Abstract
The condition of oxidative stress arises when oxidant production exceeds antioxidant activity in cells and plasma. The overabundance of oxidants is mechanistically connected with the multifactorial etiology of insulin resistance, primarily in skeletal muscle tissue, and the subsequent development of type 2 diabetes. Two important mechanisms for this oxidant excess are 1) the mitochondrial overproduction of hydrogen peroxide and superoxide ion in conditions of energy surplus and 2) the enhanced activation of cellular NADPH oxidase via angiotensin II (AT1) receptors. Several recent studies are reviewed that support the concept that direct exposure of mammalian skeletal muscle to an oxidant stress (including hydrogen peroxide) results in stimulation of the serine kinase p38 mitogen-activated protein kinase (p38 MAPK), and that the engagement of this stress-activated p38 MAPK signaling is mechanistically associated with diminished insulin-dependent stimulation of insulin signaling elements and glucose transport activity. The beneficial interactions between the antioxidant α-lipoic acid and the advanced glycation end product inhibitor pyridoxamine to ameliorate oxidant stress-associated defects in whole-body and skeletal muscle insulin action in the obese Zucker rat, a model of pre-diabetes, are also addressed. Overall, this review highlights the importance of oxidative stress in the development of insulin resistance in mammalian skeletal muscle tissue, at least in part via a p38 MAPK-dependent mechanism, and indicates that interventions that reduce this oxidative stress and oxidative damage can improve insulin action in insulin-resistant animal models. Strategies to prevent and ameliorate oxidative stress remain important in the overall treatment of insulin resistance and type 2 diabetes.
Keywords: Hydrogen peroxide, skeletal muscle, glucose transport, obese Zucker rat, lipoic acid
Introduction
The long-term maintenance of blood glucose levels within a normal range is a key physiological function in mammalian species. One criterion for normal glucose homeostasis in humans is the maintenance of blood glucose levels below 100 mg/dl following an overnight fast. If fasting blood glucose rises above 100 mg/dl, but does not surpass 126 mg/dl, these individual are considered to be “pre-diabetic”. Moreover, if glucoregulation deteriorates further, and fasting blood glucose exceeds 126 mg/dl, these individuals can be diagnosed with frank diabetes [1]. The vast majority (90–95%) of diabetic cases are classified as type 2 diabetes, a condition hallmarked both by reduced action of the hormone insulin to activate the glucose transport system in skeletal muscle (referred to as “insulin resistance”) and an inadequate compensatory hyperinsulinemia to overcome this insulin resistance [1]. This insulin resistance in skeletal muscle is a critical, early defect leading to the initial development of impaired glucose tolerance (reduced ability to dispose of an oral glucose load) in pre-diabetes and subsequently to the conversion from pre-diabetes to overt type 2 diabetes. Therefore, insulin resistance is a common metabolic impairment affecting individuals with either pre-diabetes (>57 million people in the United States) or overt type 2 diabetes, estimated to afflict ~24 million Americans and increasing [2].
The foregoing observations underscore the need for understanding how normal glucose homeostasis is achieved in humans, and what specific defects can lead to impairments in the cellular and systemic mechanisms required for this homeostatic process. In this context, the present review will briefly cover how insulin action at the level of skeletal muscle contributes to the maintenance of normal blood glucose levels and will subsequently focus on the role of one important factor, oxidative stress, involved in the multifactorial etiology of insulin resistance of glucose transport activity in skeletal muscle. The discussion of oxidative stress in this review will be limited to a few selected cellular factors engaged by oxidants (including glycogen synthase kinase-3 (GSK-3) and p38 mitogen-activated protein kinase (p38 MAPK)) or that themselves are involved in the production of oxidants (angiotensin II (ANG II), and are mechanistically linked to the development of insulin resistance in skeletal muscle.
Brief overview of normal glucoregulation in mammalian species
Systemic glucose homeostasis is achieved by the coordinated functions of several organ systems, including skeletal muscle, the liver, the endocrine pancreas, adipose tissue, and specific hypothalamic neurons [3]. The liver contributes to glucoregulation primarily via appropriate alterations in hepatic glucose production. The adipose tissue is a site of insulin-dependent glucose disposal and also acts as an endocrine organ releasing adipokines. The pancreatic α- and β-cells are the sites of the synthesis and secretion of insulin and glucagon, respectively. The hypothalamus functions in the neural regulation of these organ systems. While the contributions of these aforementioned organ systems are clearly of great importance to overall glucoregulation, the focus of this review will be on skeletal muscle, as this tissue represents the primary site of glucose disposal after a meal or during a bout of exercise [4, 5]. In this article, we will review 1) the regulation of the insulin-dependent glucose transport system in skeletal muscle under normal physiological conditions and 2) the dysregulation of this system caused specifically by oxidative stress that leads to diminished insulin-mediated glucose removal from the bloodstream, collectively termed insulin resistance.
The glucose transport system in mammalian skeletal muscle is acutely regulated by the hormone insulin through a mechanism involving the sequential engagement of several intracellular proteins [for reviews, see refs. 6–9]. Insulin binding to the α-subunits of the insulin receptor (IR) increases the receptor β-subunit tyrosine kinase activity, which can phosphorylate IR substrates (IRS; IRS-1 and IRS-2 in skeletal muscle). The tyrosine-phosphorylated IRS proteins then interact with the SH2 domains of the p85 regulatory subunit of phosphatidylinositol-3-kinase (PI3-kinase) and activate the p110 catalytic subunit of PI3-kinase. The phosphoinositide moieties produced will then allosterically activate 3-phosphoinositide-dependent kinases, which will subsequently phosphorylate Akt, a serine/threonine kinase, at Thr308. In addition, insulin-dependent engagement of the mTOR complex 2 is associated with phosphorylation of Akt on Ser473 [10, 11]. More recently, it has been shown that one target of Akt is AS160 (also known as TBC1D4), which contains a Rab-GTPase domain and has been shown to modulate the translocation of the glucose transporter protein isoform GLUT-4 to the sarcolemmal membrane [9, 12]. Once GLUT-4 is placed into the sarcolemma, glucose transport can occur via a facilitative diffusion process. It should be pointed out here that serine phosphorylation of IRS proteins via various insulin-regulated serine kinases is a normal means by which the insulin signaling cascade mediates negative feedback control upon itself [13]. However, overactivity of these serine kinases is one mechanism for the induction or exacerbation of insulin resistance (see below).
Select mechanisms for overproduction of oxidants: mitochondrial dysfunction and overactivation of NADPH oxidase
Oxidative stress develops from an imbalance between oxidant production and antioxidant activity in cells and in the plasma. This overabundance of oxidants is associated with the multifactorial etiology of insulin resistance, primarily in skeletal muscle tissue [14–18]. Correlative evidence in humans indicates an association between plasma markers of oxidative stress and damage and the degree of insulin resistance [19, 20]. While many possible cellular sites exist for the overproduction of reactive oxygen species (ROS) implicated in this relationship between oxidative stress and insulin resistance, there is surprisingly little definitive evidence in the literature on this issue. There are numerous systemic and cellular dysfunctions that can potentially contribute to ROS overproduction (including hyperglycemia, dyslipidemia, endoplasmic reticulum (ER) stress, advanced glycation end-products (AGEs), nitric oxide synthase, and lipid peroxides) and may activate factors associated with reduced insulin action [reviewed in ref. 21]. In addition, increased carbonylation and nitrosylation of proteins in insulin-sensitive tissues may be mechanistically linked to the etiology of insulin resistance in various animal models [22–26]. However, a comprehensive discussion of all of these potential mechanisms is beyond the scope of this brief review. Two specific mechanisms of ROS production relevant to the etiology of insulin resistance, mitochondrial hydrogen peroxide (H2O2) production and NADPH oxidase activation, are discussed in more detail below.
Mitochondrial H2O2
One cellular site with a high capacity for the production of oxidants, such as H2O2, is the mitochondrion [27]. A recent seminal investigation provides convincing evidence demonstrating a mechanistic relationship between elevated mitochondrial oxidant production and insulin resistance in mammalian skeletal muscle under conditions of metabolic imbalance [28]. In skeletal muscle obtained both from rats made insulin-resistant by being placed on a high-fat diet and from insulin-resistant, morbidly obese human subjects, the potential for mitochondrial H2O2 emission was enhanced, the redox state was more oxidized, and the redox-buffering capacity was diminished compared to that in skeletal muscle of the respective lean, insulin-sensitive control groups. In addition, treatment of high fat-fed rats with a cell-permeable small peptide antioxidant (SS31) resulted in attenuation of the excessive mitochondrial H2O2-emitting potential and prevented the development of whole-body and skeletal muscle insulin resistance. In mice in which the H2O2 scavenger catalase was overexpressed specifically in skeletal muscle, the effect of the high-fat diet to induce insulin resistance was prevented [28]. Collectively, these results support the concept that excess mitochondrial H2O2 production plays an important mechanistic role in the development of insulin resistance in skeletal muscle of individuals subjected to an energy surplus via increased dietary fat.
Cellular NADPH oxidase
The renin-angiotensin system plays critical roles in the normal regulation of several physiological processes, including blood pressure, cardiac mass and contractility, and fluid homeostasis [29]. Overactivity of the renin-angiotensin system is linked not only with the multifactorial etiology of hypertension, but is also known to be associated with the development of skeletal muscle insulin resistance and type 2 diabetes [29, 30]. ANG II, a distal element of this system, can bind to ANG II type 1 receptors (AT1R) in various cell types, including skeletal muscle [31, 32] and cardiomyocytes [33], and activate NADPH oxidase, producing superoxide ions via a one-electron reduction of oxygen and the oxidation of NADPH. Evidence for a mechanistic role of NADPH-derived superoxide in ANG II-induced insulin resistance comes from a study in cultured L6 myotubes, in which the effect of ANG II to impair insulin signaling and GLUT-4 translocation was prevented by the NADPH inhibitor apocynin [32]. Further information on the impact of oxidants on insulin action in mammalian skeletal muscle is presented below.
Recent findings on mechanisms underlying oxidant-induced insulin resistance in mammalian skeletal muscle
In vitro oxidant production: experimental approach to investigate oxidant-induced insulin resistance in isolated rat skeletal muscle
Over the last five years, we have utilized an in vitro approach, based on previous studies in cultured 3T3L1 adipocytes [34, 35] and L6 myotubes [36], to investigate the potential cellular mechanisms underlying the deleterious effects of the oxidant H2O2 on insulin signaling and glucose transport activity in mammalian skeletal muscle. This approach involves preparation of rat soleus muscle into strips that are suitable for in vitro incubations [37]. Subsequently, the enzyme glucose oxidase is added at specific concentrations in order to produce a constant target level of H2O2 from glucose present in the incubation medium. Initial studies utilized 2 hr incubations with the addition of 100 mU/ml glucose oxidase, which produced H2O2 at 60–90 µM [38]. In the absence of insulin, this concentration of H2O2 induced an increase in basal glucose transport activity that was associated with increased engagement of insulin signaling factors (IR, PI3-kinase, and Akt), but was preventable with the PI3-kinase inhibitor wortmannin. The 2-hr exposure to this level of H2O2 also increased activation of AMPK and p38 MAPK. Importantly, the selective inhibition of p38 MAPK with A304000 (which did not alter the oxidant-induced increases in AMPK or Akt phosphorylation) completely prevented the oxidant-induced increase in basal glucose transport activity in the isolated soleus, indicating a critical role of p38 MAPK in the action of H2O2 on basal glucose transport [38].
We have also investigated the impact of this 2-hr exposure to 60–90 µM H2O2 on insulin action in isolated soleus muscle [39]. The oxidant caused a substantial loss of insulin stimulation of both proximal (IR tyrosine phosphorylation) and distal (Akt and GSK-3β serine phosphorylation) insulin signaling elements and of glucose transport activity. A longer exposure (4 hr) of isolated soleus muscle to this level of H2O2 was associated with a selective loss of IRS-1 and IRS-2 protein, exacerbating the loss of insulin action in response to the oxidant stress [40]. Phosphorylation of Ser307 on IRS-1 was also increased under these conditions. These findings are in agreement with other investigations showing that oxidant stress leads to increased IRS proteolysis [41]. Interestingly, the loss of IRS-1 (and the enhanced IRS-1 Ser307 phosphorylation), but not of IRS-2, could be partially prevented when the oxidant-induced phosphorylation and activation of p38 MAPK was inhibited with A304000, and insulin-stimulated glucose transport activity was improved [40]. These results again underscore the important role of p38 MAPK in mediating the action of the oxidant stress, but in this case p38 MAPK is mediating the effect of H2O2 to induce insulin resistance in skeletal muscle, consistent with previous findings in cultured muscle cells [36, 42]. While our data do indicate a role of p38 MAPK in the stimulation of basal glucose transport and in insulin-stimulated glucose transport activity, these effects of p38 MAPK may involve different p38 MAPK isoforms (α, β, and γ-isoforms exist in myocytes) and/or intracellular sites of action/signaling factors from the p38 MAPK isoform(s) mediating oxidant-associated insulin resistance in skeletal muscle.
Because of the potentially confounding effects of this exposure to H2O2 (60–90 µM for 2–4 hr) on basal glucose transport in the interpretation of data on insulin resistance, we designed experiment conditions that eliminated alterations in basal glucose transport while still inducing insulin resistance. We have found that a 6-hr exposure to a lower degree of oxidant stress (30–40 µM H2O2 produced by the addition of 50 mU/ml glucose oxidase) does not result in enhanced basal glucose transport, but does induce marked reductions in insulin-stimulated Akt and GSK-3β phosphorylation and glucose transport activity [43]. Furthermore, this study provides more definitive evidence that an oxidant stress can directly induce insulin resistance in mammalian skeletal muscle via a p38 MAPK-dependent mechanism.
Related to this issue of the role of p38 MAPK in oxidant-induced insulin resistance in skeletal muscle, our collaborators from Mahidol University in Thailand have made the important observation that prior exercise training by rats attenuates the response of skeletal muscle to oxidant-induced activation of p38 MAPK and insulin resistance [44]. These findings are consistent with the concept that one mechanism by which endurance exercise training can enhance insulin sensitivity of skeletal muscle is via an enhanced capacity to resist the oxidant stress-associated activation of p38 MAPK that normally diminishes insulin action.
It should be noted that other stress-activated serine kinases may also contribute to the etiology of oxidant-induced insulin resistance in skeletal muscle [reviewed in ref. 21]. For example, c-jun N-terminal kinase (JNK) (specifically JNK1, one of three JNK isoforms that belong to the MAPK superfamily) can be activated by several inputs, including ROS and ER stress [21], and JNK activity is elevated in tissues of obese mice [45]. Moreover, global knockout of JNK1 reduces adiposity in mice placed on a high fat diet and improves glucose tolerance and insulin sensitivity, supporting the connection between JNK1 and insulin resistance [45]. We have also found an important role of JNK activation in the mechanism of oxidant-induced insulin resistance in mammalian skeletal muscle [43, and unpublished data]. The serine kinase IκB kinase β (IKKβ) regulates the functionality of the redox-sensitive transcription factor NF-κB, leading to increased expression of various proinflammatory genes and impacting immune responses, cell survival, and metabolic regulation [21]. IKKβ is activated by ROS and ER stress [21], and inhibition of IKKβ has been associated with improved insulin sensitivity [46]. However, unlike JNK, more definitive evidence is needed linking IKKβ mechanistically with impaired insulin signaling and reduced stimulation of glucose transport activity due to oxidative stress in skeletal muscle.
ANG II and oxidant stress-induced insulin resistance in isolated rat skeletal muscle
Several lines of investigation have demonstrated that excess ANG II action both in vivo and in vitro will induce a state of whole-body and skeletal muscle insulin resistance. Systemic administration of ANG II to normal rats will cause decreased systemic insulin sensitivity and reduced insulin-stimulated glucose transport activity in vitro in skeletal muscle [47]. TG(mREN2) rats, which overexpress the mouse renin-2 gene and display ANG II overactivity, are markedly insulin-resistant [48, 49] and this insulin insensitivity can be ameliorated by treatment with AT1R blockers [48, 50, 51]. Likewise, insulin resistance in obese Zucker rats can be enhanced by treatment with either an ACE inhibitor or an AT1R blocker [52, 53].
Direct evidence for a role of ANG II in oxidant-induced insulin resistance has come more recently from in vitro investigations involving cultured muscle cells or isolated mammalian skeletal muscle preparations. As mentioned above, chronic exposure of cultured L6 myotubes to ANG II impairs IRS-1 tyrosine phosphorylation and Akt serine phosphorylation and reduces insulin-dependent GLUT-4 translocation, effects prevented by inhibition of NADPH oxidase [32], by the superoxide dismutase mimetic tempol [51], or by pharmacological inhibition or knockdown of NF-κB [51]. Moreover, we have recently demonstrated that chronic exposure of rat soleus muscle to ANG II also impairs Akt and GSK-3 signaling and induces insulin resistance of glucose transport activity [54]. Importantly, the ANG II-induced insulin resistance of glucose transport activity could be partially rescued by co-treatment with the free radical scavenger tempol [54]. Collectively, these investigations provide support for the concept that overactivity of the renin-angiotensin system, in particular ANG II acting via the AT1 receptor coupled with NADPH oxidase, can mediate skeletal muscle insulin resistance, at least in part via a mechanism involving ROS. The potential role of NF-kB in linking NADPH oxidase-mediated ROS and insulin resistance in mammalian skeletal muscle is intriguing, and requires further investigation.
Interactions of the antioxidant ALA and the AGE inhibitor pyridoxamine in insulin resistance
The effectiveness of the antioxidant α-lipoic acid (ALA) to ameliorate oxidative stress and damage and improve systemic and skeletal muscle glucose disposal in conditions of insulin resistance in well documented in several investigative models [reviewed in refs. 18, 55, 56], including 3T3-L1 adipocytes [57–59], L6 myotubes [27, 60], and isolated rat skeletal muscle [61], various rat models of insulin resistance [22, 62–68], and in type 2 diabetic humans [69–71]. ALA can reduce the formation of AGE, markers of oxidative damage that are associated with impaired insulin action in skeletal muscle [72] and with the development of diabetic complications [73, 74]. Pyridoxamine (PYR) is a compound that directly inhibits AGE formation [75, 76], and chronic administration of PYR to streptozotocin-diabetic rodents [77, 78] or the pre-diabetic obese Zucker rats [79], both models characterized by oxidative stress, will reduce tissue AGE and plasma triglycerides and improve renal function.
We have recently addressed the potential interactions of the antioxidant R-(+)-ALA (the R-enantiomer of ALA) and the AGE inhibitor PYR on glucose tolerance, insulin sensitivity, and regulation of skeletal muscle glucose transport activity in the obese Zucker rat [25, 26]. After 6 weeks of systemic treatment, R-ALA and PYR alone or in combination lowered muscle protein carbonyls and urine conjugated dienes, both markers of oxidative damage. The combination of R-ALA and PM reduced fasting glucose, insulin, and free fatty acids in plasma and triglycerides in muscle and enhanced whole-body insulin sensitivity and insulin-stimulated skeletal muscle glucose transport activity to a greater extent than with either compound individually [25]. These improvements in metabolic regulation in the obese Zucker rat were essentially maintained with more prolonged (22 weeks) treatments with R-ALA and PYR in combination [26]. Collectively, the results of these studies indicate that there are beneficial interactions between the antioxidant R-ALA and the AGE inhibitor PYR for ameliorating whole-body and skeletal muscle insulin resistance in pre-diabetic obese Zucker rats.
Role of oxidative stress in diabetic complications
In addition to its documented contributions to the etiology of insulin resistance and type 2 diabetes, oxidative stress has been implicated in the development of diabetic complications, including diabetic retinopathy, nephropathy, peripheral neuropathy, and cardiovascular disease. Specific cells types, including endothelial cells, in tissues susceptible to diabetic complications are unable to regulate intracellular glucose levels [80], and hyperglycemia-induced overproduction of superoxide by the mitochondria in the microvasculature is an underlying feature of each of the diseases mentioned above [81]. Increased activity of local renin-angiotensin systems can also result in increased oxidative stress via AT1 receptor-mediated activation of NADPH oxidase in tissues susceptible to diabetic complications [82]. This overabundance of cellular oxidants and their contribution to diabetic complications are exacerbated by decreased expression of proteins that neutralize oxidants, including superoxide dismutase and catalase, in diabetes [83]. Oxidative stress and its downstream effects, including increased production and action of AGEs, alter gene expression, signaling pathways, and protein functionality leading to disruptions of normal cellular and tissue morphology and function [81].
A more thorough explanation of the many and complex ways in which oxidative stress contributes to diabetic complications is beyond the scope of this review, and readers are referred to recent reviews of the role of oxidative stress in diabetic cardiovascular disease [84–87], nephropathy [88, 89], retinopathy [90], and peripheral neuropathy [91]. Treatment with antioxidants has been hypothesized to prevent or delay the development of diabetic complications. Despite the preponderance of evidence from animal studies supporting this hypothesis, large scale, prospective studies using antioxidant treatments in humans have been disappointingly inconclusive, likely due to issues with dosage and bioavailability of the antioxidants and other confounding factors [84, 92].
Conclusions and perspectives
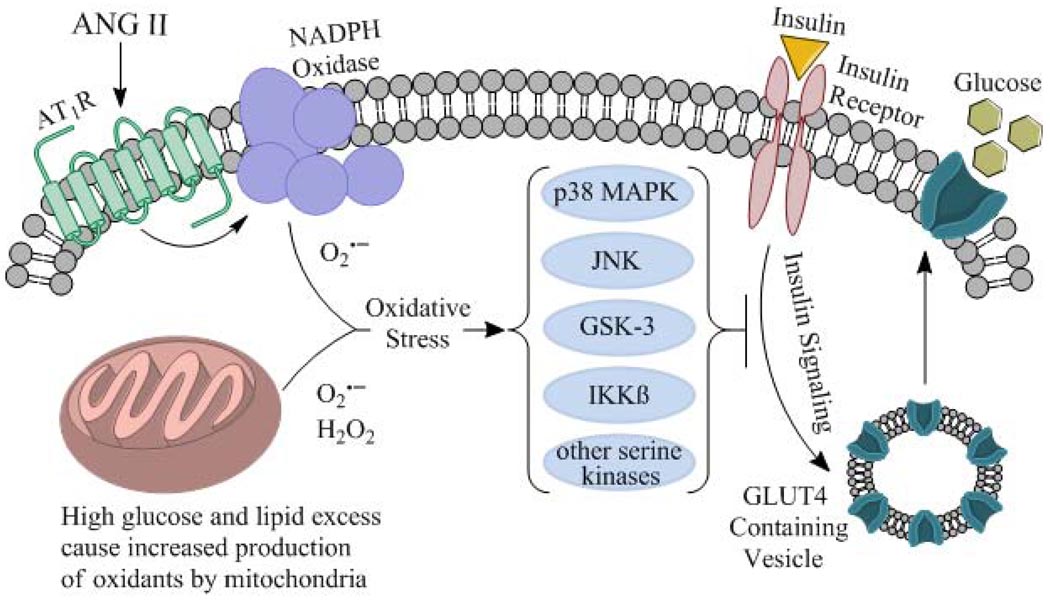
Results reviewed herein support the contribution of oxidative stress to the multifactorial etiology of insulin resistance in the whole body and specifically in the insulin-dependent glucose transport system in skeletal muscle. An overall mechanism for this oxidant-associated deterioration of insulin action in skeletal muscle is depicted in Fig. 1. In this schema, oxidant overproduction can ensue from either enhanced engagement of NADPH oxidase through an AT1R-mediated event or from excessive mitochondrial oxidant production due to energy surplus (e.g., resulting from a high fat diet). These oxidants can then, at least in part through a p38 MAPK-dependent mechanism, impair the engagement of insulin signaling factors that regulate GLUT-4 translocation, ultimately reducing the capacity for insulin-dependent glucose transport activity in the myocytes. It should be emphasized that p38 MAPK is only one of many stress-activated kinases (including JNK, GSK-3, and potentially IKKβ) involved in the development of insulin resistance in skeletal muscle due to oxidative stress (Fig. 1).
Figure 1.
Schematic representation of the overproduction of oxidants from NADPH oxidase and mitochondrial sources in mammalian skeletal muscle, with subsequent enhanced engagement of p38 MAPK and other stress-activated kinases, including JNK, GSK-3, IKKβ, and others, associated with diminished insulin-stimulated insulin signaling and reduced glucose transport activity.
Recent evidence in obesity-associated rat models of oxidative stress and insulin resistance also provides further support for the utility of interventions that reduce oxidative stress and oxidative damage for improving insulin action on whole-body and skeletal muscle glucose disposal. Antioxidant treatments in these animal models clearly mediate improvements in glucose tolerance and insulin sensitivity associated with enhancement functionality of the skeletal muscle glucose transport system. What has been more problematic has been the translation of these antioxidant intervention strategies to the effective treatment of insulin-resistant states in human subjects. An important goal of future clinical investigations should be the development and implementation of antioxidant interventions with improved oral bioavailability targeted to the mitochondria in skeletal muscle, as this appears to be a critical site of oxidant overproduction in conditions of insulin resistance.
Acknowledgments
The work of the authors cited in this article was supported in part by grants DK063967 and T32 HL07249 from the National Institutes of Health and a grant from BASF AG (Ludwigshafen, Germany).
List of abbreviations
- AGE
advanced glycation end-products
- ALA
α-lipoic acid
- ANG II
angiotensin II
- AT1R
angiotensin type 1 receptor
- ER
endoplasmic reticulum
- GSK-3β
glycogen synthase kinase-3β
- H2O2
hydrogen peroxide
- IKKβ
IκB kinase β
- IR
insulin receptor
- IRS
insulin receptor substrate
- JNK
c-jun N-terminal kinase
- p38 MAPK
p38 mitogen-activated protein kinase
- PI3-kinase
phosphatidylinositol-3-kinase
- PYR
pyridoxamine
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health: National Diabetes Statistics. [date of access: 18 August 2010]; Reference available from: http://diabetes.niddk.nih.gov/dm/pubs/statistics/#3.
- 3.Henriksen EJ. Dysregulation of glycogen synthase kinase-3 and the etiology of insulin resistance and type 2 diabetes. Curr. Diab. Rev. 2010;6:285–293. doi: 10.2174/157339910793360888. [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA, Ferrannini E, Hendler R, Felig P, Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983;32:32–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am. J. Physiol. Endocrinol. Metab. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd PR, Kahn BB. Glucose transporters and insulin action. Implications for insulin resistance and diabetes mellitus. N. Eng. J. Med. 1999;341:248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 7.Zierath JR, Krook A, Wallberg-Henriksson H. Insulin action and insulin resistance in human skeletal muscle. Diabetologia. 2000;43:821–835. doi: 10.1007/s001250051457. [DOI] [PubMed] [Google Scholar]
- 8.Henriksen EJ. Invited review: Effects of acute exercise and exercise training on insulin resistance. J. Appl. Physiol. 2002;93:788–796. doi: 10.1152/japplphysiol.01219.2001. [DOI] [PubMed] [Google Scholar]
- 9.Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl. Physiol. Nutr. Metabol. 2007;32:557–566. doi: 10.1139/H07-026. [DOI] [PubMed] [Google Scholar]
- 10.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signaling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleiman E, Carter G, Ghansah T, Patel NA, Cooper DR. Developmentally spliced PKCbetaII provides a possible link between mTORC2 and Akt kinase to regulate 3T3-L1 adipocyte insulin-stimulated glucose transport. Biochem. Biophys. Res. Comm. 2009;388:554–559. doi: 10.1016/j.bbrc.2009.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi CM, Brice Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell. Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 14.Henriksen EJ. Oxidative stress and antioxidant treatment: effects on muscle glucose transport in animal models of type 1 and type 2 diabetes. In: Packer L, Rösen P, Tritschler HJ, King GL, Azzi A, editors. Antioxidants in Diabetes Management. New York: Marcell Dekker, Inc.; 2000. pp. 303–318. [Google Scholar]
- 15.Henriksen EJ. Therapeutic effects of lipoic acid on hyperglycemia and insulin resistance. In: Cadenas E, Packer L, editors. Handbook of Antioxidants. New York: Marcell Dekker, Inc.; 2001. pp. 535–548. [Google Scholar]
- 16.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocrin. Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 17.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Henriksen EJ. Exercise training and the antioxidant alpha-lipoic acid in the treatment of insulin resistance and type 2 diabetes. Free Radic. Biol. Med. 2006;40:3–12. doi: 10.1016/j.freeradbiomed.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Paolisso G, D'Amore A, Volpe C, Balbi V, Saccomanno F, Galzerano D, Giugliano D, Varricchio M, D'Onofrio F. Evidence for a relationship between oxidative stress and insulin action in non-insulin-dependent (type II) diabetic patients. Metabolism. 1994;43:1426–1429. doi: 10.1016/0026-0495(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 20.Nourooz-Zadeh J, Rahimi A, Tajaddini-Sarmadi J, Tritschler H, Rösen P, Halliwell B, Betteridge DJ. Relationships between plasma measures of oxidative stress and metabolic control in NIDDM. Diabetologia. 1997;40:647–653. doi: 10.1007/s001250050729. [DOI] [PubMed] [Google Scholar]
- 21.Solinas G, Karin M. JNK1 and IKKβ: molecular links between obesity and metabolic dysfunction. FASEB J. 2010;24:2596–2611. doi: 10.1096/fj.09-151340. [DOI] [PubMed] [Google Scholar]
- 22.Saengsirisuwan V, Kinnick TR, Schmit MB, Henriksen EJ. Interactions of exercise training and lipoic acid on skeletal muscle glucose transport in obese Zucker rats. J. Appl. Physiol. 2001;91:145–153. doi: 10.1152/jappl.2001.91.1.145. [DOI] [PubMed] [Google Scholar]
- 23.Kaneki M, Shimizu N, Yamada D, Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid. Redox Signal. 2007;9:319–329. doi: 10.1089/ars.2006.1464. [DOI] [PubMed] [Google Scholar]
- 24.Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muellenbach EA, Diehl CJ, Teachey MK, Lindborg KA, Archuleta TL, Harrell NB, Hasselwander O, Andersen G, Somoza V, Matuschek M, Henriksen EJ. Interactions of the AGE inhibitor pyridoxamine and the antioxidant alpha-lipoic acid on insulin resistance of the obese Zucker rat. Metabolism. 2008;57:1465–1472. doi: 10.1016/j.metabol.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muellenbach EA, Diehl CJ, Teachey MK, Lindborg KA, Hasselwander O, Matuschek M, Henriksen EJ. Metabolic interactions of the AGE inhibitor pyridoxamine and the antioxidant alpha-lipoic acid following 22 weeks of treatment in obese Zucker rats. Life Sci. 2009;84:563–568. doi: 10.1016/j.lfs.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid. Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 28.Anderson EJ, Lustig ME, Boyle CE, Woodlief TL, Kane DA, Lin C-T, Price WJ, III, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietze GJ, Henriksen EJ. Angiotensin I-converting enzyme in skeletal muscle: sentinel of blood pressure control and glucose homeostasis. J. Renin-Angiotensin-Aldosterone Syst. 2008;9:75–88. doi: 10.3317/jraas.2008.011. [DOI] [PubMed] [Google Scholar]
- 30.Henriksen EJ. Improvement of insulin sensitivity by antagonism of the renin-angiotensin system. Am. J. Physiol. Regulatory Integrative Comp. Physiol. 2007;293:R974–R980. doi: 10.1152/ajpregu.00147.2007. [DOI] [PubMed] [Google Scholar]
- 31.Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am. J. Respir. Crit. Care Med. 2002;165:412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- 32.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J. Biol. Chem. 2006;281:35137–35146. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- 33.Sowers JR. Insulin resistance and hypertension. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1597–H1602. doi: 10.1152/ajpheart.00026.2004. [DOI] [PubMed] [Google Scholar]
- 34.Rudich A, Kozlovsky N, Potashnik R, Bashan N. Oxidant stress reduces insulin responsiveness in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 1997;272:E935–E940. doi: 10.1152/ajpendo.1997.272.5.E935. [DOI] [PubMed] [Google Scholar]
- 35.Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes. 1998;47:1562–1569. doi: 10.2337/diabetes.47.10.1562. [DOI] [PubMed] [Google Scholar]
- 36.Maddux BA, See W, Lawrence JC, Jr, Goldfine AL, Goldfine ID, Evans JL. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by micromolar concentrations of α-lipoic acid. Diabetes. 2001;50:404–410. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- 37.Henriksen EJ, Holloszy JO. Effect of diffusion distance on measurement of glucose transport in rat skeletal muscles in vitro. Acta Physiol. Scand. 1991;143:381–386. doi: 10.1111/j.1748-1716.1991.tb09249.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim JS, Saengsirisuwan V, Sloniger JA, Teachey MK, Henriksen EJ. Oxidant stress and skeletal muscle glucose transport: roles of insulin signaling and p38 MAPK. Free Radic. Biol. Med. 2006;41:818–824. doi: 10.1016/j.freeradbiomed.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 39.Dokken BB, Saengsirisuwan V, Kim JS, Teachey MK, Henriksen EJ. Oxidative stress-induced insulin resistance in skeletal muscle: role of glycogen synthase kinase-3. Am. J. Physiol. Endocrinol. Metab. 2008;294:E615–E621. doi: 10.1152/ajpendo.00578.2007. [DOI] [PubMed] [Google Scholar]
- 40.Archuleta TL, Lemieux AM, Saengsirisuwan V, Teachey MK, Lindborg KA, Kim JS, Henriksen EJ. Oxidant stress-induced loss of IRS-1 and IRS-2 proteins in rat skeletal muscle: role of p38 MAPK. Free Radic. Biol. Med. 2009;47:1486–1493. doi: 10.1016/j.freeradbiomed.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Potashnik R, Bloch-Damti A, Bashan N. IRS1 degradation and increased serine phosphorylation cannot predict the degree of metabolic insulin resistance induced by oxidative stress. Diabetologia. 2003;46:639–648. doi: 10.1007/s00125-003-1097-5. [DOI] [PubMed] [Google Scholar]
- 42.Blair AS, Hajduch E, Litherland GJ, Hundal HS. Regulation of glucose transport and glycogen synthesis in L6 muscle cells during oxidative stress. Evidence for cross-talk between the insulin and SAPK2/p38 mitogen-activated protein kinase signaling pathways. J. Biol. Chem. 1999;274:36293–36299. doi: 10.1074/jbc.274.51.36293. [DOI] [PubMed] [Google Scholar]
- 43.Diamond-Stanic MK, Marchionne EM, Durazo DE, Schoolmaster EJ, Teachey MK, Kim JS, Henriksen EJ. Critical role of transient activation of p38 MAPK in the etiology of skeletal muscle insulin resistance caused by low-level in vitro oxidant stress. Diabetes. 2010;59 Suppl. 1:A368. doi: 10.1016/j.bbrc.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vichaiwong K, Henriksen EJ, Toskulkao C, Prasannarong M, Bupha-Intr T, Saengsirisuwan V. Attenuation of oxidant-induced muscle insulin resistance and p38 MAPK by exercise training. Free Radic. Biol. Med. 2009;47:593–599. doi: 10.1016/j.freeradbiomed.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 45.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 46.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 47.Ogihara T, Asano T, Ando K, Chiba Y, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Katagiri K, Fukushima Y, Kikuchi M, Noguchi N, Aburatani H, Komuro I, Fujita T. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension. 2002;40:872–879. doi: 10.1161/01.hyp.0000040262.48405.a8. [DOI] [PubMed] [Google Scholar]
- 48.Blendea MC, Jacobs D, Stump CS, McFarlane SI, Ogrin C, Bahtyiar G, Stas S, Kumar P, Sha Q, Ferrario CM, Sowers JR. Abrogation of oxidative stress improves insulin sensitivity in the Ren-2 rat model of tissue angiotensin II overexpression. Am. J. Physiol. Endocrinol. Metab. 2005;288:E353–E359. doi: 10.1152/ajpendo.00402.2004. [DOI] [PubMed] [Google Scholar]
- 49.Sloniger JA, Saengsirisuwan V, Diehl CJ, Dokken BB, Lailerd N, Lemieux AM, Kim JS, Henriksen EJ. Defective insulin signaling in skeletal muscle of the hypertensive TG(mREN2)27 rat. Am. J. Physiol. Endocrinol. Metab. 2005;288:E1074–E1081. doi: 10.1152/ajpendo.00396.2004. [DOI] [PubMed] [Google Scholar]
- 50.Sloniger JA, Saengsirisuwan V, Diehl CJ, Kim JS, Henriksen EJ. Selective angiotensin II receptor antagonism enhances whole-body insulin sensitivity and muscle glucose transport in hypertensive TG(mREN2)27 rats. Metabolism. 2005;54:1659–1668. doi: 10.1016/j.metabol.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 51.Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kB activation via NADPH oxidase. Am. J. Physiol. Endocrinol. Metab. 2008;294:E345–E351. doi: 10.1152/ajpendo.00456.2007. [DOI] [PubMed] [Google Scholar]
- 52.Henriksen EJ, Jacob S. Effects of captopril on glucose transport activity in skeletal muscle of obese Zucker rats. Metabolism. 1995;44:267–272. doi: 10.1016/0026-0495(95)90276-7. [DOI] [PubMed] [Google Scholar]
- 53.Henriksen EJ, Jacob S, Kinnick TR, Teachey MK, Krekler M. Selective angiotensin II receptor antagonism reduces insulin resistance in obese Zucker rats. Hypertension. 2001;38:884–890. doi: 10.1161/hy1101.092970. [DOI] [PubMed] [Google Scholar]
- 54.Diamond-Stanic MK, Henriksen EJ. Direct inhibition by angiotensin II of insulin-dependent glucose transport activity in mammalian skeletal muscle involves a ROS-dependent mechanism. Arch. Physiol. Biochem. 2010b;116:88–95. doi: 10.3109/13813451003758703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Rad. Biol. Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 56.Packer L, Kraemer K, Rimbach G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition. 2001;17:888–895. doi: 10.1016/s0899-9007(01)00658-x. [DOI] [PubMed] [Google Scholar]
- 57.Estrada DE, Ewart HS, Tsakiridis T, Volchuk A, Ramlal T, Tritschler H, Klip A. Stimulation of glucose uptake by the natural coenzyme alpha-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes. 1996;45:1798–1804. doi: 10.2337/diab.45.12.1798. [DOI] [PubMed] [Google Scholar]
- 58.Rudich A, Tirosh A, Potashnik R, Khamaisi M, Bashan N. Lipoic acid protects against oxidative stress induced impairment in insulin stimulation of protein kinase B and glucose transport in 3T3-L1 adipocytes. Diabetologia. 1999;42:949–957. doi: 10.1007/s001250051253. [DOI] [PubMed] [Google Scholar]
- 59.Yaworsky K, Somwar R, Ramlal T, Tritschler HJ, Klip A. Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by alpha-lipoic acid in 3T3-L1 adipocytes. Diabetologia. 2000;43:294–303. doi: 10.1007/s001250050047. [DOI] [PubMed] [Google Scholar]
- 60.Konrad D, Somwar R, Sweeney G, Yaworsky K, Hayashi M, Ramlal T, Klip A. The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes. 2001;50:1464–1471. doi: 10.2337/diabetes.50.6.1464. [DOI] [PubMed] [Google Scholar]
- 61.Henriksen EJ, Jacob S, Streeper RS, Fogt DL, Hokama JY, Tritschler HJ. Stimulation by α-lipoic acid of glucose transport activity in skeletal muscle of lean and obese Zucker rats. Life Sci. 1997;61:805–812. doi: 10.1016/s0024-3205(97)00562-6. [DOI] [PubMed] [Google Scholar]
- 62.Jacob S, Streeper RS, Fogt DL, Hokama JY, Tritschler HJ, Dietze GJ, Henriksen EJ. The antioxidant alpha-lipoic acid enhances insulin-stimulated glucose metabolism in insulin-resistant skeletal muscle. Diabetes. 1996;45:1024–1029. doi: 10.2337/diab.45.8.1024. [DOI] [PubMed] [Google Scholar]
- 63.Khamaisi M, Potashnik R, Tirosh A, Demshchak E, Rudich A, Tritschler H, Wessel K, Bashan N. Lipoic acid reduces glycemia and increases muscle GLUT4 content in streptozotocin-diabetic rats. Metabolism. 1997;46:763–768. doi: 10.1016/s0026-0495(97)90120-7. [DOI] [PubMed] [Google Scholar]
- 64.Streeper RS, Henriksen EJ, Jacob S, Hokama JY, Fogt DL, Tritschler HJ, Dietze GJ. Differential effects of stereoisomers of alpha-lipoic acid on glucose metabolism in insulin-resistant rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 1997;273:E185–E191. doi: 10.1152/ajpendo.1997.273.1.E185. [DOI] [PubMed] [Google Scholar]
- 65.El Midaoui A, de Champlain J. Prevention of hypertension, insulin resistance, and oxidative stress by alpha-lipoic acid. Hypertens. 2002;39:303–307. doi: 10.1161/hy0202.104345. [DOI] [PubMed] [Google Scholar]
- 66.Saengsirisuwan V, Perez FR, Sloniger JA, Maier T, Henriksen EJ. Interactions of exercise training and R-(+)-alpha-lipoic acid on insulin signaling in skeletal muscle of obese Zucker rats. Am. J. Physiol. Endocrinol. Metab. 2004;287:E529–E536. doi: 10.1152/ajpendo.00013.2004. [DOI] [PubMed] [Google Scholar]
- 67.Song K-H, Lee WJ, Koh J-M, Kim HS, Youn J-Y, Park H-S, Koh EH, Kim M-S, Youn JH, Lee K-U, Park J-Y. α-Lipoic acid prevents diabetes mellitus in diabetes-prone obese rats. Biochem. Biophys. Res. Commun. 2004;326:197–202. doi: 10.1016/j.bbrc.2004.10.213. [DOI] [PubMed] [Google Scholar]
- 68.Gupte AA, Bomhoff GL, Morris JK, Gorres BK, Geiger PC. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J. Appl. Physiol. 2009;106:1425–1434. doi: 10.1152/japplphysiol.91210.2008. [DOI] [PubMed] [Google Scholar]
- 69.Jacob S, Henriksen EJ, Schiemann AL, Simon I, Clancy DE, Tritschler HJ, Jung WI, Augustin HJ, Dietze GJ. Enhancement of glucose disposal in patients with Type 2 diabetes by alpha-lipoic acid. Drug Res. 1995;45:872–874. [PubMed] [Google Scholar]
- 70.Jacob S, Henriksen EJ, Tritschler HJ, Augustin HJ, Dietze GJ. Improvement of insulin-stimulated glucose disposal in type 2 diabetes after repeated parenteral administration of thioctic acid. Expt. Clin. Endocrinol. Diab. 1996;104:284–288. doi: 10.1055/s-0029-1211455. [DOI] [PubMed] [Google Scholar]
- 71.Jacob S, Ruus P, Hermann R, Tritschler HJ, Maerker E, Renn W, Augustin HJ, Dietze GJ, Rett K. Oral administration of rac-α-lipoic acid modulates insulin sensitivity in patients with type 2 diabetes mellitus - a placebo controlled pilot trial. Free Rad. Biol. Med. 1999;27:309–314. doi: 10.1016/s0891-5849(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 72.Cassese A, Esposito I, Fiory F, Barbagallo AP, Paturzo F, Mirra P, Ulianich L, Giacco F, Iadicicco C, Lombardi A, Oriente F, Van Obberghen E, Beguinot F, Formisano P, Miele C. In skeletal muscle advanced glycation end products (AGEs) inhibit insulin action and induce the formation of multimolecular complexes including the receptor for AGEs. J. Biol. Chem. 2008;283:36088–36099. doi: 10.1074/jbc.M801698200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thorpe SR, Baynes JW. Role of the Maillard reaction in diabetes mellitus and diseases of aging. Durgs Ageing. 1996;9:69–77. doi: 10.2165/00002512-199609020-00001. [DOI] [PubMed] [Google Scholar]
- 74.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog. Horm. Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 75.Metz TO, Alderson NL, Chachich ME, Thorpe SR, Baynes JW. Pyridoxamine traps intermediates in lipid peroxidation reactions in vivo. Evidence on the role of lipids in chemical modification of proteins and development of diabetic complications. J. Biol. Chem. 2003;278:42012–42019. doi: 10.1074/jbc.M304292200. [DOI] [PubMed] [Google Scholar]
- 76.Metz TO, Alderson NL, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation and lipoxidation reactions: a novel therapy for treatment of diabetic complications. Arch. Biochem. Biophys. 2003;419:41–49. doi: 10.1016/j.abb.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 77.Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, Thorpe SR, Baynes JW. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002;61:939–950. doi: 10.1046/j.1523-1755.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- 78.Stitt A, Gardiner TA, Alderson NL, Canning P, Frizzell N, Duffy N, Boyle C, Januszewski AS, Chachich. M, Baynes JW, Thorpe SR. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51:2826–2832. doi: 10.2337/diabetes.51.9.2826. [DOI] [PubMed] [Google Scholar]
- 79.Alderson NL, Chachich ME, Youssef NN, Beattie RJ, Nachtigal M, Thorpe SR, Baynes JW. The AGE inhibitor pyridoxamine inhibits lipemia and development of renal and vascular disease in Zucker obese rats. Kidney Int. 2003;63:2123–2133. doi: 10.1046/j.1523-1755.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 80.Orasanu G, Plutzky J. The continuum of diabetic vascular disease: From macro- to micro- J. Am. Coll. Cardiol. 2009;53:S35–S42. doi: 10.1016/j.jacc.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 82.Steckelings UM, Rompe F, Kaschina E, Unger T. The evolving story of the RAAS in hypertension, diabetes and CV disease: moving from macrovascular to microvascular targets. Fundam. Clin. Pharmacol. 2009;23:693–703. doi: 10.1111/j.1472-8206.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 83.Maritim AC, Sanders RA, Watkins JB., III Diabtes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 84.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc. Diabetol. 2005;4:1–11. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boudina S, Able ED. Diabetic cardiomyopathy, causes and effect. Rev. Endocr. Metab. Disord. 2010;11:31–39. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fatehi-Hassanabad Z, Chan CB, Furman BL. Reactive oxygen species and endothelial function in diabetes. Eur. J. Pharmacol. 2010;636:8–17. doi: 10.1016/j.ejphar.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 87.Mellor KM, Ritchie RH, Delbridge L. Reactive oxygen species and insulin-resistant cardiomyopathy. Clin. Exp. Pharmacol. Physiol. 2010;37:222–228. doi: 10.1111/j.1440-1681.2009.05274.x. [DOI] [PubMed] [Google Scholar]
- 88.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 89.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: Mechanisms of renal disease progression. Exp. Bio. Med. 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 90.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Rev. Endocr. Metab. Disord. 2008;9:315–327. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- 91.Figueroa-Romero C, Sadid M, Feldman EL. Mechanisms of disease: The oxidative stress theory of diabetic neuropathy. Rev. Endocr. Metab. Disord. 2008;9:301–314. doi: 10.1007/s11154-008-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pazdro R, Burgess JR. The role of vitamin E and oxidative stress in diabetes complications. Mech. Ageing Dev. 2010;131:276–286. doi: 10.1016/j.mad.2010.03.005. [DOI] [PubMed] [Google Scholar]