Abstract
The site-specific identification of α-aminoadipic semialdehyde (AAS) and γ-glutamic semialdehyde (GGS) residues in proteins is reported. Semialdehydic protein modifications result from the metal-catalyzed oxidation of Lys or Arg and Pro residues, respectively. Most of the analytical methods for the analysis of protein carbonylation measure change to the global level of carbonylation and fail to provide details regarding protein identity, site, and chemical nature of the carbonylation. In this work, we used a targeted approach, which combines chemical labeling, enrichment, and tandem mass spectrometric analysis, for the site-specific identification of AAS and GGS sites in proteins. The approach is applied to in vitro oxidized glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and an untreated biological sample, namely cardiac mitochondrial proteins. The analysis of GAPDH resulted in the site-specific identification of two AAA and four GGS residues. Computational evaluation of the identified AAS and GGS sites in GAPDH indicated that these sites are located in flexible regions, show high solvent accessibility values, and are in proximity with possible metal ion binding sites. The targeted proteomic analysis of semialdehydic modifications in cardiac mitochondria yielded nine AAS modification sites which were unambiguously assigned to distinct lysine residues in the following proteins: ATP/ATP translocase isoforms 1 and 2, ubiquinol cytochrome-c reductase core protein 2, and ATP synthase α-subunit.
Keywords: Aldehyde-reactive probe, Protein carbonyls, Mass spectrometry, Metal-catalyzed oxidation, α-Aminoadipic semialdehyde, γ-Glutamic semialdehyde
Introduction
The oxidative modification of proteins is widely thought to be an important factor in the etiology and progression of several diseases [1–3]. Protein oxidation occurs via many different pathways including the direct oxidation of amino acid side chains by reactive oxygen species (ROS), cleavage of the peptide backbone, protein cross-linking, or adduction by reactive intermediates such as α,β-unsaturated aldehydes. Oxidative protein modifications have been linked to alterations of intracellular signaling pathways, protein dysfunction, protein misfolding, and aberrant protein processing and degradation. Oxidative modification may alter a protein so that it is more susceptible to proteolytic degradation; however, some oxidatively modified proteins are not only resistant to proteolytic degradation, they also may inhibit the ability of some proteases to degrade other oxidatively modified proteins [4–6]. Therefore, by an either increased rate of formation and/or decreased rate of degradation the steady-state concentration of oxidatively modified proteins increases during conditions of oxidative stress [1]. Protein carbonyl contents of tissues and bodily fluids are commonly used for assessing levels of oxidative damage to proteins. Many studies have identified α-aminoadipic semialdehyde (AAS) and γ-glutamic semialdehyde (GGS) residues to be major products of direct oxidation of amino acid side chains in proteins [7]. Both AAS and GGS are readily formed through metal-catalyzed oxidation (MCO) reactions in vitro and in vivo. AAS is formed by oxidative deamination of lysine residues, while GGS originates from the oxidation of arginine and proline residues (Scheme 1).
Scheme 1.
a Metal-catalyzed oxidation of Lys, Arg, and Pro residue to α-aminoadipic semialdehyde (AAS) and γ-glutamic semialdehyde (GGS) and b subsequent aldehyde/keto-specific affinity labeling using 2-N′-aminooxymethylcarbonyl hydrazine-D-biotin as aldehyde-reactive probe (ARP)
Some of the earliest developed and most widely employed methods for measuring protein carbonyls rely on spectrophotometric analysis following derivatization with thiobarbituric acid (TBARS) or 2,4-dinitrophenylhydrazine (DNPH) [8, 9]. While these methods have proven useful for measuring the total levels of protein carbonyls, they lack specificity and are unable to provide information on the chemical nature of the modification or identify the amino acid residue on which the modification occurs. Recently, several new methods have been developed which overcome some of these problems. Akagawa et al. developed a technique using p-aminobenzoic acid (ABA) derivatization and high-performance liquid chromatography (HPLC) with fluorometric detection for the quantitative analysis of AAS and GGS [10, 11]. Recently, Estévez et al. applied ABA labeling in conjunction with liquid chromatography–electrospray ionization–mass spectrometry (LC–ESI–MS) analysis for the analysis of AAS and GGS in food proteins [12]. A limitation of the current ABA-based methods is that they rely on complete acid hydrolysis of the proteins, and, therefore, information about the site of modification is lost. Tandem mass spectrometry (MS/MS)-based approaches allow the identification of protein carbonyls and the possible localization of carbonyl modifications in proteins to specific residues [13–15]. However, the low abundance of oxidatively modified proteins demands a targeted approach for the efficient tandem-mass-spectrometry-based analysis of oxidized proteins in complex biological samples. Here, we report the identification and characterization of proteins containing AAS and GGS residues by utilizing a biotinylated hydroxylamine as aldehyde-reactive probe (ARP) which enables affinity enrichment and subsequent tandem mass spectrometric analysis of AAS- and GGS-containing peptides. We describe the site-specific identification of distinct AAS and GGS sites in GAPDH, a prominent target of oxidative stress modifications in diverse pathologies, after in vitro MCO, as well as in an untreated biological sample, namely proteins obtained from subsarcolemmal mitochondria isolated from rat heart tissue.
Experimental section
Chemicals
N′-aminooxymethylcarbonyl hydrazine-D-biotin (ARP, purity >95% (HPLC)) was purchased from Dojindo Molecular Technologies Inc. (Rockville, MD). Rabbit muscle glyceraldehyde-3-phosphate dehydrogenase (GAPDH, purity: protein >85% (biuret)) and β-nicotinamide adenine dinucleotide (NAD, purity: ≥ 99%) were obtained from Sigma-Aldrich (St. Luis, MO). H2O2 (30% aqueous solution) was from Mallinckrodt Baker, Inc. (Phillipsburg, NJ). All other chemicals were of analytical grade and were purchased from Sigma-Aldrich (St. Luis, MO). Sequencing-grade modified trypsin was purchased from Promega (Madison, WI). Pierce UltraLink® monomeric avidin was obtained from Thermo Scientific (Rockford, IL). Solvents for the LC–MS/MS analyses were from Honeywell Burdick and Jackson (Muskegon, MI).
Metal-catalyzed oxidation, ARP labeling, and proteolysis of GAPDH
GAPDH was dissolved at a concentration of 5 mg/mL in a 50 mM sodium phosphate buffer at pH 7.4. The metal-catalyzed oxidation was accomplished in the presence of (a) 100 μM FeCl2 and 1 mM H2O2 or (b) 100 μM FeCl2, 1 mM H2O2, and 1 mM NAD [10]. Oxidation reactions were carried out at 37 °C for 2 h. Protein samples were then transferred to a Biomax centrifugal ultrafiltration device (10 kDa MWCO) and washed four times with 500 μL of freshly prepared 50 mM sodium phosphate buffer. The oxidized GAPDH samples were labeled with ARP at a final concentration of 5 mM [16] for 1 h at room temperature in the Biomax filter device. Excess ARP was removed by ultrafiltration using three wash cycles with 500 μL 50 mM sodium phosphate buffer in each cycle. After the final centrifugal step, a buffer exchange into 100 mM ammonium bicarbonate (pH 8.3) was performed. Protein samples were digested with trypsin, using 1:50 (wt/wt) enzyme-to-substrate ratio, for 16 h at 37 °C. Digestion took place within the filtration device. Peptides were finally filtered through the 10 kDa MWCO filter and recovered from the filtrate tube.
Preparation of ARP-labeled carbonylated protein samples from rat cardiac mitochondria
Subsarcolemmal mitochondria samples were isolated from the heart tissue of 24-month-old male Fisher 344 rats according to the procedures of Palmer [17] with minor modifications by Suh et al. [18]. The total protein concentration was determined using the Pierce Coomassie Plus protein assay. Mitochondrial samples containing 1 mg total protein were washed twice with 50 mM sodium phosphate buffer pH 7.4. The mitochondrial membranes were disrupted by resuspending the mitochondria in 50 mM sodium phosphate buffer pH 7.4 containing 1% (w/v) Triton X-100. The ARP labeling reaction was carried out using a final concentration of 5 mM ARP for 1 h at room temperature. Excess ARP was removed by pelleting the remaining membrane fragments at 14,000×g for 15 min and transferring the supernatant to a Biomax 10-kDa ultrafiltration device. Ultrafiltration was performed using 50 mM sodium phosphate buffer and followed by buffer transfer into 100 mM ammonium bicarbonate. The ARP-labeled mitochondrial protein samples were digested with trypsin as described above for GAPDH.
Affinity enrichment of ARP-labeled peptides
ARP-labeled peptide samples were enriched using UltraLink® monomeric avidin as described previously [19]. Briefly, protein samples were added to 100 μL avidin columns and washed extensively first with PBS buffer (10 mM sodium phosphate, 150 mM sodium chloride, pH 7.4). To remove the non-labeled peptides, three washes were performed with 50 mM ammonium bicarbonate containing 20% methanol. The column was then washed with Milli-Q water. Subsequently, the ARP-labeled peptides were released with 30% aqueous acetonitrile to which 0.4% formic acid was added. ARP-labeled peptide fractions were concentrated using vacuum centrifugation and stored at −80 °C until subjected to mass spectrometric analysis.
Mass spectrometry
The enriched peptides were analyzed by LC–MS/MS using a quadrupole orthogonal time-of-flight mass spectrometer (Q-TOF Ultima Global, Micromass/Waters, Manchester, UK) equipped with a NanoAcquity UPLC system. Peptides were separated by a 100-μm i.d.×200-mm-long bridged ethyl hybrid column (1.7-μm particle size, 130-Å pore size, Waters, Milford, MA) using a linear gradient of a binary solvent system consisting of solvent A (2% aqueous acetonitrile acidified with 0.1% formic acid) and solvent B (acetonitrile containing 0.1% formic acid). The electrospray source was operated in the positive mode with the spray voltage set to 3.5 kV. The mass spectrometer was operated in the data-dependent MS/MS mode, performing 0.6-s MS scans followed by 2.4 s MS/MS scans, on the three most abundant precursor ions detected in the MS scan. A 60-s dynamic exclusion of previously selected ions was used. The MS/MS collision energy (25–65 eV) was dynamically adjusted based on the charge state of the precursor ion selected by the quadrupole analyzer. Mass spectra were calibrated using fragment ions of Glu1-fibrinopeptide B. Lock spray mass correction was performed on the doubly charged ion of Glu1-fibrinopeptide B ([M+2 H]2+ 785.8426 Th) every 30 s during the MS/MS runs.
Additionally, an Applied Biosystems 4700 Proteomics Analyzer matrix-assisted laser desorption/ionization (MALDI)–TOF/TOF instrument in combination with a Dionex/LC Packings Ultimate nano-LC system equipped with a Probot target spotter was used. The experimental parameters for peptide separation, spotting, and MALDI–MS/MS analyses were as previously described [16].
Identification of ARP-labeled oxidized peptides
Tandem mass spectrometric data generated on the Q-TOF–MS were processed into peak list files using ProteinLynx Global Server v2.3 (Waters, Manchester, UK). MALDI–MS/MS data were processed into peak list files using the Peaks-to-Mascot tool in the 4000 Series Explorer v3.0 software (Applied Biosystems). Peak list files of the tandem mass spectrometric data were analyzed with Mascot v2.1 (Matrix Science, London, UK) and searched against the Swiss Prot database v50 (270,778 sequences, 99,412,397 residues) using the following parameters: taxonomy rodentia (20,991 sequences), ± 0.2 Da mass tolerances for the precursor and fragment ions, possibility of three missed proteolytic cleavage sites, with trypsin/P or semitrypsin selected as the digesting enzyme. ARP-α-aminoadipic semialdehyde (C18H28N6O5S, monoisotopic residue mass 440.1842 Da) (K), ARP-γ-glutamic semialdehyde (C17H26N6O5S, monoisotopic residue mass 426.1685 Da) (R, P), and methionine oxidation (M) were selected as variable modifications at the residues specified in parentheses. Because this study focused on the site-specific identification of AAS and GGS residues using ARP as carbonyl-reactive probe, other oxidative modifications were not considered.
Calculation of B-factor and residue accessible area
The 3D coordinates of rabbit muscle glyceraldeyde-3-phosphate dehydrogenase (GATPH, pdb 1J0X) were retrieved from the Protein Data Bank [20]. The structure was energetically refined in the internal coordinate space with Molsoft ICM v3.5-1p [21, 22]. The ICM PocketFinder tool was used to locate the NAD ligand binding pocket in the refined GAPDH model [23]. The B-factor (BF) and residue accessible area (RAA) values for arginine, lysine, and proline residues of a GATPH monomer in the presence and in the absence of NAD+ were calculated using the program ICM v3.5-1p. The normalized B-factor values were derived for each monomer from the atomic crystallographic data of the tetramer, and the average for each residues was calculated [20]. The atomic accessible surfaces are calculated using a faster modification of the Shrake and Rupley algorithm [24] with a water probe radius of 1.4 Å. The relative RAA is expressed by an integer number in a scale from 0 to 9 (0, fully buried; 9, fully exposed). The average RAA value of each residue was then normalized to the highest value obtained.
Results and discussion
Site-specific identification of AAS and GGS residues in GAPDH
GAPDH has been identified to be a major target of oxidative modifications in many different biological matrices and pathological conditions. GAPDH is a classical glycolytic enzyme for which also intriguing roles have been described in NO and oxidative stress sensing [25, 26] as well as in oxidative stress-induced cell death [27]. Most studies report on the susceptibility of GAPDH to adduct formation with reactive lipid peroxidation products. The lipid-derived aldehydic products, 4-hydroxy-2-nonenal (HNE) and 4-hydroxy-2-hexenal, have been shown to modify the active site cysteine residue, trigger degradation (22), and induce conformational changes that are accompanied by reduction in biological activity of GAPDH (23). However, less is known about the susceptibility of GAPDH to metal-catalyzed oxidation reactions.
We subjected GAPDH to MCO using Fenton chemistry (Fe2+/H2O2). Protein oxidation was stopped after 2 h by ultrafiltration. Subsequently, the carbonylated residues were chemically labeled using a carbonyl-specific hydroxylamine-based biotinylation probe (a.k.a. aldehyde/keto reactive probe, Scheme 1). We then digested the labeled protein with trypsin, enriched the ARP-labeled peptides using an immobilized monomeric avidin column, and sequenced the peptides by LC–MS/MS analysis. Using this approach, we identified six amino acid residues that had been oxidized to AAS or GGS and were derivatized by ARP (Table 1). The identified sites are summarized in Fig. 1 and included the following residues: Lys-3 and Lys-192 being oxidized to AAS and Arg-11, Arg-78, Pro-122, and Pro-127 being oxidized to GGS. The same six residues were identified with AAS and GGS modifications, irrespective of the presence of the NAD cofactor during MCO. The modification of Lys and Arg residues always resulted in a missed tryptic cleavage site occurring at the modified residue, as would be expected. Both Pro oxidations occurred on the same tryptic peptide sequence spanning residues 117 to 137. However, this peptide was detected with a single modification occurring either on Pro-122 or Pro-127, but not with both modifications present simultaneously on the same peptide. The latter observation, however, does not exclude the possibility that both proline residues, 122 and 127, are concurrently present as semialdehydes. Possible factors that may have contributed to the fact that a di-ARP-modified peptide was not identified may include less favorable mass spectrometric characteristics of the di-biotinylated peptide and/or a possible steric hindrance of two ARP tags if two carbonyl sites are in proximity. Tandem mass spectra of the identified ARP-labeled, semialdehyde-modified peptides are included in the Electronic Supplementary Material (Figs. S1–S11). Examination of the tandem mass spectra of the ARP-labeled semialdehyde-containing peptides revealed some common features aiding in the manual interpretation of the tandem mass spectra but, on the other hand, also complicating the automated interpretation of tandem mass spectra using search algorithms, such as Mascot software. The first of these features is the neutral loss of the ARP moiety from the y-ion in which the ARP-labeled residue is the N-terminal residue. This results in the respective y-ion appearing at −331 m/z from its expected value. In some cases, the neutral loss is not complete, and the respective y-ion also appears at the expected m/z including the mass of the ARP tag. The other two features commonly observed in the tandem mass spectra of ARP-labeled peptides are the fragment ions present at m/z 227 and m/z 332 which originate from the ARP tag as previously described [19].
Table 1.
ARP-labeled carbonylated peptides of GAPDH identified by LC–tandem mass spectrometry
| Peptide | Modificationa | ESI Q-TOF MS | MALDI TOF/TOF MS |
|---|---|---|---|
| VK*VGVNGFGR | AAS (K3) | ✓ | ✓ |
| VGVNGFGR*IGR | GGS (R11) | ✓ | ✓ |
| AITIFQER*DPANIK | GGS (R78) | ✓ | ✓ |
| VIISAP*SADAPMFVMGVNHEK | GGS (P122) | ✓ | ✓ |
| VIISAPSADAP*MFVMGVNHEK | GGS (P127) | ✓ | |
| TVDGPSGK*LWR | AAS (K192) | ✓ | ✓ |
The tandem mass spectra of the oxidatively modified peptides are available in the Electronic Supplementary Material
Sequence numbering according to Swiss Prot P46406 (G3P_RABIT)
Fig. 1.
Tandem mass spectrum of the ARP-labeled GGS-containing GAPDH peptide encompassing the residues 4–14 (according to G3P_RABIT; P46406). ESI–MS/MS analysis was performed on the doubly protonated precursor ion, [M+2 H]2+, at m/z 701.3. Fragment ions marked with an asterisk retained the ARP moiety during collision-induced fragmentation and allowed the unequivocal localization of the GGS residue to position 11. The fragment ion peak at m/z 440.2 represents the y4-ion after loss of the ARP moiety associated with the proposed formation of an azacyclopentene moiety (see also Fig. S19). Non-peptide fragmentation of the ARP tag results in the ions at m/z 227.1 (ARP F1) and 332.1 ([ARP+H]+). Proposed structures for the ARP-related fragment ions have been discussed previously [19]
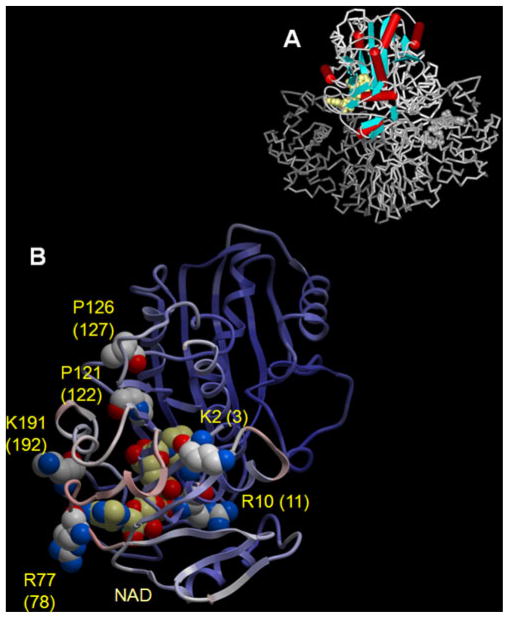
We identified six semialdehyde residues out of 47 possible sites, containing Lys, Arg, and Pro residues, per GAPDH monomer. The fact that only 12.7% of all possible sites were found as semialdehydes suggests a certain level of selectivity for Fenton-chemistry-based MCO. BF and solvent accessibility (SA) values were calculated for all Lys, Arg, and Pro residues in the GAPDH. All six modified residues (Lys-3, Arg-11, Arg-78, Pro-122, Pro-127, and Lys-192) are located in proximity to the NAD-binding pocket (Fig. 2a). Except for Lys-192, all other modified residues are part of the NAD-binding Rossmann-fold domain. Residue Lys-192 has a BF of 48.2 and normalized SA value of 9 in concurrence with its location on a flexible loop protruding the cofactor binding pocket (Fig. 2).
Fig. 2.
Identified semialdehydic residues in GAPDH after in vitro MCO using Fe2+/H2O2. a The crystal structure of rabbit muscle GAPDH (pdb 1J0X) shows four monomeric units; subunit P is highlighted. b The monomeric subunit P of GAPDH is displayed with the protein backbone shown as ribbon and colored by B-Factor values (ICM v3.5-1p). The residues identified with semialdehyde modifications are displayed in CPK style and colored by atom type (C: white, O: red, N: blue). The NAD cofactor is shown in CPK style (C: pale yellow, O: red, N: blue). The residues are numbered according to the pdb file and the corresponding annotations used in the Swiss Prot database for P46406 (G3P_RABIT) are given in parentheses
Other notable structural features are the close proximity of acidic residues to the identified oxidatively modified sites. Acidic residues have been described to serve as metal binding sites promoting Fenton chemistry and oxidative insult [15]. In the crystal structure (pdb 1J0X), two acidic residues, Asp-48 and Glu-315, are sterically close to Arg-11 with distances of 2.4 and 2.8 Å, respectively. Additionally, Arg-78 is located directly between two acidic residues, namely Glu-77 and Asp-79. The proximity of Asp-125 and Asp-138 to Pro-122 and Pro-127 could in part explain why these Pro residues were found oxidized. In proximity to Lys-3, two acidic residues are present, Asp-27 and Glu-91, which possibly may serve as metal ion binding sites, thereby promoting oxidative deamination leading to carbonyl formation. The identified sites are supportive of a conceptual framework in that a combination of surface exposure and proximity of possible transition metal binding sites favors distinct sites to undergo MCO, conferring, at least to a certain degree, specificity to MCO-derived carbonylation reactions.
Identification of semialdehydic modifications in mitochondrial proteins
There is a large body of evidence that links protein oxidation to mitochondrial dysfunction. Mitochondria serve as the cellular power plants but are also known to be a major source of ROS within the cell and are important regulators of oxidative stress [28–30]. Oxidative damage to mitochondrial proteins has been implicated in a host of human pathological states, including cancer, heart disease, and neurological disorders [31]. We attempted the identification of semialdehydic modification in mitochondrial proteome samples derived from rat heart tissues.
Mitochondrial protein samples were treated with ARP, excess reagent was removed, and after digestion, the ARP-labeled peptides were enriched, and the resulting peptide mixture was subjected to LC–MS/MS analysis. MS/MS data were searched with Mascot software using residue masses that allowed searching for the ARP-labeled AAS and ARP-labeled GGS modifications. The searches yielded nine sites with AAS modifications occurring on four mitochondrial proteins. The respective tandem mass spectra are compiled in the Electronic Supplementary Material (Figs. S12–S21). The identifications and sites of modification are summarized in Table 2. Six peptides from ATP/ATP translocase 1 were identified, and each peptide contained a Lys residue that was oxidized to AAS and labeled with ARP. One peptide from ATP/ATP translocase 2 was detected in which the Lys residue at position 43 was oxidized to AAS and amenable to labeling with ARP. A peptide encompassing the residues 172 to 182 of ATP synthase α chain was identified with the Lys residue at position 175 oxidized to AAS and labeled with ARP. A peptide from the ubiquinol cytochrome-c reductase complex core protein 2 was identified which contained an ARP-labeled AAS residue at position 161. In each case, the modified Lys residue resulted in a missed tryptic cleavage site. The tandem mass spectra displayed ARP–AAS-specific fragment ions in each case. The LC–ESI–MS/MS spectrum of the peptide encompassing the residues 61–72 of ADP/ATP translocase 1 (ADT1_RAT) is shown in Fig. 3 to illustrate the low-energy collision-induced dissociation behavior of ARP-labeled AAS-containing peptides. For the ARP-labeled AAS-containing peptides, the yn-ion with the AAS residue at the N-terminal position showed neutral loss of the ARP moiety resulting in a yn-331.17 fragment ion. In most tandem mass spectra, additional characteristic fragment ions at m/z 227.1 (ARP F1) and m/z 332.19 ([ARP+H]+) were observed, which substantiated the presence of the ARP tag in the identified peptides.
Table 2.
Mitochondrial proteins identified with semialdehydic modifications
| Biological | Swiss Prot ID | UniProt accession | Protein name | Peptide sequence | Modification |
|---|---|---|---|---|---|
| Respiratory chain | ATPA_RAT | P15999 | ATP synthase subunit alpha | VGLK*APGIIPRa,b | AAS (K175) |
| QCR2_RAT | P32551 | Ubiquinol–cytochrome-c reductase complex core protein 2 | IDK*AVAFQNPQTRb | AAS (K161) | |
| Membrane transport | ADT1_RAT | Q05962 | ADP/ATP translocase 1 | VK*LLLQVQHASKb | AAS (K33) |
| YK*QIFLGGVDRa | AAS (K52) | ||||
| IPK*EQGFLSFWRa,b | AAS (K63) | ||||
| YFPTQALNFAFK*DKb | AAS (K91) | ||||
| HK*QFWRa | AAS (K107) | ||||
| VLVLYDEIK*Ka | AAS (K295) | ||||
| ADT2_RAT | Q09073 | ADP/ATP translocase 2 | LLLQVQHASK*QITADKa | AAS (K43) |
The tryptic peptide sequences are listed with the modified residue marked with an asterisk. The location of the modification is provided in parentheses. Letters “a” and “b” indicate whether the peptide was detected by ESI–Q-TOF or MALDI–TOF/TOF mass spectrometry, respectively.
Fig. 3.
Tandem mass spectrum of the ARP-labeled AAS-containing peptide IPKEQGFLSFWR from ADT1_RAT encompassing the residues 61–72. ESI–MS/MS analysis of the [M+2 H]2+ ion at m/z 910.5 yields a complete yn-ion series. Fragment ions marked with an asterisk retained the ARP moiety during collision-induced fragmentation and allowed the unequivocal localization of the AAS residue to position 63. The fragment ion peak at m/z 1,278.7.2 represents the y10-ion after loss of the ARP moiety associated with the proposed formation of an azacyclo-hexane moiety (see also Fig. S19). Fragment ions specific for the ARP tag are observable at m/z 227.1 (ARP F1) and m/z 332.1 ([ARP+H]+)
Identified AAS-containing proteins are involved in mitochondrial energy production
ADP/ATP translocase functions to transport ADP and ATP across the inner mitochondrial membrane (IMM). The abundance of ANT has been estimated to constitute up to 10% of the IMM proteins, making it the single most abundant protein in the IMM [32, 33]. In the current work, six Lys residues (out of 24 Lys residues) were modified by MCO and identified as AAS residues in ADP/ATP translocase 1. These residues appear to be susceptible sites to damage by ROS which could, through sterically induced conformational changes, lead to decreased activity of this membrane transport protein. In their recent study, Luo et al. showed that acrolein inhibits ADP/ATP translocase activity which could lead to an overall increase in the production of ROS in the mitochondria, resulting in a detrimental cycle of increased oxidative stress damage [34]. It has been hypothesized by Wallace that a decreased translocase activity would reduce the concentration of ADP in the mitochondrial matrix, limiting ADP-dependant proton transport through ATP synthase (complex V), which would hyperpolarize the IMM and inhibit the electron transport chain [35]. An accumulation of electrons would then result in an increased production of ROS and, in turn, increased oxidative stress.
Two proteins from the mitochondrial electron transport chain (ETC) complexes were identified with AAS modifications: the α-subunit of ATP synthase (complex V) and the core 2 protein of the ubiquinol–cytochrome-c reductase complex (complex III). Both ETC complex constituents have been described previously as targets of carbonylation reactions [36, 37]. The ATP synthase α-subunit forms part of the catalytic core of the F1 complex of ATP synthase, consisting of three copies of the α-subunit and three copies of the β-subunit. ATP synthase α-subunit was identified with an AAS modification to Lys-175. The α-subunit of ATP synthase has been shown previously to be a target of oxidative stress-induced damage, leading to a decrease in ATP synthase activity in the early stages of Alzheimer’s disease [38]. In the second protein identified, the core protein 2 of the ubiquinol–cytochrome-c reductase complex (complex III), Lys-161 was oxidized to an AAS residue. Complex III is widely considered to be a major source of ROS within the mitochondria. Complex III contains two ROS-generating centers: the Qo center, oriented toward the intermembrane space, and the Qi center that faces the mitochondrial matrix [39]. While the core protein 2 has no catalytic activity, it is required for assembly of the complex. Being in close proximity to the Qi site may promote oxidative modification of core protein 2 and Lys-161 as susceptible target site. Complex III has been found as a constituent of supramolecular complexes together with ETC complexes I and V [40, 41], allowing speculations that proximity of complex III to complex V may provide a possible route for oxidative modifications caused by ROS emanating from complex III.
Considerations on the applicability of the ARP strategy for profiling protein carbonyls in complex matrices
The described analytical strategy using the biotinylated hydroxyl amine, ARP, as a chemical probe for labeling and enrichment of carbonylated proteins is applicable to a wide array of chemically diverse protein carbonyls, including proteins containing AAS and GGS residues (this study) and protein adducts of reactive lipid peroxidation products, such as 2-enals, e.g., HNE [19]. However, sample matrices containing relatively high concentrations of other small molecule carbonyls, e.g., in the form of oxidized lipids, methylglyoxal, pyridoxal, etc., may interfere with the analysis of protein-bound carbonyls. By removing carbonyl-containing small molecules by dialysis, ultrafiltration, gel filtration, or other methods and adding an excess of ARP, efficient labeling of protein carbonyls should be possible.
It should be noted that due to the low natural abundance of carbonylated proteins and the large dynamic range in protein concentrations, with the current technique, we are still limited to only detecting the most abundant of the in vivo oxidatively modified proteins. In this context, a critical step of the analytical strategy is the efficient capture and release of the ARP-labeled peptides from the immobilized monomeric avidin prior to mass spectrometric analysis. Additionally, a potential caveat of targeted approaches is that identification of the protein target is usually based on assignment of a single peptide derived from tandem mass spectral data. Therefore, accurate peptide mass determination in combination with content-rich fragment ion spectra and the observation of diagnostic non-peptide fragment ions originating from the chemical probe are crucial for increasing the confidence level with which target assignments can be performed [19].
Conclusion
A targeted tandem-mass-spectrometry-based approach was used for obtaining site-specific assignments of AAS and GGS residues in GAPDH and mitochondrial proteins. The unambiguous identification of semialdehydic modifications using peptide tandem mass spectra was aided by yn-ions that indicated loss of the chemical tag and the observation of fragment ions specific for the aldehyde/keto-reactive tagging probe.
Carbonyl-specific derivatization using a biotinylated hydroxylamine and the subsequent targeted analysis by LC–MS/MS has also been used successfully by us and others for the site-specific identification of protein modifications by lipid peroxidation products [16, 19, 42]. Thus, carbonyl-specific labeling methods may allow identification of chemically diverse groups of protein carbonylation modifications in one LC–MS/MS run [43].
The findings and the described method will be useful additions to the field of proteomics of oxidative stress modifications. The reported method is broadly applicable, and it is expected that the approach will provide critical information for researchers with interests in redox biology, toxicology, and aging. The analytical strategy may also be useful for monitoring oxidative modifications introduced as a consequence of food processing in nutritional protein preparations.
Supplementary Material
Acknowledgments
This study was supported by NIH/NIA grant R01AG025372. We acknowledge the use of Oregon State University’s Mass Spectrometry Facility supported in part by the Environmental Health Sciences Center (NIH/NIEHS grant P30ES00210).
Abbreviations
- AAS
α-Aminoadipic semialdehyde
- ARP
Aldehyde-reactive probe
- ESI
Electrospray ionization
- GGS
γ-Glutamic semialdehyde
- MCO
Metal-catalyzed oxidation
- MS/MS
Tandem mass spectrometry
- TOF
Time-of-flight
- UPLC
Ultra-performance liquid chromatography
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00216-010-4289-0) contains supplementary material, which is available to authorized users.
Contributor Information
Juan D. Chavez, Department of Chemistry, Oregon State University, Corvallis, OR 97331, USA
William H. Bisson, Department of Environmental and Molecular Toxicology, Oregon State University, Corvallis, OR 97331, USA
Claudia S. Maier, Email: claudia.maier@oregonstate.edu, Department of Chemistry, Oregon State University, Corvallis, OR 97331, USA
References
- 1.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 2.Korolainen MA, Goldsteins G, Alafuzoff I, Koistinaho J, Pirttila T. Proteomic analysis of protein oxidation in Alzheimer’s disease brain. Electrophoresis. 2002;23(19):3428–3433. doi: 10.1002/1522-2683(200210)23:19<3428::AID-ELPS3428>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 4.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329(1–2):23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 5.Nystrom T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24(7):1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grune T, Jung T, Merker K, Davies KJA. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int J Biochem Cell Biol. 2004;36(12):2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Requena JR, Chao CC, Levine RL, Stadtman ER. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc Natl Acad Sci USA. 2001;98 (1):69–74. doi: 10.1073/pnas.011526698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95 (2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 9.Fields R, Dixon HB. Micro method for determination of reactive carbonyl groups in proteins and peptides, using 2,4-dinitrophenylhydrazine. Biochem J. 1971;121(4):587–589. doi: 10.1042/bj1210587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akagawa M, Sasaki D, Ishii Y, Kurota Y, Yotsu-Yamashita M, Uchida K, Suyama K. New method for the quantitative determination of major protein carbonyls, alpha-aminoadipic and gamma-glutamic semialdehydes: investigation of the formation mechanism and chemical nature in vitro and in vivo. Chem Res Toxicol. 2006;19(8):1059–1065. doi: 10.1021/tx060026p. [DOI] [PubMed] [Google Scholar]
- 11.Akagawa M, Suyama K, Uchida K. Fluorescent detection of alpha-aminoadipic and gamma-glutamic semialdehydes in oxidized proteins. Free Radic Biol Med. 2009;46(6):701–706. doi: 10.1016/j.freeradbiomed.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Estevez M, Ollilainen V, Heinonen M. Analysis of protein oxidation markers alpha-aminoadipic and gamma-glutamic semi-aldehydes in food proteins using liquid chromatography (LC)–electrospray ionization (ESI)–multistage tandem mass spectrometry (MS) J Agric Food Chem. 2009;57(9):3901–3910. doi: 10.1021/jf804017p. [DOI] [PubMed] [Google Scholar]
- 13.Mirzaei H, Regnier F. Affinity chromatographic selection of carbonylated proteins followed by identification of oxidation sites using tandem mass spectrometry. Anal Chem. 2005;77(8):2386–2392. doi: 10.1021/ac0484373. [DOI] [PubMed] [Google Scholar]
- 14.Mirzaei H, Regnier F. Identification and quantification of protein carbonylation using light and heavy isotope labeled Girard’s P reagent. J Chromatogr A. 2006;1134(1–2):122–133. doi: 10.1016/j.chroma.2006.08.096. [DOI] [PubMed] [Google Scholar]
- 15.Temple A, Yen TY, Gronert S. Identification of specific protein carbonylation sites in model oxidations of human serum albumin. J Am Soc Mass Spectrom. 2006;17(8):1172–1180. doi: 10.1016/j.jasms.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Chavez J, Wu J, Han B, Chung WG, Maier CS. New role for an old probe: affinity labeling of oxylipid protein conjugates by N′-aminooxymethylcarbonylhydrazino d-biotin. Anal Chem. 2006;78 (19):6847–6854. doi: 10.1021/ac0607257. [DOI] [PubMed] [Google Scholar]
- 17.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252(23):8731–8739. [PubMed] [Google Scholar]
- 18.Suh JH, Heath SH, Hagen TM. Two subpopulations of mitochondria in the aging rat heart display heterogenous levels of oxidative stress. Free Radic Biol Med. 2003;35(9):1064–1072. doi: 10.1016/s0891-5849(03)00468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavez J, Chung WG, Miranda CL, Singhal M, Stevens JF, Maier CS. Site-specific protein adducts of 4-hydroxy-2(E)-nonenal in human THP-1 monocytic cells: protein carbonylation is diminished by ascorbic acid. Chem Res Toxicol. 2010;23(1):37–47. doi: 10.1021/tx9002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowan-Jacob SW, Kaufmann M, Anselmo AN, Stark W, Grutter MG. Structure of rabbit-muscle glyceraldehyde-3-phosphate dehydrogenase. Acta Crystallogr D Biol Crystallogr. 2003;59(Pt 12):2218–2227. doi: 10.1107/s0907444903020493. [DOI] [PubMed] [Google Scholar]
- 21.Abagyan R, Kuznetsov T. A new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J Comp Chem. 1994;15:488–506. [Google Scholar]
- 22.Cardozo T, Totrov M, Abagyan R. Homology modeling by the ICM method. Proteins. 1995;23(3):403–414. doi: 10.1002/prot.340230314. [DOI] [PubMed] [Google Scholar]
- 23.An J, Totrov M, Abagyan R. Pocketome via comprehensive identification and classification of ligand binding envelopes. Mol Cell Proteomics. 2005;4(6):752–761. doi: 10.1074/mcp.M400159-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Shrake A, Rupley JA. Environment and exposure to solvent of protein atoms. Lysozyme and insulin. J Mol Biol. 1973;79(2):351–371. doi: 10.1016/0022-2836(73)90011-9. [DOI] [PubMed] [Google Scholar]
- 25.Hara MR, Cascio MB, Sawa A. GAPDH as a sensor of NO stress. Biochim Biophys Acta. 2006;1762(5):502–509. doi: 10.1016/j.bbadis.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Hwang NR, Yim SH, Kim YM, Jeong J, Song EJ, Lee Y, Lee JH, Choi S, Lee KJ. Oxidative modifications of glyceraldehyde-3-phosphate dehydrogenase play a key role in its multiple cellular functions. Biochem J. 2009;423(2):253–264. doi: 10.1042/BJ20090854. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima H, Amano W, Kubo T, Fukuhara A, Ihara H, Azuma YT, Tajima H, Inui T, Sawa A, Takeuchi T. Glyceraldehyde-3-phosphate dehydrogenase aggregate formation participates in oxidative stress-induced cell death. J Biol Chem. 2009;284(49):34331–34341. doi: 10.1074/jbc.M109.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(3–4):222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 29.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 30.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12(5):913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 31.Gibson BW. The human mitochondrial proteome: oxidative stress, protein modifications and oxidative phosphorylation. Int J Biochem Cell Biol. 2005;37(5):927–934. doi: 10.1016/j.biocel.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Fiore C, Trezeguet V, Le Saux A, Roux P, Schwimmer C, Dianoux AC, Noel F, Lauquin GJ, Brandolin G, Vignais PV. The mitochondrial ADP/ATP carrier: structural, physiological and pathological aspects. Biochimie. 1998;80(2):137–150. doi: 10.1016/s0300-9084(98)80020-5. [DOI] [PubMed] [Google Scholar]
- 33.Klingenberg M, Nelson DR. Structure–function relationships of the ADP/ATP carrier. Biochim Biophys Acta. 1994;1187 (2):241–244. doi: 10.1016/0005-2728(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 34.Luo J, Shi R. Acrolein induces oxidative stress in brain mitochondria. Neurochem Int. 2005;46(3):243–252. doi: 10.1016/j.neuint.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283(5407):1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 36.Feng J, Xie H, Meany DL, Thompson LV, Arriaga EA, Griffin TJ. Quantitative proteomic profiling of muscle type-dependent and age-dependent protein carbonylation in rat skeletal muscle mitochondria. J Gerontol A Biol Sci Med Sci. 2008;63(11):1137–1152. doi: 10.1093/gerona/63.11.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prokai L, Yan LJ, Vera-Serrano JL, Stevens SM, Jr, Forster MJ. Mass spectrometry-based survey of age-associated protein carbonylation in rat brain mitochondria. J Mass Spectrom. 2007;42(12):1583–1589. doi: 10.1002/jms.1345. [DOI] [PubMed] [Google Scholar]
- 38.Terni B, Boada J, Portero-Otin M, Pamplona R, Ferrer I. Mitochondrial ATP-synthase in the entorhinal cortex is a target of oxidative stress at stages I/II of Alzheimer’s disease pathology. Brain Pathol. 2009;20:222–223. doi: 10.1111/j.1750-3639.2009.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278(38):36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 40.Gomez LA, Monette JS, Chavez JD, Maier CS, Hagen TM. Supercomplexes of the mitochondrial electron transport chain decline in the aging rat heart. Arch Biochem Biophys. 2009;490(1):30–35. doi: 10.1016/j.abb.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schagger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19 (8):1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slade PG, Williams MV, Brahmbhatt V, Dash A, Wishnok JS, Tannenbaum SR. Proteins modified by the lipid peroxidation aldehyde 9, 12-dioxo-10(E)-dodecenoic acid in MCF7 breast cancer cells. Chem Res Toxicol. 2009;23(3):557–567. doi: 10.1021/tx9002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madian AG, Regnier FE. Profiling carbonylated proteins in human plasma. J Proteome Res. 2010;9(3):1330–1343. doi: 10.1021/pr900890k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.