Abstract
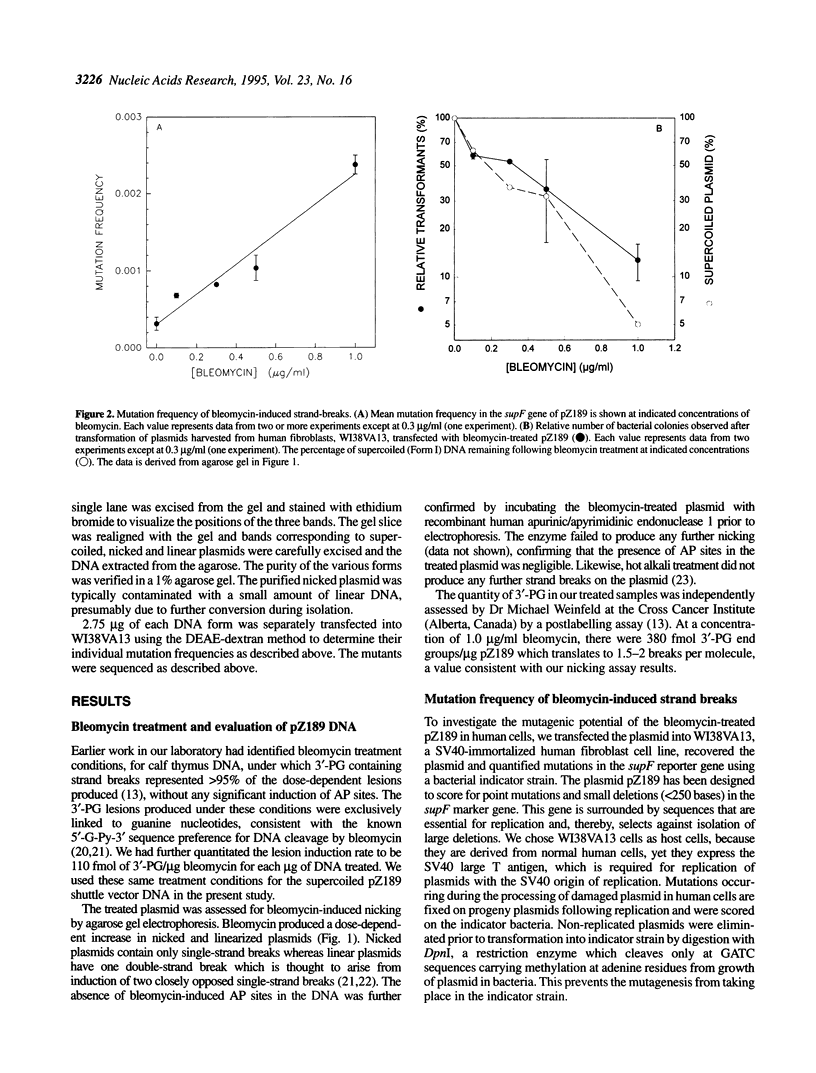
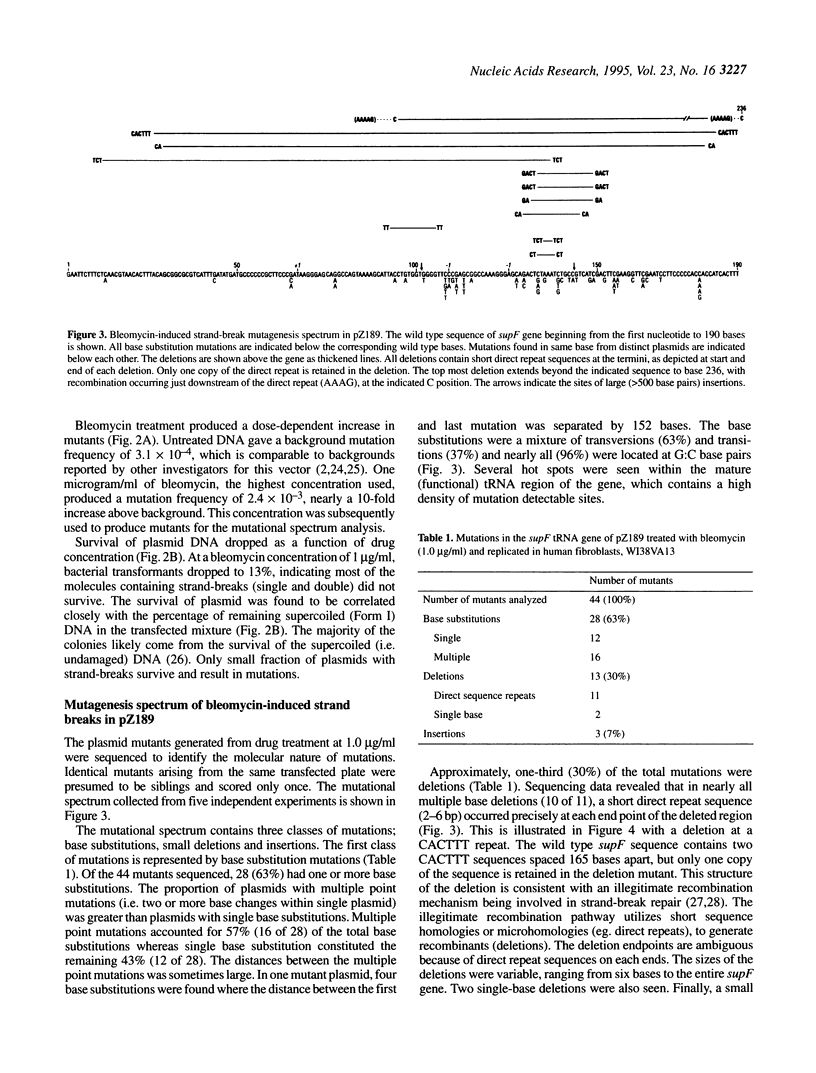
Using the radiomimetic drug, bleomycin, we have determined the mutagenic potential of DNA strand breaks in the shuttle vector pZ189 in human fibroblasts. The bleomycin treatment conditions used produce strand breaks with 3'-phosphoglycolate termini as > 95% of the detectable dose-dependent lesions. Breaks with this end group represent 50% of the strand break damage produced by ionizing radiation. We report that such strand breaks are mutagenic lesions. The type of mutation produced is largely determined by the type of strand break on the plasmid (i.e. single versus double). Mutagenesis studies with purified DNA forms showed that nicked plasmids (i.e. those containing single-strand breaks) predominantly produce base substitutions, the majority of which are multiples, which presumably originate from error-prone polymerase activity at strand break sites. In contrast, repair of linear plasmids (i.e. those containing double-strand breaks) mainly results in deletions at short direct repeat sequences, indicating the involvement of illegitimate recombination. The data characterize the nature of mutations produced by single- and double-strand breaks in human cells, and suggests that deletions at direct repeats may be a 'signature' mutation for the processing of DNA double-strand breaks.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett R. A., Swerdlow P. S., Povirk L. F. Spontaneous cleavage of bleomycin-induced abasic sites in chromatin and their mutagenicity in mammalian shuttle vectors. Biochemistry. 1993 Mar 30;32(12):3188–3195. doi: 10.1021/bi00063a034. [DOI] [PubMed] [Google Scholar]
- Bredberg A., Kraemer K. H., Seidman M. M. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning S., Dryja T. P. Short, direct repeats at the breakpoints of deletions of the retinoblastoma gene. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5044–5048. doi: 10.1073/pnas.86.13.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuyper J., Piette J., Merville M. P., Van de Vorst A. Termini generated at the site of the DNA breakage mediated by photoexcited promazines. Biochem Pharmacol. 1986 Apr 15;35(8):1345–1350. doi: 10.1016/0006-2952(86)90280-7. [DOI] [PubMed] [Google Scholar]
- DiCioccio R., Srivastava B. I. Effect of bleomycin on deoxynucleotide-polymerizing enzymes from human cells. Cancer Res. 1976 May;36(5):1664–1668. [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinkwater N. R., Miller E. C., Miller J. A. Estimation of apurinic/apyrimidinic sites and phosphotriesters in deoxyribonucleic acid treated with electrophilic carcinogens and mutagens. Biochemistry. 1980 Oct 28;19(22):5087–5092. doi: 10.1021/bi00563a023. [DOI] [PubMed] [Google Scholar]
- Ganesh A., North P., Thacker J. Repair and misrepair of site-specific DNA double-strand breaks by human cell extracts. Mutat Res. 1993 May;299(3-4):251–259. doi: 10.1016/0165-1218(93)90101-i. [DOI] [PubMed] [Google Scholar]
- Gentil A., Cabral-Neto J. B., Mariage-Samson R., Margot A., Imbach J. L., Rayner B., Sarasin A. Mutagenicity of a unique apurinic/apyrimidinic site in mammalian cells. J Mol Biol. 1992 Oct 20;227(4):981–984. doi: 10.1016/0022-2836(92)90513-j. [DOI] [PubMed] [Google Scholar]
- Giloni L., Takeshita M., Johnson F., Iden C., Grollman A. P. Bleomycin-induced strand-scission of DNA. Mechanism of deoxyribose cleavage. J Biol Chem. 1981 Aug 25;256(16):8608–8615. [PubMed] [Google Scholar]
- Henner W. D., Rodriguez L. O., Hecht S. M., Haseltine W. A. gamma Ray induced deoxyribonucleic acid strand breaks. 3' Glycolate termini. J Biol Chem. 1983 Jan 25;258(2):711–713. [PubMed] [Google Scholar]
- Keyse S. M., Amaudruz F., Tyrrell R. M. Determination of the spectrum of mutations induced by defined-wavelength solar UVB (313-nm) radiation in mammalian cells by use of a shuttle vector. Mol Cell Biol. 1988 Dec;8(12):5425–5431. doi: 10.1128/mcb.8.12.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner C. U., Patil C. K., Evans J. W., Cuomo C. A., Fried L. M., Carter T., Oettinger M. A., Brown J. M. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995 Feb 24;267(5201):1178–1183. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- Kow Y. W., Faundez G., Melamede R. J., Wallace S. S. Processing of model single-strand breaks in phi X-174 RF transfecting DNA by Escherichia coli. Radiat Res. 1991 Jun;126(3):357–366. [PubMed] [Google Scholar]
- Kunkel T. A., Alexander P. S. The base substitution fidelity of eucaryotic DNA polymerases. Mispairing frequencies, site preferences, insertion preferences, and base substitution by dislocation. J Biol Chem. 1986 Jan 5;261(1):160–166. [PubMed] [Google Scholar]
- Kunkel T. A. Mutational specificity of depurination. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1494–1498. doi: 10.1073/pnas.81.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller S. P., Godbout R., Chan D. W., Weinfeld M., Day R. S., 3rd, Barron G. M., Allalunis-Turner J. Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science. 1995 Feb 24;267(5201):1183–1185. doi: 10.1126/science.7855602. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Morris T., Thacker J. Formation of large deletions by illegitimate recombination in the HPRT gene of primary human fibroblasts. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1392–1396. doi: 10.1073/pnas.90.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullenders L. H., Hazekamp-van Dokkum A. M., Kalle W. H., Vrieling H., Zdzienicka M. Z., van Zeeland A. A. UV-induced photolesions, their repair and mutations. Mutat Res. 1993 May;299(3-4):271–276. doi: 10.1016/0165-1218(93)90103-k. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Maidhof A., Arendes J., Geurtsen W., Zahn R. K., Schmidseder R. Additive effects of bleomycin and neocarzinostatin on degradation of DNA, inhibition of DNA polymerase beta, and cell growth. Cancer Res. 1979 Sep;39(9):3768–3773. [PubMed] [Google Scholar]
- Niwa O., Moses R. E. Synthesis by DNA polymerase I on bleomycin-treated deoxyribonucleic acid: a requirement for exonuclease III. Biochemistry. 1981 Jan 20;20(2):238–244. doi: 10.1021/bi00505a002. [DOI] [PubMed] [Google Scholar]
- Phillips J. W., Morgan W. F. Illegitimate recombination induced by DNA double-strand breaks in a mammalian chromosome. Mol Cell Biol. 1994 Sep;14(9):5794–5803. doi: 10.1128/mcb.14.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Bennett R. A., Wang P., Swerdlow P. S., Austin M. J. Single base-pair deletions induced by bleomycin at potential double-strand cleavage sites in the aprt gene of stationary phase Chinese hamster ovary D422 cells. J Mol Biol. 1994 Oct 21;243(2):216–226. doi: 10.1006/jmbi.1994.1649. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Han Y. H., Steighner R. J. Structure of bleomycin-induced DNA double-strand breaks: predominance of blunt ends and single-base 5' extensions. Biochemistry. 1989 Jul 11;28(14):5808–5814. doi: 10.1021/bi00440a016. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Porter T. N., Wilson J. H. Mechanisms of nonhomologous recombination in mammalian cells. Mol Cell Biol. 1985 Oct;5(10):2599–2607. doi: 10.1128/mcb.5.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouet P., Smih F., Jasin M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol. 1994 Dec;14(12):8096–8106. doi: 10.1128/mcb.14.12.8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan K. Ionizing radiation and genetic risks. III. Nature of spontaneous and radiation-induced mutations in mammalian in vitro systems and mechanisms of induction of mutations by radiation. Mutat Res. 1991 Jul;258(1):75–97. doi: 10.1016/0165-1110(91)90029-u. [DOI] [PubMed] [Google Scholar]
- Schwartz J. L., Mustafi R., Beckett M. A., Czyzewski E. A., Farhangi E., Grdina D. J., Rotmensch J., Weichselbaum R. R. Radiation-induced DNA double-strand break frequencies in human squamous cell carcinoma cell lines of different radiation sensitivities. Int J Radiat Biol. 1991 Jun;59(6):1341–1352. doi: 10.1080/09553009114551211. [DOI] [PubMed] [Google Scholar]
- Seidman M. M., Bredberg A., Seetharam S., Kraemer K. H. Multiple point mutations in a shuttle vector propagated in human cells: evidence for an error-prone DNA polymerase activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4944–4948. doi: 10.1073/pnas.84.14.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikpi M. O., Dry S. M., Freedman M. L., Lurie A. G. Mutations caused by gamma-radiation-induced double-strand breaks in a shuttle plasmid replicated in human lymphoblasts. Int J Radiat Biol. 1992 Nov;62(5):555–562. doi: 10.1080/09553009214552471. [DOI] [PubMed] [Google Scholar]
- Steighner R. J., Povirk L. F. Bleomycin-induced DNA lesions at mutational hot spots: implications for the mechanism of double-strand cleavage. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8350–8354. doi: 10.1073/pnas.87.21.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. C., Sikpi M. O., Preston R. J., Mitra S., Jaberaboansari A. Mutations induced by ionizing radiation in a plasmid replicated in human cells. I. Similar, nonrandom distribution of mutations in unirradiated and X-irradiated DNA. Radiat Res. 1991 Aug;127(2):190–201. [PubMed] [Google Scholar]
- Weinfeld M., Soderlind K. J. 32P-postlabeling detection of radiation-induced DNA damage: identification and estimation of thymine glycols and phosphoglycolate termini. Biochemistry. 1991 Jan 29;30(4):1091–1097. doi: 10.1021/bi00218a031. [DOI] [PubMed] [Google Scholar]
- Whitaker S. J., Ung Y. C., McMillan T. J. DNA double-strand break induction and rejoining as determinants of human tumour cell radiosensitivity. A pulsed-field gel electrophoresis study. Int J Radiat Biol. 1995 Jan;67(1):7–18. doi: 10.1080/09553009514550021. [DOI] [PubMed] [Google Scholar]
- Winters T. A., Weinfeld M., Jorgensen T. J. Human HeLa cell enzymes that remove phosphoglycolate 3'-end groups from DNA. Nucleic Acids Res. 1992 May 25;20(10):2573–2580. doi: 10.1093/nar/20.10.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]