Abstract
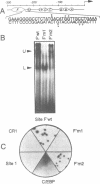
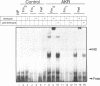
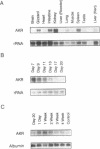
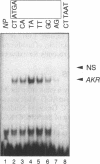
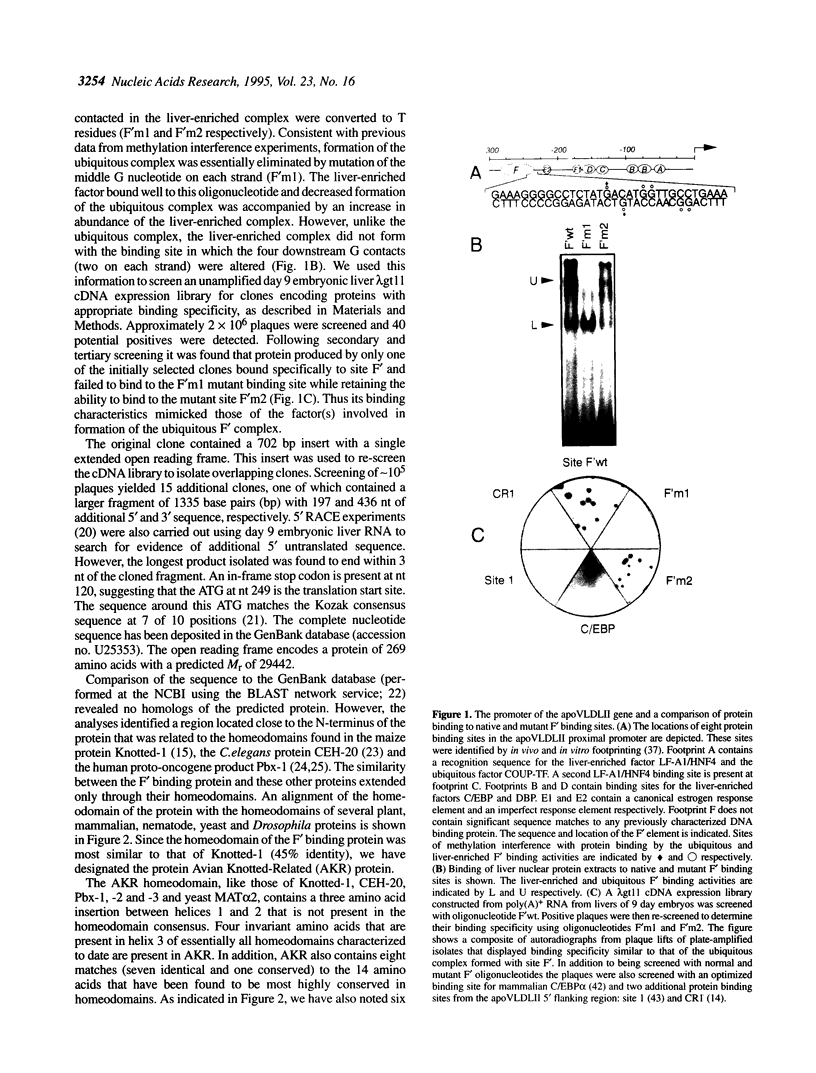
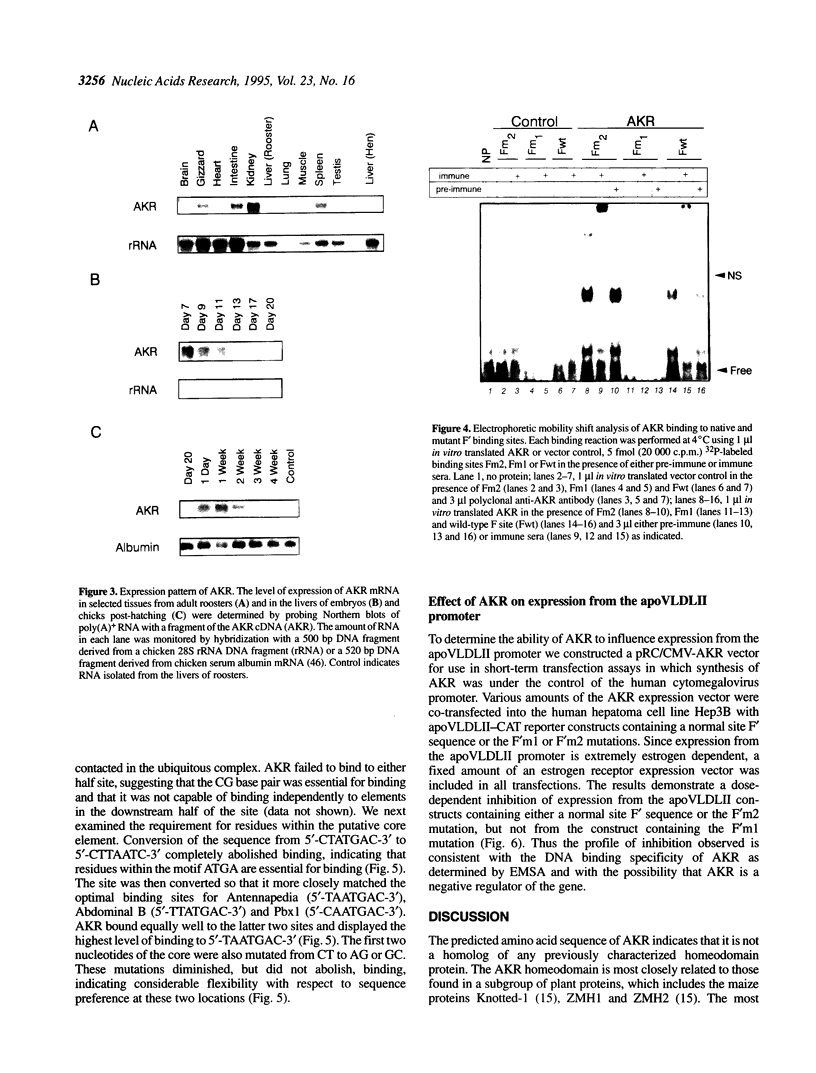
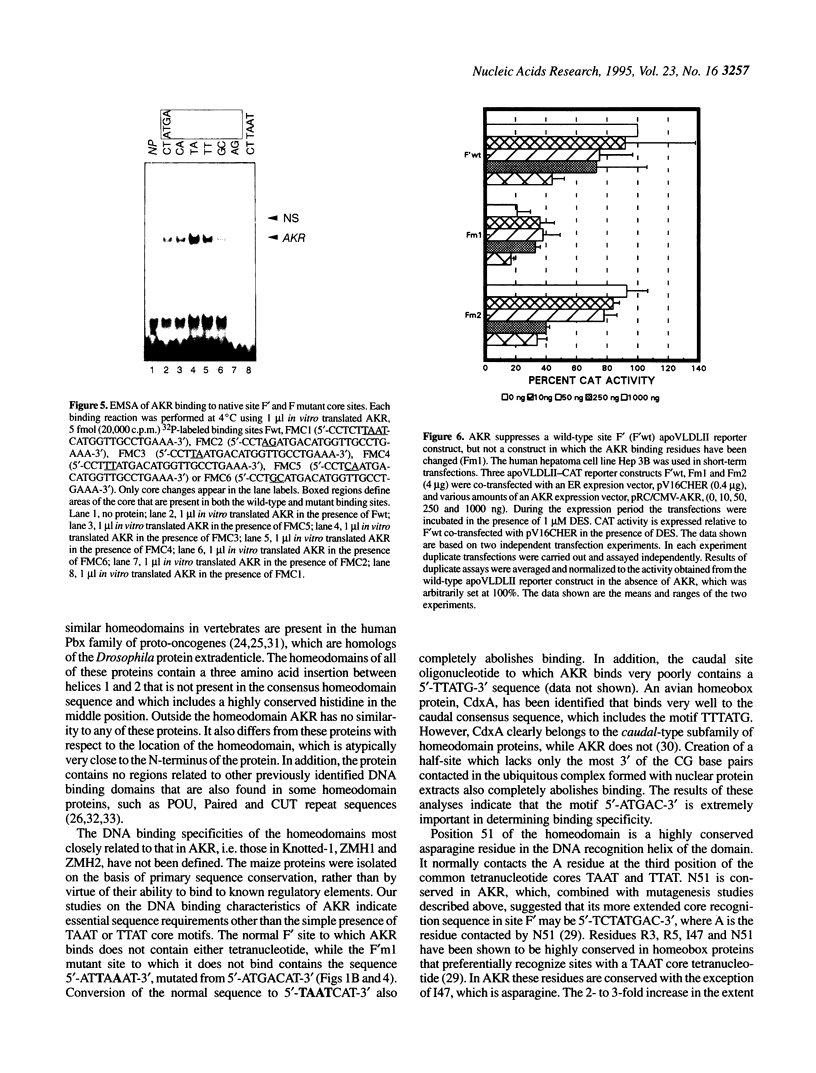
Expression of the avian apoVLDLII gene is liver specific and completely dependent on estrogen. Previous analyses of protein binding sites in the apoVLDLII promoter revealed interactions between liver-enriched and ubiquitous factors at a location, site F', between nucleotides -229 and -260 relative to the major transcriptional start site. Site-directed mutagenesis of G residues contacted by these factors decreased expression from the promoter approximately 5-fold in the avian hepatoma cell line LMH2A. We have used this site to screen a cDNA expression library constructed from day 9 embryonic liver RNA. One of the two DNA binding factors isolated is a novel homeodomain protein. With the exception of the homeodomain itself, which is atypically located close to the protein N-terminus, the factor displays little similarity to any known DNA binding protein. Its homeodomain is most similar to that of the maize protein Knotted-1, while the most closely related vertebrate domain is that of the human proto-oncoprotein Pbx1. We demonstrate that the DNA binding specificity of the factor is consistent with its involvement in the ubiquitous complex formed with site F' and that it is capable of suppressing expression from the apoVLDLII promoter in short-term transfection experiments. These studies, combined with its DNA binding specificity, the tissue distribution of its mRNA and its developmental regulation, suggest a role as a negative regulator of gene expression in non-hepatic tissues and in the liver early during embryogenesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Ahlstrom-Jonasson L., Smith M., Tatchell K., Nasmyth K. A., Hall B. D. The sequence of the DNAs coding for the mating-type loci of Saccharomyces cerevisiae. Cell. 1981 Nov;27(1 Pt 2):15–23. doi: 10.1016/0092-8674(81)90356-1. [DOI] [PubMed] [Google Scholar]
- Aurora R., Herr W. Segments of the POU domain influence one another's DNA-binding specificity. Mol Cell Biol. 1992 Feb;12(2):455–467. doi: 10.1128/mcb.12.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman J. M., Wijnholds J., Schippers I. J., Pot W., Gruber M., Ab G. Regulatory elements and DNA-binding proteins mediating transcription from the chicken very-low-density apolipoprotein II gene. Nucleic Acids Res. 1991 Oct 11;19(19):5371–5377. doi: 10.1093/nar/19.19.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson G. V., Nguyen T. H., Maas R. L. The expression pattern of the murine Hoxa-10 gene and the sequence recognition of its homeodomain reveal specific properties of Abdominal B-like genes. Mol Cell Biol. 1995 Mar;15(3):1591–1601. doi: 10.1128/mcb.15.3.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz E. A., Evans M. I. Functional analysis of regulatory regions upstream and in the first intron of the estrogen-responsive chicken very low density apolipoprotein II gene. J Biol Chem. 1992 Apr 5;267(10):7134–7138. [PubMed] [Google Scholar]
- Brennan R. G., Matthews B. W. The helix-turn-helix DNA binding motif. J Biol Chem. 1989 Feb 5;264(4):1903–1906. [PubMed] [Google Scholar]
- Bürglin T. R., Ruvkun G. New motif in PBX genes. Nat Genet. 1992 Aug;1(5):319–320. doi: 10.1038/ng0892-319. [DOI] [PubMed] [Google Scholar]
- Chan L., Jackson R. L., O'Malley B. W., Means A. R. Synthesis of very low density lipoproteins in the cockerel. Effects of estrogen. J Clin Invest. 1976 Aug;58(2):368–379. doi: 10.1172/JCI108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan V., Elbrecht A., Goldman P., Lazier C. B., Deeley R. The avian apoprotein II very low density lipoprotein gene. Methylation patterns of 5' and 3' flanking regions during development and following induction by estrogen. J Biol Chem. 1982 Dec 10;257(23):14453–14460. [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The sequence specificity of homeodomain-DNA interaction. Cell. 1988 Sep 23;54(7):1081–1090. doi: 10.1016/0092-8674(88)90123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker S. C., Jackson D. G., von Kessler D. P., Sun B. I., Young K. E., Beachy P. A. The degree of variation in DNA sequence recognition among four Drosophila homeotic proteins. EMBO J. 1994 Aug 1;13(15):3551–3560. doi: 10.1002/j.1460-2075.1994.tb06662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker S. C., von Kessler D. P., Beachy P. A. Differential DNA sequence recognition is a determinant of specificity in homeotic gene action. EMBO J. 1992 Nov;11(11):4059–4072. doi: 10.1002/j.1460-2075.1992.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbrecht A., Lazier C. B., Protter A. A., Williams D. L. Independent developmental programs for two estrogen-regulated genes. Science. 1984 Aug 10;225(4662):639–641. doi: 10.1126/science.6740331. [DOI] [PubMed] [Google Scholar]
- Elbrecht A., Williams D. L., Blue M. L., Lazier C. B. Differential ontogeny of estrogen responsiveness in the chick embryo liver. Can J Biochem. 1981 Aug;59(8):606–612. doi: 10.1139/o81-084. [DOI] [PubMed] [Google Scholar]
- Evans M. I., Silva R., Burch J. B. Isolation of chicken vitellogenin I and III cDNAs and the developmental regulation of five estrogen-responsive genes in the embryonic liver. Genes Dev. 1988 Jan;2(1):116–124. doi: 10.1101/gad.2.1.116. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. I., Burns A. T., Christmann J. L., Deeley R. G. Cloning of a double-stranded cDNA that codes for a portion of chicken preproalbumin. A general method for isolating a specific DNA sequence from partially purified mRNA. J Biol Chem. 1978 Dec 10;253(23):8629–8639. [PubMed] [Google Scholar]
- Grant C. E., Deeley R. G. Cloning and characterization of chicken YB-1: regulation of expression in the liver. Mol Cell Biol. 1993 Jul;13(7):4186–4196. doi: 10.1128/mcb.13.7.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes S. D., Brent R. A genetic model for interaction of the homeodomain recognition helix with DNA. Science. 1991 Jan 25;251(4992):426–430. doi: 10.1126/science.1671176. [DOI] [PubMed] [Google Scholar]
- Harada R., Dufort D., Denis-Larose C., Nepveu A. Conserved cut repeats in the human cut homeodomain protein function as DNA binding domains. J Biol Chem. 1994 Jan 21;269(3):2062–2067. [PubMed] [Google Scholar]
- Hoey T., Levine M. Divergent homeo box proteins recognize similar DNA sequences in Drosophila. Nature. 1988 Apr 28;332(6167):858–861. doi: 10.1038/332858a0. [DOI] [PubMed] [Google Scholar]
- Hoodless P. A., Roy R. N., Ryan A. K., Haché R. J., Vasa M. Z., Deeley R. G. Developmental regulation of specific protein interactions with an enhancerlike binding site far upstream from the avian very-low-density apolipoprotein II gene. Mol Cell Biol. 1990 Jan;10(1):154–164. doi: 10.1128/mcb.10.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Murre C., Sun X. H., Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990 Feb 23;60(4):547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Hoey T. Homeobox proteins as sequence-specific transcription factors. Cell. 1988 Nov 18;55(4):537–540. doi: 10.1016/0092-8674(88)90209-7. [DOI] [PubMed] [Google Scholar]
- Margalit Y., Yarus S., Shapira E., Gruenbaum Y., Fainsod A. Isolation and characterization of target sequences of the chicken CdxA homeobox gene. Nucleic Acids Res. 1993 Oct 25;21(21):4915–4922. doi: 10.1093/nar/21.21.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W., Garber R. L., Wirz J., Kuroiwa A., Gehring W. J. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984 Jun;37(2):403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Levine M. S., Hafen E., Kuroiwa A., Gehring W. J. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. 1984 Mar 29-Apr 4Nature. 308(5958):428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- Monica K., Galili N., Nourse J., Saltman D., Cleary M. L. PBX2 and PBX3, new homeobox genes with extensive homology to the human proto-oncogene PBX1. Mol Cell Biol. 1991 Dec;11(12):6149–6157. doi: 10.1128/mcb.11.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourse J., Mellentin J. D., Galili N., Wilkinson J., Stanbridge E., Smith S. D., Cleary M. L. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990 Feb 23;60(4):535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- Rauskolb C., Wieschaus E. Coordinate regulation of downstream genes by extradenticle and the homeotic selector proteins. EMBO J. 1994 Aug 1;13(15):3561–3569. doi: 10.1002/j.1460-2075.1994.tb06663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A. K., Schrader T. J., Wright R. B., Buchanan L., Deeley R. G. Characterization of protein interactions with positive and negative elements regulating the apoVLDLII gene. DNA Cell Biol. 1994 Oct;13(10):987–999. doi: 10.1089/dna.1994.13.987. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Yamamoto M., Kuroiwa A. Cell type dependent transcription regulation by chick homeodomain proteins. Mech Dev. 1992 Mar;37(1-2):25–36. doi: 10.1016/0925-4773(92)90012-9. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Singh H., Clerc R. G., LeBowitz J. H. Molecular cloning of sequence-specific DNA binding proteins using recognition site probes. Biotechniques. 1989 Mar;7(3):252–261. [PubMed] [Google Scholar]
- Skalnik D. G., Strauss E. C., Orkin S. H. CCAAT displacement protein as a repressor of the myelomonocytic-specific gp91-phox gene promoter. J Biol Chem. 1991 Sep 5;266(25):16736–16744. [PubMed] [Google Scholar]
- Treisman J., Gönczy P., Vashishtha M., Harris E., Desplan C. A single amino acid can determine the DNA binding specificity of homeodomain proteins. Cell. 1989 Nov 3;59(3):553–562. doi: 10.1016/0092-8674(89)90038-x. [DOI] [PubMed] [Google Scholar]
- Van Dijk M. A., Voorhoeve P. M., Murre C. Pbx1 is converted into a transcriptional activator upon acquiring the N-terminal region of E2A in pre-B-cell acute lymphoblastoid leukemia. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6061–6065. doi: 10.1073/pnas.90.13.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- Vollbrecht E., Veit B., Sinha N., Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991 Mar 21;350(6315):241–243. doi: 10.1038/350241a0. [DOI] [PubMed] [Google Scholar]
- Wijnholds J., Muller E., Ab G. Oestrogen facilitates the binding of ubiquitous and liver-enriched nuclear proteins to the apoVLDL II promoter in vivo. Nucleic Acids Res. 1991 Jan 11;19(1):33–41. doi: 10.1093/nar/19.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskocil R., Bensky P., Dower W., Goldberger R. F., Gordon J. I., Deeley R. G. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk M. A., Murre C. extradenticle raises the DNA binding specificity of homeotic selector gene products. Cell. 1994 Aug 26;78(4):617–624. doi: 10.1016/0092-8674(94)90526-6. [DOI] [PubMed] [Google Scholar]