Abstract
Purpose
The impact of an Antibiotic Restriction Program (ARP) on the patterns of antibiotic use in the treatment of community-acquired pneumonia (CAP) was examined. We also evaluated the association between the ARP and the length of hospital stay in regard to CAP treatment and cost savings associated with the implementation of the ARP.
Methods
A retrospective cohort study of patients admitted with CAP was conducted at two six-month periods, one prior to the ARP and one after the ARP. The health system’s Computerized Patient Record System (CPRS) was used to obtain demographics, length of hospital stays, readmission rates, blood culture results, co–morbidities, antibiotic use, and durations of therapy. A total of 130 patients met the inclusion criteria for the final analyses. Average drug costs, employee salaries, and the cost of laboratory procedures were used to assess cost savings associated with the ARP.
Results
From a total of 132 antibiotics that were ordered to treat CAP in the pre-ARP period, 28 were restricted (21.2%). However, the number of restricted antibiotics ordered was significantly reduced to 12 out of 114 (10.2%) antibiotics ordered in the post-ARP period (P = 0.024). In post-ARP implementation, mean length of hospital stay was also significantly reduced from 7.6 to 5.8 days (P = 0.017), and although not statistically significant, 30-day readmission rates declined from 16.9% to 6.2% (P = 0.097). The ARP was also associated with $943 savings per patient treated for CAP.
Conclusions
In addition to a decrease in the antibiotic utilization and the mean length of hospital stay, the ARP may have yielded cost savings and reduced the readmission rates for those patients admitted and treated for CAP.
Keywords: Antibiotic restriction program, infection, antibiotics, hospital
Introduction
Antimicrobial agents are among the most frequently and inappropriately prescribed medications worldwide [1]. Broad evidence indicates that approximately 64% of all hospitalized patients receive antibiotic drugs during their hospital stay [2]. It has been reported that more than half of antibiotics may be inappropriately prescribed [3]. With the high volume of broad spectrum antibiotics prescribed, there may be severe consequences if simple management of prescribing habits are not initiated. Unnecessary use of antibiotics can contribute to the development of antimicrobial resistance leading to possible development of suprainfections [4, 5], increased time and effort to care for these infections, and consequent increased health care costs [3, 6]. Despite published guidelines, efforts to curtail unnecessary use of antimicrobial agents, surveys of individual hospitals have implicated frequent inadequacy in implementing these guidelines [6]. Medical centers have increasingly initiated programs to restrict the use of antibiotics [7]. However, the degree to which these programs can decrease healthcare costs and the development of resistance is uncertain [8, 9].
In September 2005 the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas implemented an Antibiotic Restriction Program (ARP) to curtail the incidence of Clostridium difficile-associated disease (CDAD) [10-12] and to reduce the spread of multidrug resistance pathogens. A secondary objective was to reduce the costs associated with the excessive use of antibiotics throughout the medical center.
In order to evaluate the impact of the ARP on the antibiotic use, we specifically chose to study a subgroup of patients with community-acquired pneumonia (CAP), an inflammatory infection of the alveoli, distal airways, and interstitium of the lungs that requires antibiotic therapy and that has well-established risk factors, symptoms, and treatment options.
An estimated 2 to 5 million cases of CAP are diagnosed in the United States alone every year leading to more than one million inpatient hospitalizations and more than 50,000 deaths [13].
Inappropriate use of antibiotics to treat CAP has been associated with higher morbidity and mortality rates [13, 14, 15].
In this study, we compared the antibiotic utilization in the treatment of CAP before and after the initiation of the ARP and evaluated the association between the ARP and the length of hospital stay in regard to CAP treatment. We also examined potential cost savings associated with the implementation of the ARP.
Methods
IRB approval
This study was approved by the Institutional Review Boards (IRB) at both Baylor College of Medicine and the Research and Development (R&D) Committee at the MEDVAMC in Houston, Texas.
Site of study
The MEDVAMC is a teaching hospital with 375 hospital beds, a 40-bed Spinal Cord Injury Center, and a 120-bed transitional care unit. This state-of-the-art facility serves as the primary health care provider for almost 120,000 veterans in southeast Texas and logged more than 800,000 outpatient visits in the fiscal year 2007.
Description
Prior to the ARP implementation, restricted antibiotics were dispensed without prior approval from a member of the Infectious Diseases (ID) Team, which was appointed after the implementation of the ARP to oversee the appropriate prescription of these antibiotics. The ID Team included a multidisciplinary team of infectious diseases staff physicians and clinical/staff pharmacists. However, after the implementation of the ARP, the ID Team’s approval was necessary prior to dispensing and initiating a course of restricted antibiotic, which included: piperacillin/tazobactam, cefepime, ertapenem, imipenem, moxifloxacin, ciprofloxacin, clindamycin, linezolid, vancomycin, daptomycin, dalfopristin/quinupristin, liposomal amphotericin B, caspofungin, voriconazole, and itraconazole. Those patients that did not receive approval for the restricted antibiotics received formulary (unrestricted) antibiotics that included ampicllin, penicillin, aztreonam metronidazole, gentamicin, amikacin, nafcillin, cefazolin, and first line agents: ceftriaxone and ampicllin/sulbactam. Orders for restricted antibiotics were not honored by the intravenous (IV) room pharmacists without a verbal telephone approval by the ID attending physician or the ID Clinical Pharmacist. Prescribing physicians were required to contact the ID attending physician via a dedicated pager to obtain approval Monday through Friday, 8:00 am to 5:00 pm. The approval process included recommendations for dosage and duration of therapy. After 5:00 pm on weekdays and on weekends, restricted antibiotics were dispensed without approval until 8:00 AM on the next working day when the ID Clinical Pharmacy Specialist reviewed all after-hour and weekend orders for appropriateness. The Clinical Pharmacist could then approve or disapprove the continuation of antibiotic use after further discussions with the ID physician on the consulting service. If the drug was disapproved, the pharmacist called the prescribing physician to discuss the reasons for disapproval (e.g. possibility of use of formulary antibiotics, erroneous microbiology laboratory diagnosis, or no further indication for infection) and to make recommendations for alternative therapy. The prescribing physician then had the option of responding by requesting a formal consultation by the ID service.
Assessment of impact
The impact of the ARP was analyzed in this retrospective cohort study by comparing antibiotic utilization in a six months period prior to the implementation of the program and a six months period after the initiation of the program. The pre-intervention period was defined from October 2004 to March 2005. The ARP was initiated on September 2005, and the post intervention period was defined from October 2005 to March 2006. It was important that the two time frames contain identical months to avoid possible selection bias due to the likelihood of increased pneumonia infections during the winter months.
Average drug costs, employee salaries, and the cost of laboratory procedures were all combined and used to calculate the average bed-care cost per day per patient throughout the various medicine wards at the MEDVAMC. Most patients with CAP are admitted to a general medicine floor, with an average bed-care cost of about $539 per day.
Patient inclusion
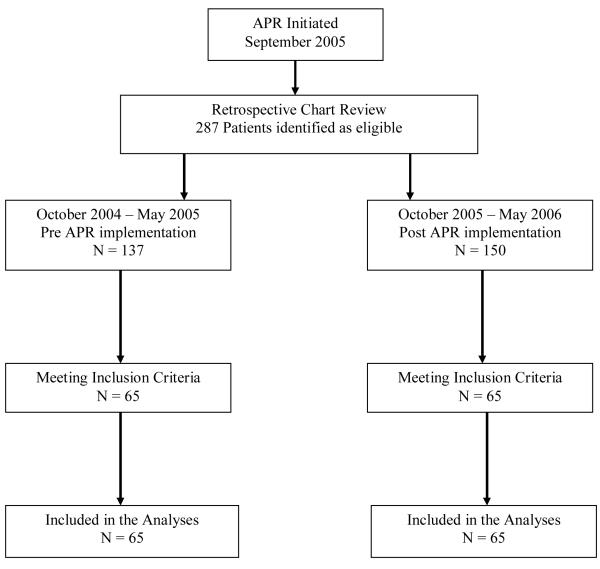
Data for this single center, observational, retrospective, cohort study were obtained by the review of electronic patient medical records from patients who were admitted to the MEDVAMC with the diagnosis of CAP, defined as infectious pneumonia in patients who had not been hospitalized in the preceding two weeks and who had been admitted (< 48 hours) previously [13, 16]. Patients were identified using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9 codes) [18] for pneumonia. We included patients in the study if they had fever, productive cough, chest pain, localized rales, wheezes on examination of the chest, and pulmonary infiltrate or consolidation on chest x–ray, assessed by the radiology report. Fever was defined as having a body temperature of ≥102°F (38.9°C) [17]. Patients already receiving antimicrobial treatment upon admission, those with prior antibiotic exposure for the past 30 days prior to admission and those with suspected hospital acquired (health care associated or aspiration pneumonia) were excluded. Furthermore, patients on antibiotic therapy with any interruption in the prescribed duration and those with >1 prescription for the same antibiotic during the course of the study were included. None of the patients included in this study was ventilated. We did not include patients that were hospitalized in the intensive care units. For consistency of the reviews, only one ID clinical pharmacist performed the chart reviews. The health system’s Computerized Patient Record System (CPRS) was used to obtain demographics as well as data on the length of hospital stays, readmission rates, blood culture results, co-morbidities, allergy status, antibiotic use (including dosage and frequency), and durations of therapy. Only those patients fulfilling the above inclusion criteria were included in the study (Figure1). Outcomes defined in this cohort study included change in the antibiotic utilization, change in the mean length of hospital stay, change in 30-day readmission rate (with an ICD-9 code for infection), and change in the health care cost post implementation of the ARP.
Figure 1.
Study Flow Chart
Statistical analyses
Statistical analyses were performed using Stata software version 8.2 for Windows (Stata, College Station, TX). Chi–square or Fisher’s exact tests were performed to compare the frequencies of restricted antibiotics ordered and to compare baseline characteristics and frequencies of co-morbidities, bacteremia, and readmission rates between pre- and post-ARP. Student’s t–tests were performed to compare the average age, mean number of co-morbidities, mean number of white blood cells (WBC), and mean length of hospital stay between the two groups. A level of significance was set at type I error of 5%.
Results
A total of 287 patients, 137 prior to ARP implementation and 150 post ARP implementation, were identified during the two established time frames using ICD - 9 codes for pneumonia. With the exclusion of patients with prior antibiotic therapy and those with nosocomial, aspiration, or health care associated pneumonia, 130 patients were included in the study. There were no statistically significant differences in the baseline characteristics between the two groups in this study (Table I). Table 2 displays the total number of antibiotics ordered during the two established time frames. Except with moxifloxacin, the use of restricted antibiotics declined post ARP implementation with the largest decline with clindamycin (from 8 to 2). The ARP was associated with a 57% reduction (n = 16) in the frequency of total restricted antibiotics administered to treat CAP. Prior to the ARP implementation, 28 of the 132 total antibiotics ordered were restricted agents (21%). Post-ARP implementation, however, the number of restricted antibiotics used was significantly reduced to 12 out of a total of 114 antibiotics (11%) (P = 0.024) considering that the total number antibiotics (restricted and non-restricted) to treat CAP was also reduced by 14%. A significant reduction in the mean length of stay for CAP treatment (P = 0.017) was also observed after the implementation of the ARP. Prior to the initiation of the program, the mean length of stay for patients being treated for CAP was 7.6 days compared to 5.8 days post implementation of the ARP. Although statistically insignificant (P = 0.097), readmission rates within 30 days of discharge with any ICD-9 code for infection were also reduced post-ARP implementation. For pre-ARP implementation, there were 11 patients (16.9%) with 30 day readmissions compared with 4 patients (6.2%) in the post-ARP implementation group. There were no significant statistical difference between the mean duration of antibiotic therapy pre-ARP (5.59 ± 3.2 days) vs. post-ARP (6.22 ± 3.0 days) (p-value=0.108; two-tailed Student’s t-test).
Table I.
Baseline demographics and clinical status before and after the implementation of antibiotic restriction program (ARP)
| Characteristic | Pre-intervention period (n=65) |
Post-intervention Period (n=65) |
p-value |
|---|---|---|---|
| Mean age, yrs (±SD) | 69.7 (±14.21) | 66.7 (±11.28) | 0.180 |
| Male sex (%) | 62 (95.4) | 64 (98.5) | 0.619 |
| Race no. (%) | |||
| Caucasian | 32 (49.2) | 40 (61.5) | 0.217 |
| African American | 26 (40.0) | 21 (32.3) | 0.465 |
| Hispanic | 6 (9.2) | 4 (9.2) | 0.744 |
| Asian | 1 (1.5) | 0 (0) | 1 |
| Mean no. of co-morbidities (±SD) | 1.52 (±0.13) | 1.57 (±0.13) | 0.803 |
| COPD (%) | 26 (40.0) | 32 (49.2) | 0.378 |
| Diabetes mellitus (%) | 23 (35.4) | 19 (29.2) | 0.574 |
| Coronary artery disease (%) | 11 (16.9) | 21 (32.3) | 0.066 |
| Malignancy (%) | 11 (16.9) | 12 (18.5) | 0.642 |
| Alcoholism (%) | 3 (4.6) | 4 (6.15) | 1 |
| Liver disease (%) | 7 (10.8) | 6 (9.2) | 1 |
| Heart failure (%) | 7 (10.8) | 10 (15.4) | 0.604 |
| Renal disease (%) | 7 (10.8) | 3 (4.62) | 0.324 |
| Mortality (%) | 4 (6.2) | 2 (3.1) | 0.680 |
| Mean WBC (1000/mm3) (±SD) | 13.4 (±0.87) | 19.5 (±4.99) | 0.227 |
| Bacteremia1 (%) | 35 (53.8) | 31 (47.7) | 0.599 |
| Citrobacter ssp. | 1 (1.5) | 0 (0) | 1 |
|
| |||
| Morganella morganii | 1 (1.5) | 0 (0) | 1 |
|
| |||
| Staphylococcus aureus | 16 (24.6) | 14 (21.5) | 0.835 |
|
| |||
| MRSA | 2 (3.0) | 1 (1.5) | 1 |
|
| |||
| Streptococcus pneumoniae | 10 (15.4) | 14 (21.5) | 0.498 |
|
| |||
| Pseudomonas ssp. | 3 (4.6) | 0 (0) | 0.244 |
|
| |||
| Escherichia coli | 1 (1.5) | 0 (0) | 1 |
|
| |||
| Haemophilus influenzae | 1 (1.5) | 2 (3.1) | 1 |
|
| |||
| Chlamydia pneumoniae | 0 (0) | 1 (1.5) | 1 |
|
| |||
| Staphylococcus epidermidis | 2 (3.1) | 0 (0) | 0.496 |
|
| |||
| None (%) | 30 (46.2) | 34 (52.3) | 0.599 |
|
| |||
| Allergy status 2 | 0 | 0 | 1 |
SD - Standard Deviation
Bacteremia was defined as the presence ≥ 1 species grown from the participants’ blood cultures
Allergies to any of the restricted antibiotics
Table II.
Antibiotic prescriptions, hospital stays, and readmissions before and after the implementation of ARP
| Attribute | Pre-intervention period (n=65) |
Post-intervention Period (n=65) |
p-value |
|---|---|---|---|
| Mean length of stay, days (±SD) | 7.55 (±0.6) | 5.80 (±0.3) | 0.017* |
| Readmission within 30 days (%) | 11 (16.9) | 4 (6.20) | 0.097 |
| Mean duration of antibiotic therapy, days (±SD) | 5.59 (±3.2) | 6.22 (±3.0) | 0.108 |
| No. of antibiotics used a | 132 | 114 | |
| No. of Restricted Antibiotics (%) b | 28 (21.2) | 12 (10.5) | 0.024* |
| Moxifloxacin | 2 | 4 | |
| Cefepime | 6 | 2 | |
| Clindamycin | 8 | 2 | |
| Vancomycin | 6 | 3 | |
| Ertapenem | 2 | 1 | |
| Piperacillin/Tazobactam | 3 | 0 | |
| Linezolid | 1 | 0 |
Total number of antibiotics ordered during the two periods
Total number of restricted antibiotics and percentages ordered during the two periods
Statistically significant with a type I error of 5%
With a reduction in length of stay from 7.6 to 5.8 days, the ARP program was associated with an average saving of about $943 per patient being treated for CAP. This number does not include the cost savings associated with a reduction in 30 day readmission rates.
Discussion
Infection control programs, although insufficient, may be important in controlling the spread of pathogens and limiting the development of antibiotic resistance [19, 20]. Uncontrolled and widespread prescription of many antibiotics, particularly broad-spectrum agents, is believed to contribute to the development of antibiotic resistance [20]. Altering antibiotic prescription practices through guideline development, restricting antibiotic usage through ARPs, and narrowing the spectrum of antibiotics have all shown varying degrees of effectiveness [21]. However, some studies have indicated that antibiotic control programs may work where traditional infection control programs fail [22].
Some antibiotic control programs have slowed the emergence of antibiotic resistance [23-24], reduced the total number of antibiotics administered, and decreased health care cost [25-26], without compromising the patient quality of care. Other studies based on biomarkers have also shown promising results in reducing the number of antibiotic prescriptions [27]. In this study, we examined the impact of an ARP on the antibiotic use, length of hospital stay, and overall cost in patients with CAP and the complications from that disease without monitoring for antibiotic susceptibility patterns as a measured outcome at the MEDVAMC.
We observed significant reductions in the antibiotic utilization and the mean length of hospital stay when compared patients with CAP during a six-month period prior to the ARP implementation with patients with CAP in a six-month period following the ARP implementation. Although moxifloxacin is the only fluoroquinolone that was increased post APR, but not statistically significant (2-tailed p-value of 0.42), the total use of restricted antibiotics significantly declined post-ARP implementation with the largest decline with clindamycin. However, Infectious Diseases Society America (IDSA) and American Thoracic Society (ATS) guidelines recommend empiric monotherapy with a fluoroquinolone with enhanced activity against S. pneumoniae (gemifloxacin, levofloxacin, moxifloxacin) as the standard of care for community acquired pneumonia in patient with risk factors for drug resistant S. pneumoniae [6].
Furthermore, the total number of antibiotics (both restricted and unrestricted) used to treat CAP also declined 14% after the implementation of the ARP. The largest decrease in the utilization of clindamycin may have been due to the duplication of therapy in the selection of this antibiotic prior to the ARP program. With the increasing antibiotic resistance rates along with the development of supra-infections due to broad-spectrum antibiotic use, a significant reduction in antibiotic utilization may help to limit emergence of resistant pathogens [23, 24].
Budget restrictions continue as healthcare costs increase. Pharmacy and medication costs are no exception. However, hospital costs are often reduced to a greater extent with a reduction in length of stay than by the expenditures of the antimicrobial agents themselves due to the high costs of bed-care cost for inpatients per day. Although it is difficult to determine to what extent the reduced mean length in hospital stay is attributed to the ARP itself, as infection control measures (such as hand washing and sanitization, utilization of protective clothing/masks/gloves, isolation of patients with MRSA, tuberculosis, or CDAD, etc.) may have influenced the outcomes of the study, the reduction in mean length of stay, not including 30 day readmissions, may have been associated with a yearly cost saving of $122,550 based on a $943 per patient savings over the six month study period after the implementation of the ARP. This number is solely in regard to the CAP treatment, and if extrapolated further to include other disease states, an even greater cost savings may have been observed.
Although in this study we examined the association between the implementation of the ARP and antibiotic utilization, duration of hospital stay, and possible cost savings, we did not assess any trends. Furthermore, the outcomes of the study may have been confounded due to several factors including the implementation of infection control measures, differences in the severity of the pneumonia, and an earlier switch to oral therapy. However, more stringent guidelines on dispensing restricted antibiotics may have lead to increased targeted therapy rather than empiric therapy after the implementation of the ARP, hence contributing to a possible shorter mean length of hospital stay.
Larger studies may be needed to further assess the associations between the outcomes in this study and the implementation of the ARP. In addition, studies investigating the possible changes in the multiresistant trends due to the implementation of the ARP may also be warranted.
Acknowledgments
This study was presented in parts at Alcalde XXII Southwest Leadership Conference for Pharmacy Residents & Fellows and The Texas Society of Health System’s Pharmacist 60th Annual Seminar, Dallas, TX, 2008.
M. D. Mansouri has received support from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID; Grant #1R21AI074010-01A2).
Footnotes
Conflict of interest None.
References
- 1.John JF, Fishman NO. Programmatic role of the infectious diseases physical in controlling antimicrobial costs in the hospital. Clin Infect Dis. 1997;24:471–485. doi: 10.1093/clinids/24.3.471. [DOI] [PubMed] [Google Scholar]
- 2.Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168:2254–2260. doi: 10.1001/archinte.168.20.2254. [DOI] [PubMed] [Google Scholar]
- 3.Tobia CC, Aspinall SL, Good CB, Fine MJ, Hanlon JT. Appropriateness of antibiotic prescribing in veterans with community-acquired pneumonia, sinusitis, or acute exacerbations of chronic bronchitis: a cross-sectional study. Clin Ther. 2008;30:1135–1144. doi: 10.1016/j.clinthera.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tacconelli E. Antimicrobial use: risk driver of multidrug resistant microorganisms in healthcare settings. Curr Opin Infect Dis. 2009;22:352–358. doi: 10.1097/QCO.0b013e32832d52e0. [DOI] [PubMed] [Google Scholar]
- 5.Pallasch TJ. Antibiotic resistance. Dent Clin North Am. 2003;47:623–639. doi: 10.1016/s0011-8532(03)00039-9. [DOI] [PubMed] [Google Scholar]
- 6.Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 7.Apisarnthanarak A, Danchaivijitr S, Khawcharoenporn T, Limsrivilai J, Warachan B, Bailey TC, Fraser VJ, Thammasart University Antibiotic Management Team Effectiveness of Education and an Antibiotic – Control Program in a Tertiary Care Hospital in Thailand. Clin Infect Dis. 2006;42:768–775. doi: 10.1086/500325. [DOI] [PubMed] [Google Scholar]
- 8.Gould IM, Jappy B. Trends in hospital antibiotic prescribing after introduction of an antibiotic policy. J Antimicrob Chemother. 1996;38:895–904. doi: 10.1093/jac/38.5.895. [DOI] [PubMed] [Google Scholar]
- 9.Klapp DL, Ramphal R. Antibiotic restriction in hospitals associated with medical schools. Am J Hosp Pharm. 1983;40:1957–1960. [PubMed] [Google Scholar]
- 10.Nuila F, Cadle RM, Logan N, Musher DM. The impact of antibiotic stewardship on Clostridium dificile disease. Infect Control Hosp Epidemiol. 2008;29:1096–1097. doi: 10.1086/591450. [DOI] [PubMed] [Google Scholar]
- 11.Muto CA, Pokrywka M, Shutt K, Mendelsohn AB, Nouri K, Posey K, Roberts T, Croyle K, Krystofiak S, Patel-Brown S, Pasculle AW, Paterson DL, Saul M, Harrison LH. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26:273–280. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 12.Price J, Cheek E, Lippett S, Cubbon M, Gerding DN, Sambol SP, Citron DM, Llewelyn M. Impact of an intervention to control Clostridium difficile infection on hospital- and community-onset disease; an interrupted time series analysis. Clin Microbiol Infect. 2010;16:1297–1302. doi: 10.1111/j.1469-0691.2009.03077.x. [DOI] [PubMed] [Google Scholar]
- 13.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG, Infectious Diseases Society of America. American Thoracic Society Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community – Acquired Pneumonia in Adults. Clin Infect Dis. 2007;44:S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polgreen PM, Chen YY, Cavanaugh JE, Ward M, Coffman S, Hornick DB, Diekema DJ, Herwaldt LA. An outbreak of severe Clostridium difficile-associated disease possibly related to inappropriate antimicrobial therapy for community-acquired pneumonia. Infect Control Hosp Epidemiol. 2007;28:212–214. doi: 10.1086/512174. [DOI] [PubMed] [Google Scholar]
- 15.Johnson D, Carriere KC, Jin Y, Marrie T. Appropriate antibiotic utilization in seniors prior to hospitalization for community-acquired pneumonia is associated with decreased in-hospital mortality. J Clin Pharm Ther. 2004;29:231–239. doi: 10.1111/j.1365-2710.2004.00553.x. [DOI] [PubMed] [Google Scholar]
- 16.Halm EA, Teirstein AS. Management of Community – Acquired Pneumonia. N Engl J Med. 2002;247:2039–2045. doi: 10.1056/NEJMcp020499. [DOI] [PubMed] [Google Scholar]
- 17.Cunha BA. Fever in the intensive care unit. Intensive Care Med. 1999;25:648–651. doi: 10.1007/s001340050925. [DOI] [PubMed] [Google Scholar]
- 18.Clinical Modification (ICD-9 codes) 9th Revision, 6th Edition Practice Management Information Corporation; 2005. International Classification of Diseases. [Google Scholar]
- 19.Ebnöther C, Tanner B, Schmid F, La Rocca V, Heinzer I, Bregenzer T. Impact of an infection control program on the prevalence of nosocomial infections at a tertiary care center in Switzerland. Infect Control Hosp Epidemiol. 2008;29:38–43. doi: 10.1086/524330. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharyya BK. Antibiotic resistance--a close look. Hindustan Antibiot Bull. 1999;41:25–31. [PubMed] [Google Scholar]
- 21.Hall CS, Ost DE. Effectiveness of programs to decrease antimicrobial resistance in the intensive care unit. Semin Respir Infect. 2003;18:112–121. [PubMed] [Google Scholar]
- 22.Paterson DL. Restrictive antibiotic policies are appropriate in intensive care units. Crit Care Med. 2003;31:S25–28. doi: 10.1097/00003246-200301001-00004. [DOI] [PubMed] [Google Scholar]
- 23.Shales DM, Gerding DN, John JF, Craig WA, Bornstein DL, Duncan RA, Eckman MR, Farrer WE, Greene WH, Lorian V, Levy S, McGowan JE, Jr, Paul SM, Ruskin J, Tenover FC, Watanakunakorn C. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the prevention of antimicrobial resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Infect Control Hosp Epidemiol. 1997;18:275–291. doi: 10.1086/647610. [DOI] [PubMed] [Google Scholar]
- 24.Gould IM. A review of the role of antibiotic policies in the control of antibiotic resistance. J Antimicrob Chemother. 1999;43:459–465. doi: 10.1093/jac/43.4.459. [DOI] [PubMed] [Google Scholar]
- 25.White AC, Jr, Atmar RL, Wilson J, Cate TR, Stager CE, Greenberg SB. Effects of Requiring Prior Authorization for Selected Antimicrobials: Expenditures, Susceptibilities, and Clinical Outcomes. Clin Infect Dis. 1997;25:230–239. doi: 10.1086/514545. [DOI] [PubMed] [Google Scholar]
- 26.Bassetti M, Di Biagio A, Rebesco B, Amalfitano ME, Topal J, Bassetti D. The effect of formulary restriction in the use of antibiotics in an Italian hospital. Eur J Clin Pharmacol. 2001;57:529–534. doi: 10.1007/s002280100338. [DOI] [PubMed] [Google Scholar]
- 27.Tang H, Huang T, Jing J, Shen H, Cui W. Effect of procalcitonin-guided treatment in patients with infections: a systematic review and meta-analysis. Infection. 2009;37:497–507. doi: 10.1007/s15010-009-9034-2. [DOI] [PubMed] [Google Scholar]