Abstract
Soluble growth factors play an important role in the coordination and integration of cell proliferation, differentiation, fate determination and morphogenesis during development of multicellular organisms. Fibroblast Growth Factors (FGFs) are a large family of polypeptide growth factors that are present in organisms ranging from nematodes to humans. RNA alternative splicing of FGFs and their receptors further enhances the complexity of this ligand-receptor system. The mouse Fgf8 gene produces eight splice variants, which encode isoform proteins with different N-termini and distinct receptor binding affinity and biological activity. In this article, we review the roles of Fgf8 in vertebrate development and summarize the recent findings on the in vivo function of different Fgf8 splice variants. We propose that multiple Fgf8 isoform proteins act in concert to regulate the overall function of Fgf8 and account for the diverse and essential role of Fgf8 during vertebrate development.
The FGF family in vertebrates
The first two FGFs, Fgf1 and Fgf2, were isolated as mitogens for fibroblast from bovine pituitary and brain (Gospodarowicz, 1975; Gospodarowicz et al., 1978; Gospodarowicz et al., 1974; Maciag et al., 1979). Subsequent studies have identified numerous FGFs in various organisms, except for unicellular organisms (Itoh and Ornitz, 2008). In mice and humans, the FGF family is comprised of 22 members, which are grouped into six or seven subfamilies based on similarity in sequence, gene location and function. Genome sequence analyses suggest that the FGF family may have expanded in two phases by gene duplication during evolution (Itoh and Ornitz, 2004; Itoh and Ornitz, 2008). The first phase was associated with early metazoan evolution to generate ancestors of 6–7 FGF subfamilies. Subsequently, large-scale genome duplication during the early evolution of vertebrates further expanded the FGF subfamilies to contain 3–4 members.
Most of FGF members are secreted proteins with a clear N-terminal signal peptide. FGFs 1, 2, 9, 16, and 20 are secreted proteins though they lack an obvious cleavable N-terminal signal sequence. FGFs 11–14 are intracellular proteins and thus called intracellular FGF (iFGF) (Itoh and Ornitz, 2008). All FGFs contain a conserved core of approximately120 amino acids arranged into a globular domain composed of 12 antiparallel β strands (Eriksson et al., 1991; Zhu et al., 1991). This core domain is important for receptor binding, while the divergent N- and C-termini of FGF can modify the receptor binding affinity and account for the different biological function of FGF (Mohammadi et al., 2005). In humans and mice, there are four FGF receptors, FGFR1-4, which are receptor tyrosine kinases and contain an extracellular ligand-binding domain with three immunoglobulin domains (I, II, and III), a single-pass transmembrane domain, and intracellular tyrosine kinase domain (Mohammadi et al., 2005; Ornitz and Itoh, 2001). Alternative splicing of FGFR1-3 genes produces two different isoforms with different immunoglobulin domain III (IIIb and IIIc), which display distinct tissue-specific expression pattern and ligand-binding affinity (Mohammadi et al., 2005). These four FGFRs mediate the function of all FGFs, except for iFGFs (Goldfarb, 2005; Olsen et al., 2003).
FGFs have binding sites for heparin and heparan sulfate, which concomitantly interact with FGF and FGFR and thereby facilitate the formation of a ternary FGF-FGFR-heparin complex (Mohammadi et al., 2005; Nugent and Edelman, 1992; Schlessinger et al., 2000). Therefore, heparin sulfate is considered a co-receptor of FGF. Because of their high affinity of binding with FGFs and the abundance of heparan sulfate proteoglycans on cell surface and in extracellular matrix, FGFs are mostly retained near the site of production (Itoh and Ornitz, 2008). However, FGFs 19, 21, 23 have low-affinity heparin binding sites and thus act in an endocrine manner (Goetz et al., 2007). The low heparin-binding affinity results in the dependence of these three FGFs on Klotho/βKlotho as their co-receptor to act only in Klotho-expressing tissues (Goetz et al., 2007).
Expression and diverse function of FGF8 during vertebrate development
FGF8 was originally identified as an androgen-induced growth factor in a mouse mammary tumor cell line, SC-3 cells (Tanaka et al., 1992). Subsequent studies have revealed its important role in different developmental processes, including cell specification, differentiation, proliferation, survival, and transformation. During mouse embryogenesis, Fgf8 is expressed at sites that are known to instruct growth and patterning, such as the primitive streak and tail bud, the apical ectodermal ridge (AER) of the limb bud, and the midbrain-hindbrain junction, anterior neural ridge (Crossley and Martin, 1995; MacArthur et al., 1995b). In addition, Fgf8 expression is found in branchial arches, the hypothalamus, and otic vesicles (Crossley and Martin, 1995; Crossley et al., 2001).
Genetic studies have demonstrated that Fgf8 plays an important role during embryogenesis in mice. Before and during gastrulation, Fgf8 is essential for morphogenetic remodeling of embryos by promoting formation and migration of the mesoderm (Guo and Li, 2007; Sun et al., 1999). Toward the end of gastrulation, the persistent Fgf8 expression in the tailbud and the posterior end of the neural tube patterns the neural tube and paraxial mesoderm (Diez del Corral et al., 2003; Dubrulle and Pourquie, 2004). Furthermore, the expression of Fgf8 in the primitive streak is essential for development of the left-right asymmetry of the internal organs (Meyers and Martin, 1999). Because of the critical role of Fgf8 during early embryogenesis, mouse embryos homozygous for Fgf8-null mutation die at embryonic day (E) 8.5 (Sun et al., 1999). Generation of hypomorphic mutation of Fgf8 and floxed conditional mutation, which allows tissue-specific knockout experiments using the Cre-loxP system, has been instrumental in investigating the function of Fgf8 in the development of multiple tissue/organ systems after E8.5 (Meyers et al., 1998). Remarkably, these experiments have revealed that Fgf8 plays a crucial role in most of the tissues or organs in which Fgf8 transcripts are present. For example, deletion of Fgf8 results in malformation of major brain structures (Garel et al., 2003; Martinez-Ferre and Martinez, 2009; Storm et al., 2003), abnormal development of olfactory neurogenesis and the nasal cavity (Kawauchi et al., 2005), cardiovascular and craniofacial structures (Frank et al., 2002; Trumpp et al., 1999), limbs (Lewandoski et al., 2000; Moon and Capecchi, 2000), inner ear (Ladher et al., 2005), and kidneys (Perantoni et al., 2005). In humans, mutations in the FGF8 genes have been implicated in congenital malformations and pathological conditions in adults. FGF8 mutations has been associated with non-syndromic cleft lip and/or palate (Riley et al., 2007). Furthermore, mutations of FGF8 cause idiopathic hypogonadotropic hypogonadism (IHH, also termed congenital gonadotropin-releasing hormone deficiency) (Falardeau et al., 2008; Trarbach et al., 2010).
Alternative splicing generates different FGF8 isoforms with different biological activities and function
Alternative splicing is known to be common in higher eukaryotic organisms. In humans, more than 98% of multi-exonic genes in the genome undergo alternative splicing, generating multiple splice variants and greatly augmenting genetic and functional complexity without a concomitant increase in the genome size (Pan et al., 2008; Wang et al., 2008). The FGF8 gene produces one of the most complex transcript variants through alternative splicing among the FGF family.
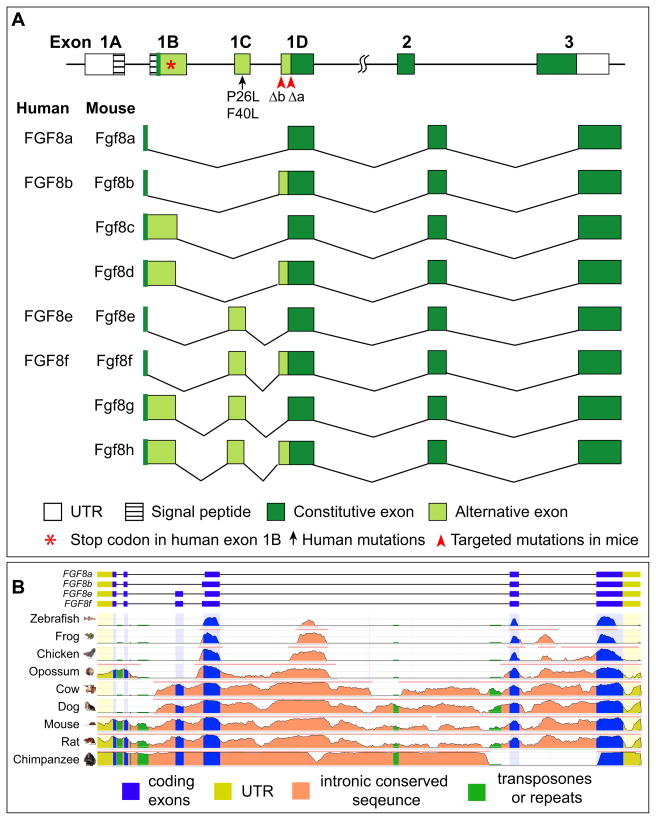
Most FGF genes contain a 3-exon genomic structure (Ornitz and Itoh, 2001). The first exon of the FGF8 gene is further subdivided into four alternatively spliced sub-exons in human and mouse (Crossley and Martin, 1995; Gemel et al., 1996; MacArthur et al., 1995b) (Fig. 1A). In mice, the Fgf8 splice variants produce eight potential isoform proteins, Fgf8 a–h, of different N-termini (Blunt et al., 1997; Crossley and Martin, 1995; MacArthur et al., 1995b). In humans, a stop codon in FGF8 exon 1B limits the number of isoforms to four, corresponding to mouse Fgf8 a, b, e and f isoforms (Gemel et al., 1996; Ghosh et al., 1996). Interestingly, FGF8a and FGF8b have 100% sequence homology between mouse and man, while FGF8 as a whole has 98% homology (Ghosh et al., 1996). Comparison of the genomic sequence of FGF8 genes in different species shows that, although the last three exons (1D, 2, and 3) are highly conserved, the upstream exons are extremely divergent (Fig. 1B). Exon 1C is absent in zebrafish, Xenopus and chicken, and only Fgf8a and Fgf8b have been identified in these organisms (Haworth et al., 2005; Inoue et al., 2006; Shim et al., 2005). The exon 1C also appears absent in opossum (Fig. 1B). Therefore, the acquisition of isoforms e, f, g, and h is probably recent in evolution and these FGF8 isoforms are only present in placental mammals.
Figure 1.
(A) Genomic structure and different splice variants of the FGF8 gene in humans and mice. In humans, there is a stop codon (asterisk) in the exon 1B. Therefore, only four potential splice variants of human FGF8 instead of eight in mice. Targeted point mutations Δa and Δb (arrowheads) disrupt the splice acceptor in exon 1D abolishing Fgf8a-containing (a, c, e, and g) and Fgf8b-containing (b, d, f, and h) splice variants in mice. Missense mutations (arrow) have been identified in FGF8 exon 1C, which is only included in FGF8e and FGF8f, in patients with idiopathic hypogonadotropic hypogonadism. (B) Genome comparison of Fgf8 genes in different species. Note that the first four exons are highly diverse while the last two exons are conserved. Exons 1C is missing in zebrafihs, frog, chicken and opossum.
Expression of different Fgf8 splice variants
Alternative splicing is often regulated, and switching production of spliceforms encoding proteins of different activity plays an important regulatory role during development. However, there is currently no evidence that the alternative splicing of FGF8 are regulated, at least during vertebrate embryogenesis. Expression studies of Fgf8 splice variants in zebrafish, Xenopus, and chick have shown that both Fgf8a and Fgf8b are co-expressed, with Fgf8b being the predominant spliceform (Inoue et al., 2006; Sato et al., 2001; Shim et al., 2005). In mice, at least seven Fgf8 splice variants were detected in various Fgf8 expressing tissues (Blunt et al., 1997; MacArthur et al., 1995b). RT-PCR using a single pair of primers that detect all Fgf8 spliceforms indicated that the relative level of different splice variants remains constant in different tissues and at different stages (Blunt et al., 1997)(Li et al, unpublished data). Although Brondani and colleagues showed that retinoic acid induces switch of FGF8b to FGF8a in the prostate cancer cell line LNCaP (Brondani and Hamy, 2000; Brondani et al., 2002), treatment with retinoic acid does not affect the relative levels of fgf8a and fgf8b transcripts in Xenopus gastrula explants or Xenopus embryos in vivo from the early gastrula to the neurula stage (Shim et al., 2005). Therefore, the overall function of FGF8 is likely controlled by the concerted action of different isoforms, depending on the availability of receptors, co-receptors, and other components of FGF signaling in the specific cellular context.
FGF8a and FGF8b have distinct activities and different binding affinities with FGF receptor
Numerous studies using different assay systems have demonstrated that two of the evolutionarily conserved FGF8 isoforms, FGF8a and FGF8b, have different biological activities. It was first reported that FGF8b had much greater transforming activity on NIH3T3 cells than FGF8a, and cells transfected with FGF8b were more tumorigenic (MacArthur et al., 1995a). Subsequently, the distinct activity of Fgf8a and Fgf8b was demonstrated by misexpression experiments in the developing midbrain and hindbrain in the mouse and chick embryo. Fgf8 is normally expressed at the midbrain-hindbrain junction, called the isthmus (Crossley and Martin, 1995). Transplantation experiments have revealed that grafts of the isthmic tissue can induce ectopic midbrain or cerebellum in competent regions of the neural tube (Alvarado-Mallart, 1993; Le Douarin, 1993). Recombinant Fgf8b protein possesses the similar inductive activity of the isthmic tissue (Crossley et al., 1996). Subsequent studies using transgenic mice or in ovo electroporation in chick embryos show that Fgf8a and Fgf8b have distinct activities in the developing midbrain. Misexpression of Fgf8a causes massive proliferation of midbrain cells and transformation of the posterior forebrain into a midbrain fate (Lee et al., 1997; Liu et al., 1999; Sato et al., 2001). On the other hand, misexpression of Fgf8b transforms midbrain cells to a hindbrain fate (Liu et al., 1999). Experiments in zebrafish and Xenopus embryos have also demonstrated different activity between fgf8a and fgf8b (Fletcher et al., 2006; Inoue et al., 2006; Shim et al., 2005).
Mitogenic studies using transfected BaF3 cell lines expressing different FGFRs have indicated that different FGF8 isoforms have distinct binding specificities with different FGFRs and their isoforms, suggesting the divergent function of FGF8a and FGF8b may result from their selective binding with FGFRs (MacArthur et al., 1995b). Given the remarkable divergence in their activity, the only difference between FGF8a and FGF8b is the presence of an additional 11 amino acids in the N-terminus of FGF8b. It was speculated that an N-glycosylation site, NFTQ, within these 11 amino acid residues might contribute to the unique function of FGF8b (Crossley and Martin, 1995; Shim et al., 2005). Indeed, glycosylation has been shown to modulate receptor binding affinity and the activity of other FGFs (Asada et al., 1999; Bellosta et al., 1993; Duraisamy et al., 2001). However, we found that mutating N31 to alanine did not affect the activity of Fgf8b, demonstrating that N-glycosylation is probably not essential for the Fgf8b-specific activity (unpublished data). Structural analysis shows that F32 mediates an additional contact with Ig domain III of the receptors FGFR1c, 2c, 3c and FGFR4, and this additional contact results in a significant increase in the binding affinity with FGFRs (Olsen et al., 2006). Moreover, substitution of F32 to alanine renders FGF8bF32A to have a similar binding affinity with FGFR1c, 2c, 3c and FGFR4 as FGF8a; forced expression of FGF8bF32A causes the same phenotype as that of FGF8a in the embryonic midbrain of chick embryos (Olsen et al., 2006). Therefore, the increased binding affinity of FGF8b with FGFRs accounts for the FGF8b-specific activity.
It remains to be determined whether the activity of Fgf8a and Fgf8b differs in the intensity or the pathway of FGF8 signaling. Electroporation of reduced concentration of Fgf8b produces the same phenotype as Fgf8a in the chick embryo (Sato et al., 2001). However, electroporation of increased concentration of Fgf8a in chick embryos (unpublished data), or injection of 100x more Fgf8a RNA does not produce the same phenotype as Fgf8b in Xenopus, suggesting that there is quality difference in the signaling generated by Fgf8a or Fgf8b (Fletcher et al., 2006). In support of this notion, only Fgf8b induces the Ras-ERK signaling pathway in chick midbrain (Sato and Nakamura, 2004). Differences in the kinetics of ligand-receptor interaction may modulate the quality of signal transduction. Interestingly, structural analysis suggests that FGF8b binding induces rotation and closer juxtaposition of cytoplasmic kinase domains of FGF-FGFR dimers, which may ultimately change the kinetics and quality of signal transduction (Olsen et al., 2006).
In vivo requirement for Fgf8a and Fgf8b isoforms during development
Loss-of-function experiments have identified the relative contribution of FGF8a and FGF8b to the overall FGF8 function during embryogenesis. Experiments in Xenopus using morpholino oligonucleotides to eliminate fgf8a and fgf8b together or fgf8a alone demonstrate that fgf8b, but not fgf8a, is required for proper gastrulation and mesoderm formation, whereas deletion of fgf8a causes a reduction in the specification of posterior neural tissues (Fletcher et al., 2006). Mice carrying a point mutation at the splice acceptor that abolishes Fgf8b-containing spliceforms (b, d, f, and h) display an identical defect in morphogenetic remodeling as embryos homozygous for Fgf8-null mutation before gastrulation (Guo and Li, 2007). However, the remaining Fgf8a-containing spliceforms (a, c, e, and g) can partially compensate for the loss of Fgf8b-containing spliceforms in mesoderm specification and migration, both of which are severely disrupted in Fgf8-null embryos, during gastrulation (Guo and Li, 2007; Sun et al., 1999). In contrast to their role in gastrulation, Fgf8b-containing spliceforms are absolutely essential for Fgf8 function in the formation of the midbrain and hindbrain (MHB) (Guo et al., 2010). Deletion of Fgf8b-containing spliceforms leads to loss of multiple key regulatory genes, including Fgf8 itself, in the isthmus. Therefore, specific inactivation of Fgf8b-containing spliceforms, similar to the complete deletion of Fgf8, in MHB progenitors results in loss of the midbrain, isthmus, and cerebellum (Chi et al., 2003; Guo et al., 2010). Conversely, deletion of Fgf8a-containing isoforms has no discernable effects on MHB development (Guo et al., 2010). Interestingly, mice lacking Fgf8a-containing spliceforms display phenotypes, such as growth retardation, reduced fertility, and partial postnatal lethality, and the severity of the phenotype is dependent on genetic backgrounds (Guo et al., 2010). These results suggest that, although Fgf8b-containing isoforms have a dominant function, Fgf8a-containing spliceforms may play a subtle role in fine-tuning Fgf8 function in tissues outside the MHB region.
Other FGF8 isoforms may also contribute to the overall FGF8 function
There is evident that, in addition to FGF8a and Fgf8b, other FGF8 isoforms may also contribute to the overall FGF8 function. Experiments using BaF3 cells expressing different FGFRs have revealed that recombinant Fgf8c and Fgf8f have a strong mitogenic activity by activating Fgfr2c, Fgfr3c and Fgfr4 (Blunt et al., 1997; MacArthur et al., 1995b). Mouse Fgf8f exhibits similar potency as human and mouse FGF8b in inducing mesoderm in Xenopus embryo explants and expanding the mesodermal domain in Xenopus embryos (Fletcher et al., 2006). Given that FGF8f contains the N-terminal sequence that is crucial for FGF8b activity, it may not be surprising that FGF8f has similar activity as FGF8b (Falardeau et al., 2008; Olsen et al., 2006). In support of the potential function FGF8f, both FGF8b and FGF8f are induced by androgens in squamous cell carcinoma line and the expression of FGF8f is detected and associated with progression of esophageal carcinomas (Tanaka et al., 2001). Furthermore, two loss-of-function mutations, P26L and F40L, that are located within exon 1C and thus affect only FGF8e and FGF8f isoforms have been indentified in patients with idiopathic hypogonadotropic hypogonadism, which is associated with deficiency of gonadotropin-releasing hormone (GnRH) (Falardeau et al., 2008). Therefore, FGF8e and/or FGF8f isoforms may be important for the function of FGF8 in the formation GnRH neurons.
Concluding remarks
Multiple FGF8 isoforms are co-expressed in various site of FGF8 expressing cells. The function of FGF8 may, therefore, reflect the combined activity of a particular sum of FGF8 isoforms. As the addition of new Fgf8 isoforms occurs in placental mammals, Fgf8c–h may play distinct function from Fgf8a and Fgf8b, and probably contribute to the evolution of mammals. It is worth to note that several different FGF family members are often expressed in the same tissue during development. Interactions among different FGFs may produce activities different from each individual FGF. Therefore, experiments to dissect the relative contribution of different FGF8 isoforms to the overall FGF8 function can provide an excellent experimental paradigm to understand the interaction among FGFs in the control of vertebrate development. The remaining challenges are to determine the molecular bases underlying the different activity of FGF8 isoforms and the potential interaction among different FGF8 isoforms.
Acknowledgments
We thank Dr. Jaime Rivera-Perez for critical reading of the manuscript. This work is supported by grants from the NIH (R01HD050474) and March of Dimes foundation to J. L.
References
- Alvarado-Mallart RM. Fate and potentialities of the avian mesencephalic/metencephalic neuroepithelium. J Neurobiol. 1993;24(10):1341–1355. doi: 10.1002/neu.480241007. [DOI] [PubMed] [Google Scholar]
- Asada M, Yoneda A, Oda Y, Ota K, Ozawa K, Fukuta K, Omae F, Asanagi M, Orikasa N, Suzuki M, Oka S, Makino T, Imamura T. Characterization of fibroblast growth factor-6 expressed by Chinese hamster ovary cells as a glycosylated mitogen for human vascular endothelial cells. Growth Factors. 1999;16(4):293–303. doi: 10.3109/08977199909069147. [DOI] [PubMed] [Google Scholar]
- Bellosta P, Talarico D, Rogers D, Basilico C. Cleavage of K-FGF produces a truncated molecule with increased biological activity and receptor binding affinity. J Cell Biol. 1993;121(3):705–713. doi: 10.1083/jcb.121.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt AG, Lawshe A, Cunningham ML, Seto ML, Ornitz DM, MacArthur CA. Overlapping expression and redundant activation of mesenchymal fibroblast growth factor (FGF) receptors by alternatively spliced FGF-8 ligands. J Biol Chem. 1997;272(6):3733–3738. doi: 10.1074/jbc.272.6.3733. [DOI] [PubMed] [Google Scholar]
- Brondani V, Hamy F. Retinoic acid switches differential expression of FGF8 isoforms in LNCaP cells. Biochem Biophys Res Commun. 2000;272(1):98–103. doi: 10.1006/bbrc.2000.2740. [DOI] [PubMed] [Google Scholar]
- Brondani V, Klimkait T, Egly JM, Hamy F. Promoter of FGF8 reveals a unique regulation by unliganded RARalpha. J Mol Biol. 2002;319(3):715–728. doi: 10.1016/S0022-2836(02)00376-5. [DOI] [PubMed] [Google Scholar]
- Chi CL, Martinez S, Wurst W, Martin GR. The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development. 2003;130(12):2633–2644. doi: 10.1242/dev.00487. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121(2):439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380(6569):66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108(2):183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40(1):65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, Pourquie O. fgf8 mRNA decay establishes a gradient that couples axial elongation to patterning in the vertebrate embryo. Nature. 2004;427(6973):419–422. doi: 10.1038/nature02216. [DOI] [PubMed] [Google Scholar]
- Duraisamy Y, Slevin M, Smith N, Bailey J, Zweit J, Smith C, Ahmed N, Gaffney J. Effect of glycation on basic fibroblast growth factor induced angiogenesis and activation of associated signal transduction pathways in vascular endothelial cells: possible relevance to wound healing in diabetes. Angiogenesis. 2001;4(4):277–288. doi: 10.1023/a:1016068917266. [DOI] [PubMed] [Google Scholar]
- Eriksson AE, Cousens LS, Weaver LH, Matthews BW. Three-dimensional structure of human basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991;88(8):3441–3445. doi: 10.1073/pnas.88.8.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118(8):2822–2831. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RB, Baker JC, Harland RM. FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development. 2006;133(9):1703–1714. doi: 10.1242/dev.02342. [DOI] [PubMed] [Google Scholar]
- Frank DU, Fotheringham LK, Brewer JA, Muglia LJ, Tristani-Firouzi M, Capecchi MR, Moon AM. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development. 2002;129(19):4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130(9):1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- Gemel J, Gorry M, Ehrlich GD, MacArthur CA. Structure and sequence of human FGF8. Genomics. 1996;35(1):253–257. doi: 10.1006/geno.1996.0349. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Shankar DB, Shackleford GM, Wu K, T'Ang A, Miller GJ, Zheng J, Roy-Burman P. Molecular cloning and characterization of human FGF8 alternative messenger RNA forms. Cell Growth Differ. 1996;7(10):1425–1434. [PubMed] [Google Scholar]
- Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque MS, Mohammadi M. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27(9):3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. Fibroblast growth factor homologous factors: evolution, structure, and function. Cytokine Growth Factor Rev. 2005;16(2):215–220. doi: 10.1016/j.cytogfr.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975;250(7):2515–2520. [PubMed] [Google Scholar]
- Gospodarowicz D, Bialecki H, Greenburg G. Purification of the fibroblast growth factor activity from bovine brain. J Biol Chem. 1978;253(10):3736–3743. [PubMed] [Google Scholar]
- Gospodarowicz D, Jones KL, Sato G. Purification of a growth factor for ovarian cells from bovine pituitary glands. Proc Natl Acad Sci U S A. 1974;71(6):2295–2299. doi: 10.1073/pnas.71.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Li JY. Distinct functions of the major Fgf8 spliceform, Fgf8b, before and during mouse gastrulation. Development. 2007;134(12):2251–2260. doi: 10.1242/dev.004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Li K, Sunmonu NA, Li JY. Fgf8b-containing spliceforms, but not Fgf8a, are essential for Fgf8 function during development of the midbrain and cerebellum. Dev Biol. 2010;338(2):183–192. doi: 10.1016/j.ydbio.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth KE, Healy C, Sharpe PT. Characterisation of the genomic structure of chick Fgf8. DNA Seq. 2005;16(3):180–186. doi: 10.1080/10425170500069973. [DOI] [PubMed] [Google Scholar]
- Inoue F, Nagayoshi S, Ota S, Islam ME, Tonou-Fujimori N, Odaira Y, Kawakami K, Yamasu K. Genomic organization, alternative splicing, and multiple regulatory regions of the zebrafish fgf8 gene. Dev Growth Differ. 2006;48(7):447–462. doi: 10.1111/j.1440-169X.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20(11):563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237(1):18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Shou J, Santos R, Hebert JM, McConnell SK, Mason I, Calof AL. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132(23):5211–5223. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 2005;19(5):603–613. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM. Embryonic neural chimaeras in the study of brain development. Trends Neurosci. 1993;16(2):64–72. doi: 10.1016/0166-2236(93)90019-i. [DOI] [PubMed] [Google Scholar]
- Lee SM, Danielian PS, Fritzsch B, McMahon AP. Evidence that FGF8 signalling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development. 1997;124(5):959–969. doi: 10.1242/dev.124.5.959. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat Genet. 2000;26(4):460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- Liu A, Losos K, Joyner AL. FGF8 can activate Gbx2 and transform regions of the rostral mouse brain into a hindbrain fate. Development. 1999;126(21):4827–4838. doi: 10.1242/dev.126.21.4827. [DOI] [PubMed] [Google Scholar]
- MacArthur CA, Lawshe A, Shankar DB, Heikinheimo M, Shackleford GM. FGF-8 isoforms differ in NIH3T3 cell transforming potential. Cell Growth Differ. 1995a;6(7):817–825. [PubMed] [Google Scholar]
- MacArthur CA, Lawshe A, Xu J, Santos-Ocampo S, Heikinheimo M, Chellaiah AT, Ornitz DM. FGF-8 isoforms activate receptor splice forms that are expressed in mesenchymal regions of mouse development. Development. 1995b;121(11):3603–3613. doi: 10.1242/dev.121.11.3603. [DOI] [PubMed] [Google Scholar]
- Maciag T, Cerundolo J, Ilsley S, Kelley PR, Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979;76(11):5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Ferre A, Martinez S. The development of the thalamic motor learning area is regulated by Fgf8 expression. J Neurosci. 2009;29(42):13389–13400. doi: 10.1523/JNEUROSCI.2625-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18(2):136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Martin GR. Differences in left-right axis pathways in mouse and chick: functions of FGF8 and SHH. Science. 1999;285(5426):403–406. doi: 10.1126/science.285.5426.403. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16(2):107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Moon AM, Capecchi MR. Fgf8 is required for outgrowth and patterning of the limbs. Nat Genet. 2000;26(4):455–459. doi: 10.1038/82601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent MA, Edelman ER. Kinetics of basic fibroblast growth factor binding to its receptor and heparan sulfate proteoglycan: a mechanism for cooperactivity. Biochemistry. 1992;31(37):8876–8883. doi: 10.1021/bi00152a026. [DOI] [PubMed] [Google Scholar]
- Olsen SK, Garbi M, Zampieri N, Eliseenkova AV, Ornitz DM, Goldfarb M, Mohammadi M. Fibroblast growth factor (FGF) homologous factors share structural but not functional homology with FGFs. J Biol Chem. 2003;278(36):34226–34236. doi: 10.1074/jbc.M303183200. [DOI] [PubMed] [Google Scholar]
- Olsen SK, Li JY, Bromleigh C, Eliseenkova AV, Ibrahimi OA, Lao Z, Zhang F, Linhardt RJ, Joyner AL, Mohammadi M. Structural basis by which alternative splicing modulates the organizer activity of FGF8 in the brain. Genes Dev. 2006;20(2):185–198. doi: 10.1101/gad.1365406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2(3):REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40(12):1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development. 2005;132(17):3859–3871. doi: 10.1242/dev.01945. [DOI] [PubMed] [Google Scholar]
- Riley BM, Mansilla MA, Ma J, Daack-Hirsch S, Maher BS, Raffensperger LM, Russo ET, Vieira AR, Dode C, Mohammadi M, Marazita ML, Murray JC. Impaired FGF signaling contributes to cleft lip and palate. Proc Natl Acad Sci U S A. 2007;104(11):4512–4517. doi: 10.1073/pnas.0607956104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Araki I, Nakamura H. Inductive signal and tissue responsiveness defining the tectum and the cerebellum. Development. 2001;128(13):2461–2469. doi: 10.1242/dev.128.13.2461. [DOI] [PubMed] [Google Scholar]
- Sato T, Nakamura H. The Fgf8 signal causes cerebellar differentiation by activating the Ras-ERK signaling pathway. Development. 2004;131(17):4275–4285. doi: 10.1242/dev.01281. [DOI] [PubMed] [Google Scholar]
- Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell. 2000;6(3):743–750. doi: 10.1016/s1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- Shim S, Bae N, Park SY, Kim WS, Han JK. Isolation of Xenopus FGF-8b and comparison with FGF-8a. Mol Cells. 2005;19(3):310–317. [PubMed] [Google Scholar]
- Storm EE, Rubenstein JL, Martin GR. Dosage of Fgf8 determines whether cell survival is positively or negatively regulated in the developing forebrain. Proc Natl Acad Sci U S A. 2003;100(4):1757–1762. doi: 10.1073/pnas.0337736100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13(14):1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Miyamoto K, Minamino N, Takeda M, Sato B, Matsuo H, Matsumoto K. Cloning and characterization of an androgen-induced growth factor essential for the androgen-dependent growth of mouse mammary carcinoma cells. Proc Natl Acad Sci U S A. 1992;89(19):8928–8932. doi: 10.1073/pnas.89.19.8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Ueo H, Mafune K, Mori M, Wands JR, Sugimachi K. A novel isoform of human fibroblast growth factor 8 is induced by androgens and associated with progression of esophageal carcinoma. Dig Dis Sci. 2001;46(5):1016–1021. doi: 10.1023/a:1010753826788. [DOI] [PubMed] [Google Scholar]
- Trarbach EB, Abreu AP, Silveira LF, Garmes HM, Baptista MT, Teles MG, Costa EM, Mohammadi M, Pitteloud N, Mendonca BB, Latronico AC. Nonsense mutations in FGF8 gene causing different degrees of human gonadotropin-releasing deficiency. J Clin Endocrinol Metab. 2010;95(7):3491–3496. doi: 10.1210/jc.2010-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpp A, Depew MJ, Rubenstein JL, Bishop JM, Martin GR. Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 1999;13(23):3136–3148. doi: 10.1101/gad.13.23.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456(7221):470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Komiya H, Chirino A, Faham S, Fox GM, Arakawa T, Hsu BT, Rees DC. Three-dimensional structures of acidic and basic fibroblast growth factors. Science. 1991;251(4989):90–93. doi: 10.1126/science.1702556. [DOI] [PubMed] [Google Scholar]