing sulf1 double-stranded RNA (dsRNA) in the P compartment of wing discs. Comparing to wild-type wing discs (Fig.3A, C), extracellular Wg was enhanced in the area where sulf1 was eliminated (Fig. 3B–B′, D). We also examined extracellular wg levels in the wing discs containing sulf1 mutant clones. Consistent with the results obtained from sulf1 RNAi, extracellular Wg level was enhanced in sulf1 mutant clones (Fig. 3E–F). However, we observed no obvious changes in Sens levels in the wing discs expressing sulf1 RNAi or sulf1 mutant clones (data now shown), suggesting that enhanced Wg levels by sulf1 RNAi or sulf1 mutant clones may not be enough to induce sens expression in these experimental conditions.
Fig. 3. Sulf1 restricts extracellular Wg distribution.
A–F: Extracellular levels of Wg in wild-type wing disc (A), in wing discs expressing sulf1 dsRNA (B) driven by En-Gal4 and in wing discs containing sulf1 mutant clones (E). The extracellular Wg staining are stronger in the sulf1 dsRNA expressing posterior compartment marked by the absence of Ci staining (B′) and in the sulf1 mutant clones marked by lack of GFP (E′). Signal profiles of extracellular Wg were obtained from selected regions in wild-type discs (C), in discs expressing sulf1 dsRNA (D) and from the boxed area across the sulf1 mutant clone (F). G–J: Extracellular levels of Wg in the discs overexpressing sulf1 in the posterior compartment (G) marked by mRFP (G′) and in the clones expressing sulf1 (I) marked by GFP (I′). Extracellular staining of Wg is reduced in the sulf1 expression area. Signal profiles (H, J) of extracellular Wg were obtained from the selected regions in G and I. In all images, wing discs were obtained from late 3rd instar larva. Anterior compartment faces left, and dorsal compartment faces up. In B, D, F, H, and J the selected regions used for analysis are shown in the box, and the color of profiles are noted at the bottom of each figure. In C, D and H, the left-right axis corresponds to the dorsal-ventral axis of the selected regions.
In a second experiment, we determined whether sulf1 over-expression can cause reduced levels of extracellular Wg. Sulf1 was over-expressed in the posterior compartment by using en-Gal4. In sulf1 over-expression region, the extracellular Wg staining was reduced (Fig. 3G–H). This reduction is more obvious in the area that is 3 to 4 cells away from DV boundary. To further confirm this, we made clones ectopically expressing sulf1 and found reduced extracellular Wg levels in clones (Fig. 3I–J). However, the over-expression of sulf1 is not enough to reduce the expression of Wg target gene such as sens or dll (data not shown).
Taken together, these results indicate that Sulf1 can negatively regulate extracellular Wg levels in the wing disc.
Sulf1 functions together with Notum to negatively regulate Wg signaling activities
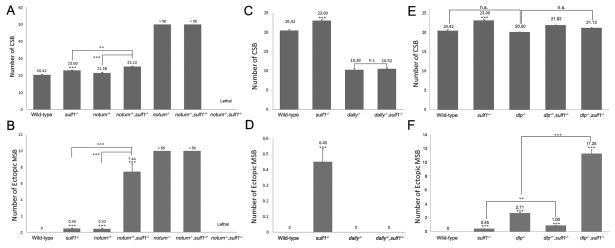
In third instar wing discs, notum is expressed along DV boundary and induced by high levels of Wg (Gerlitz and Basler, 2002; Giraldez et al., 2002). Notum homozygous mutant is viable but exhibits strikingly increased numbers of CSBs and ectopic MSBs (Gerlitz and Basler, 2002; Giraldez et al., 2002). These data lead to a conclusion that Notum functions as an antagonist of Wg. As both Notum and Sulf1 are negative feedback regulators of Wg signaling, we suspect that they may function together in Wg signaling. We tested this by performing the genetic interaction experiment between notum and sulf1. In notum mutant, around 20% homozygous notum mutant can be found. However more than 90% notum−/−sulf1−/+ flies are lethal. The survived flies show more CSBs and ectopic MSBs on wing margin comparing to the notum−/− flies. Notum and sulf1 double mutant are lethal, which may result from further increase of Wg signaling. On the other hand, notum heterozygous mutant flies have less increased number of CSBs and MSBs than notum homozygous mutant. Compared with notum heterozygous mutant, notum−/+ sulf1−/− flies show more increased numbers of CSBs and ectopic MSBs (Fig. 4A and B). On the basis of these data, we argue that Notum and Sulf1 work together to negatively regulate Wg signaling activity.
Fig. 4. Genetic interaction between sulf1, notum and dally.
A–F: Quantification of number of chemosensory bristles (CSB), and ectopic mechanosensory bristles (MSB). Shown are means of the number of CSB and ectopic MSB with standard error. Statistical significance comparing with wild-type is shown on the top of the bar. Other comparisons are marked by horizontal bars, and statistical significance is indicated. (***p < 0.0001, **p < 0.01, n.s. for non significant difference.)
Sulf1 regulates Wg signaling by modulating Dally activity
Sulf1 functions as 6-O endosulfatase, which modulates the sulfation levels on HS chains by removing sulfate groups (Dhoot et al., 2001). Because two Drosophila glypicans Dally and Dlp have effect on Wg distribution and signaling activities (Lin and Perrimon, 1999; Tsuda et al., 1999; Baeg et al., 2001), they are potential substrates of Sulf1. Dally is known as a positive regulator of Wg signaling activity and dally homozygous mutant flies show reduced number of bristles on wing margin (Han et al., 2005). The results of our genetic interaction experiments suggested that Sulf1 may restrict Wg signaling mainly by modulating Dally activity. As shown in Figure 4C and D, sulf1 mutant shows an increased number of CSBs and exhibits ectopic MSBs. However, in dally-sulf1 double mutant, the number of CSBs along wing margin is reduced and no ectopic MSB is found (Fig. 4C–D). Thus dally-sulf1 double mutant exhibits similar phenotypes that were showed in dally mutant, suggesting Dally might be downstream substrate of Sulf1.
We also examined the genetic interaction of dlp with sulf1. Dlp inhibits Wg signaling activity at DV boundary and dlp homozygous mutant flies show increased number of bristles on wing margin (Franch-Marro et al., 2005; Han et al., 2005). In dlp mutant, the number of ectopic MSB, but not CSB, is increased (Fig. 4E–F). If Sufl1 acts mainly through Dlp to regulate negatively Wg signaling, we would expect to see no further increase of ectopic MSB in dlp-sulf1 mutant. However, in dlp-sulf1 double mutant, the number of ectopic MSBs is obviously increased (Fig. 4E and F), arguing that Sulf1 is likely to act through other substrate other than Dlp. This data further support the view that Dally is likely to be a main substrate for Sulf1.
Discussion
The formation of Wg gradient is a complex process involving a number of cell-surface and extracellular molecules. Here we show that Wg is both necessary and sufficient to activate the expression of sulf1. Our loss-of-function and gain-of-function experiments argue that Sulf1 restricts Wg distribution. Genetic interaction experiments further suggest that Sulf1 may work together with Notum to regulate Wg signaling, probably through modifying Dally. Thus, Sulf1 is an extracellular feedback antagonist of Wg distribution and signaling activity.
Feedback regulation of Wg signaling
One of the important findings in this work is that Sulf1 acts as a feedback regulator of Wg signaling. Although previous studies showed that enzymes involved in HSPGs biosynthesis are required for formation of proper Wg gradient, none of them is regulated by Wg signaling activity. Here we show that the expression of sulf1 is diminished when Wg signaling is inhibited, and is enhanced when Wg signaling is over-activated (Fig. 1E–H). These results clearly demonstrate that sulf1 is a Wg target gene.
In the wing disc, Wg signaling is regulated by several feedback regulators. One good example is notum. Notum is expressed along DV boundary, and its expression is activated by Wg signal (Gerlitz and Basler, 2002; Giraldez et al., 2002). Notum homozygous mutant show dramatically increased CSBs and ectopic MSBs in the anterior wing margin. This phenotype is much stronger than those of sulf1 homozygous mutants. This indicates that Notum may function as a major antagonist of Wg signal while Sulf1 acts as a minor co-worker. A previous study showed that Notum inhibited Wg signaling mainly by modifying Dlp (Gerlitz and Basler, 2002; Giraldez et al., 2002). They demonstrated that Notum can cleave the GPI anchor of Dlp, and proposed that Notum and Dlp collaborate each other to inhibit Wg signaling (Kirkpatrick et al., 2004; Kreuger et al., 2004). However, our previous experiments suggested that the target of Notum might be Dally rather than Dlp (Han et al., 2005). Our recent study further supports our conclusion (Yan et al., 2009).
Our genetic interaction experiments showed that sulf1-dally mutant exhibit decreased number of CSBs, which is similar to dally single mutant. This indicates that Dally might be the substrate of Sulf1. Since sulf1 encodes for 6-O-endosulfatase (Ai et al., 2003), it might acts to reduce the sulfation levels of Dally by removing sulfate group from the GAG chains. Together, our experiments suggest that both Notum and Sufl1 may regulate Wg signaling and distribution by modulating the activities of Dally.
Wg gradient activity is regulated by multiple mechanisms
Wg distribution and signaling activity is also regulated by another two feedback loops through Glypican genes dally and dlp (Baeg et al., 2004; Kirkpatrick et al., 2004; Kreuger et al., 2004; Franch-Marro et al., 2005; Han et al., 2005; Hufnagel et al., 2006). Wg signaling activates expression of dally, but suppresses expression of dlp (Fujise et al., 2001; Han et al., 2005). Dally is a positive regulator of Wg distribution and signaling activity, while Dlp exhibits biphasic activity in Wg signaling and distribution (Yan et al., 2009). Here we provide evidence that Dally might control Wg signaling through another negative feedback loop on post-translational level. High level Wg activates expression of sulf1. This secreted endosulfatase stays in the extracellular matrix and removes sulfate group from Dally, thereby reducing the Dally’s activities in Wg signaling and distribution. These two feedback loops may collaborate each other to control proper Wg signaling activities. Thus, our findings provide novel insights into the mechanisms of how Wg forms its precise gradient during development.
Material and Methods
Fly strains
The following null alleles of dally, dlp, notum were used: dally80 (Han et al., 2004b); dlpMH20 (Franch-Marro et al., 2005), wf141(Gerlitz and Basler, 2002). The following UAS constructs were used to overexpress the corresponding transgenes in the posterior compartment of the wing disc: UAS-TCFDN (van de Wetering et al., 1997). UAS-sulf1 RNAi (developed in this study), UAS-sulf1 (developed in this study). To generate the UAS-sulf1 RNAi construct, the entire third exon was cloned into pWIZ vector. For the UAS-sulf1 constuct, the entire sulf1 gene was cloned into pUAST vector. The following lines were also used: En-Gal4 UAS-dcr2, Hh-Gal4 tub1-Gal80ts (McGuire et al., 2003; McGuire et al., 2004). Act>y+>Gal4 UAS-GFP (Ito et al., 1997) was used to drive the expression of UAS-Armact (Pai et al., 1997) and UAS-sulf1 in random clones marked with GFP.
Generation of sulf1 mutant allele
Sulf1 mutant was generated by imprecise excision of P-element inserted in the first intron of sulf1 gene. The original P-element line w1118; P{GT1}Sulf1GT-000656 (Bloomington Stock Center BL20991) was crossed with w*; ry506 Sb1 P{Δ2-3}99B/TM6B, Tb1 (BL1798) to induce P-element jump. Lines with P-element excision were selected by lack of eye color and then examined by PCR to detect deletion. Sulf1Δ1 was found to contain a deletion of 268 bp in the first exon and the first intron. This deletion causes a frame shift. The mRNA transcript in sulf1Δ1 was examined by reverse transcriptase PCR and sequencing. The sequence of the transcript confirmed the frame shift. In both embryo and wing discs, Sulf1 is not detected by anti-Sulf1 antibody staining.
Generation of mutant clones and ectopic expression
Mutant clones in the wing disc were generated by the FLP-FRT method (Golic, 1991; Xu and Rubin, 1993) and induced by giving first or second instar larvae a heat-shock at 37°C for 1.5 hour. Clones ectopically expressing Armact and sulf1 in the wing disc were generated by FLP-OUT technique (Ito et al., 1997). First or second instar larvae were given heat-shock at 37°C for 0.5 hours and dissected in the third instar stage. Genotype of flies to generate sulf1 mutant clones marked by the absence of GFP is y w hsp70-flp/+ or Y; hsp70-Myc-GFP M(3)i55 FRT82B/sulf1 FRT82B. Genotype of clones expressing Armact and sulf1 marked by GFP is y w hsp70-flp/UAS-armact; act>y+>Gal4 UAS-GFP/+ and y w hsp70-flp/+ or Y; act>y+>Gal4 UAS-GFP/UAS-sulf1
Antibodies and immunofluorescence
Guinea Pig anti-Sulf1 antibody was developed in this study. To generate the antibody, the sulf1 fragment corresponding to Amino Acid position 395 through 575 was cloned in vector Pgex-4T-1 and expressed by bacteria. The anti-Sulf1 antibody was made by Proteintech Group Inc. Chicago.
Imaginal discs fixation and antibody staining were performed as described (Belenkaya et al., 2002). Primary antibodies were used at the following dilutions: mouse anti-Wg 4D4 (1:5) (Iowa Developmental Studies Hybridoma Bank; IDSHB), rat anti-Ci (1:10) (Motzny and Holmgren, 1995), guinea pig anti-Sulf1 (1:1000) and rabbit anti-GFP Alexa Fluor 488 at 1:1000 (Molecular Probes). Extracellular Sulf1 and Wg staining was performed as described (Strigini and Cohen, 2000; Baeg et al., 2001). Primary antibodies were used at the following dilutions in extracellular staining: mouse anti-Wg 4D4 at 1:3 (IDSHB), and guinea pig anti-Sulf1 at 1:500. The primary antibodies were detected by fluorescent-conjugated secondary antibodies from Jackson ImmunoResearch Laboratories.
Confocal observation and image processing
Samples with fluorescent immuno-staining were observed by the Zeiss LSM510 on an Axio observer inverted microscope. All images were taken at a single optical section. For the analysis of expression of sulf1 (Fig. 1H) and extracellular levels of Wg (Fig. 3C, D, F, H and J) the original images taken by confocal were exported in tiff format. The plot values of selected regions in the images were measured by ImageJ software and then used to generate plot profiles in Microsoft Excel. For each experiment, at least three samples were analyzed in this way and the similar results were obtained.
Quantification of chemosensory bristles (CSBs) and Ectopic mechanosensory bristles (MSBs)
The anterior wing margins were examined by microscope and the numbers of CSB and MSB were calculated. Ectopic MSB is a thick bristle located directly adjacent to the wild-type row of MSB. For each genotype, at least 20 wings were examined. Student t-test was used to examine the statistical significance. Differences of P<0.01 were considered as significant.
Acknowledgments
We thank H. Bellen, S. Cumberledge and the Iowa Developmental Studies Hybridoma Bank (DSHB) for antibodies; the Bloomington Stock Center for Drosophila stocks; L. Ray for comments on the manuscript. This work was supported partially by NIH grants (2R01 GM063891 and 1R01GM087517) and the Knowledge Innovation Program of the Chinese Academy of Sciences KSCX2-YW-R-263 to X. L.
References
- Ai X, Do AT, Kusche-Gullberg M, Lindahl U, Lu K, Emerson CP., Jr Substrate specificity and domain functions of extracellular heparan sulfate 6-O-endosulfatases, QSulf1 and QSulf2. J Biol Chem. 2006;281:4969–4976. doi: 10.1074/jbc.M511902200. [DOI] [PubMed] [Google Scholar]
- Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP., Jr QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol. 2003;162:341–351. doi: 10.1083/jcb.200212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- Baeg GH, Selva EM, Goodman RM, Dasgupta R, Perrimon N. The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors. Dev Biol. 2004;276:89–100. doi: 10.1016/j.ydbio.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J, Lin X. pygopus Encodes a nuclear protein essential for wingless/Wnt signaling. Development. 2002;129:4089–4101. doi: 10.1242/dev.129.17.4089. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bienz M. Homeotic genes and positional signalling in the Drosophila viscera. Trends Genet. 1994;10:22–26. doi: 10.1016/0168-9525(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Bornemann DJ, Duncan JE, Staatz W, Selleck S, Warrior R. Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development. 2004;131:1927–1938. doi: 10.1242/dev.01061. [DOI] [PubMed] [Google Scholar]
- Campbell G, Weaver T, Tomlinson A. Axis specification in the developing Drosophila appendage: the role of wingless, decapentaplegic, and the homeobox gene aristaless. Cell. 1993;74:1113–1123. doi: 10.1016/0092-8674(93)90732-6. [DOI] [PubMed] [Google Scholar]
- Couso JP, Bate M, Martinez-Arias A. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science. 1993;259:484–489. doi: 10.1126/science.8424170. [DOI] [PubMed] [Google Scholar]
- Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP., Jr Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001;293:1663–1666. doi: 10.1126/science.293.5535.1663. [DOI] [PubMed] [Google Scholar]
- Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- Franch-Marro X, Marchand O, Piddini E, Ricardo S, Alexandre C, Vincent JP. Glypicans shunt the Wingless signal between local signalling and further transport. Development. 2005;132:659–666. doi: 10.1242/dev.01639. [DOI] [PubMed] [Google Scholar]
- Fujise M, Izumi S, Selleck SB, Nakato H. Regulation of dally, an integral membrane proteoglycan, and its function during adult sensory organ formation of Drosophila. Dev Biol. 2001;235:433–448. doi: 10.1006/dbio.2001.0290. [DOI] [PubMed] [Google Scholar]
- Gerlitz O, Basler K. Wingful, an extracellular feedback inhibitor of Wingless. Genes Dev. 2002;16:1055–1059. doi: 10.1101/gad.991802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ, Copley RR, Cohen SM. HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev Cell. 2002;2:667–676. doi: 10.1016/s1534-5807(02)00180-6. [DOI] [PubMed] [Google Scholar]
- Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Bourillot PY. Morphogen gradientinterpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X, Lin X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development. 2004a;131:1563–1575. doi: 10.1242/dev.01051. [DOI] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Wang B, Lin X. Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Development. 2004b;131:601–611. doi: 10.1242/dev.00958. [DOI] [PubMed] [Google Scholar]
- Han C, Yan D, Belenkaya TY, Lin X. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development. 2005;132:667–679. doi: 10.1242/dev.01636. [DOI] [PubMed] [Google Scholar]
- Hufnagel L, Kreuger J, Cohen SM, Shraiman BI. On the role of glypicans in the process of morphogen gradient formation. Dev Biol. 2006;300:512–522. doi: 10.1016/j.ydbio.2006.08.076. [DOI] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CA, Dimitroff BD, Rawson JM, Selleck SB. Spatial regulation of Wingless morphogen distribution and signaling by Dally-like protein. Dev Cell. 2004;7:513–523. doi: 10.1016/j.devcel.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Kreuger J, Perez L, Giraldez AJ, Cohen SM. Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Dev Cell. 2004;7:503–512. doi: 10.1016/j.devcel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Lamanna WC, Baldwin RJ, Padva M, Kalus I, Ten Dam G, van Kuppevelt TH, Gallagher JT, von Figura K, Dierks T, Merry CL. Heparan sulfate 6-O-endosulfatases: discrete in vivo activities and functional co-operativity. Biochem J. 2006;400:63–73. doi: 10.1042/BJ20060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G. Morphogens, compartments, and pattern: lessons from drosophila? Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–6021. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature. 1999;400:281–284. doi: 10.1038/22343. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem. 2002;277:49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzny CK, Holmgren R. The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech Dev. 1995;52:137–150. doi: 10.1016/0925-4773(95)00397-j. [DOI] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development. 1997;124:871–880. doi: 10.1242/dev.124.4.871. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Pai LM, Orsulic S, Bejsovec A, Peifer M. Negative regulation of Armadillo, a Wingless effector in Drosophila. Development. 1997;124:2255–2266. doi: 10.1242/dev.124.11.2255. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Bernfield M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature. 2000;404:725–728. doi: 10.1038/35008000. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Hacker U. Wingless, hedgehog and heparan sulfate proteoglycans. Development. 2004;131:2509–2511. doi: 10.1242/dev.01225. author reply 2511–2503. [DOI] [PubMed] [Google Scholar]
- Phillips RG, Whittle JR. wingless expression mediates determination of peripheral nervous system elements in late stages of Drosophila wing disc development. Development. 1993;118:427–438. doi: 10.1242/dev.118.2.427. [DOI] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr Biol. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- Takei Y, Ozawa Y, Sato M, Watanabe A, Tabata T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development. 2004;131:73–82. doi: 10.1242/dev.00913. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kamimura K, Nakato H, Archer M, Staatz W, Fox B, Humphrey M, Olson S, Futch T, Kaluza V, Siegfried E, Stam L, Selleck SB. The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature. 1999;400:276–280. doi: 10.1038/22336. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Williams JA, Paddock SW, Carroll SB. Pattern formation in a secondary field: a hierarchy of regulatory genes subdivides the developing Drosophila wing disc into discrete subregions. Development. 1993;117:571–584. doi: 10.1242/dev.117.2.571. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yan D, Wu Y, Feng Y, Lin SC, Lin X. The core protein of glypican Dally-like determines its biphasic activity in wingless morphogen signaling. Dev Cell. 2009;17:470–481. doi: 10.1016/j.devcel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]