Abstract
Background/Objectives
Live attenuated influenza vaccine (LAIV) protects against influenza by mucosal activation of the immune system. Studies in animals and adults have demonstrated that probiotics improve the immune response to mucosally delivered vaccines. We hypothesized that Lactobacillus GG (LGG) would act as an immune adjuvant to increase rates of seroconversion after LAIV administration.
Subjects/Methods
We conducted a randomized double-blind placebo-controlled pilot study to determine if LGG improved rates of seroconversion after administration of LAIV. We studied 42 healthy adults during the 2007–8 influenza season. All subjects received LAIV and then were randomized to LGG or placebo twice daily for 28 days. HAI titers were assessed at baseline, day 28, and day 56 to determine rates of seroconversion. Subjects were assessed for adverse events throughout the study period.
Results
39 subjects completed the per protocol analysis. Both LGG and LAIV were well tolerated. Protection rates against the vaccine H1N1 and B strains was similar suboptimal in subjects receiving LGG and placebo. For the H3N2 strain, 84% receiving LGG vs. 55% receiving placebo had a protective titer 28 days after vaccination (odds of having a protective titer was 1.84 95% CI 1.04–3.22, P=0.048).
Conclusion
Lactobacillus GG is potential as an important adjuvant to improve influenza vaccine immunogenicity. Future studies of probiotics as immune adjuvants may need to consider specifically examining vaccine naïve or seronegative subjects, target mucosal immune responses, or focus on groups known to have poor response to influenza vaccines.
Keywords: Live attenuated Influenza vaccine, antibody responses, Probiotics, Lactobacillus GG
INTRODUCTION
The trivalent live attenuated influenza vaccine (LAIV) was licensed in June 2003 as an alternative to inactivated, trivalent influenza vaccine (TIV) for healthy individuals aged between 2 and 49 years. The effectiveness of LAIV in preventing influenza illness varies by age, prior influenza immunization and match between circulating influenza virus and vaccine strains (Nichol et al., 2006). While studies in children have demonstrated superior or equivalent efficacy between LAIV and TIV in children, recent studies have not consistently demonstrated the same robust results in healthy adults. Monto et al. reported sub-optimal protection against laboratory confirmed symptomatic influenza from LAIV compared with TIV for the 2007–2008 influenza season (Monto et al., 2009). In a study of military personnel aged 17–49 years over three influenza seasons, receipt of TIV was associated with a significantly lower rate of healthcare visits for pneumonia and influenza compared with those who received LAIV (Wang et al., 2009). However, among new recruits being vaccinated for the first time, the incidence of health care visits was similar. Systemic immune responses to LAIV previously have been found to be lower than TIV (Beyer et al., 2002) and mucosal immunologic correlates of protection after receipt of LAIV may be more informative but are difficult to measure (Treanor et al., 2003).
The recent outbreak of pandemic H1N1 influenza has heightened the need to improve vaccine responses. Because of its convenient administration and stimulation of mucosal immunity, LAIV remains an important part of the armamentarium to prevent influenza infection. This has led to increasing interest in adjuvants to improve LAIV immunogenicity. Probiotics are defined as live microorganisms that confer a health benefit when administered in adequate amounts. Probiotics may enhance innate and adaptive immunity and have previously been demonstrated to augment the immune response to mucosally delivered vaccines (Fang et al., 2000). Mice fed Lactobacillus prior to an influenza virus challenge had higher levels of influenza specific IgG and greater protection against illness (Yasui et al., 2004). Intranasal administration of LGG decrease morbidity and mortality in mice infection with H1N1 (Harata et al., 2010). Probiotic Lactobacillus fermentum (CECT5716) (Olivares et al., 2007) and a fermented dairy drink containing Lactobacillus caseii DN-114 (CNCMI-1518) Streptococcus thermophilus and Lactobacillus bulgaricus (Boge et al., 2009) improved the immunogenicity of TIV in two human trials. However, there are no human studies of probiotics as an immune adjuvant for mucosally delivered LAIV. We conducted a proof of concept study to evaluate whether the probiotic Lactobacillus GG had any immune-adjuvant effect on serum influenza antibody titers and increased rates of seroconversion after administration of LAIV to healthy adults during a single influenza season.
MATERIAL AND METHODS
Study design
In the 2007–2008 influenza season, we conducted a double-blind randomized placebo controlled clinical trial to assess the safety and immunogenicity to LAIV in healthy subjects 18–49 years of age while also receiving an oral probiotic - Lactobacillus LGG (ATCC 53101, Culturelle™) or matching placebo. The study was approved by the Tufts Medical Center Institutional Review Board, the Tufts Clinical Research Center and registered on Clinical Trials.gov (NCT00620412). The study was supported in part by NIH grant M01RR00054 and Amerifit Brands Inc. Amerifit Brands Inc. had no role the design, conduct, analysis or interpretation of the results.
Subjects for whom LAIV was contraindicated, who had received the 2007–2008 influenza vaccine or who had used any probiotic in 4 weeks before enrollment were ineligible to participate. Receipt of influenza vaccine in prior influenza seasons and yogurt consumption were not exclusion criteria, but subjects were asked to avoid consumption of any yogurt or probiotic during the first 4 weeks of the study. Subjects were recruited from the local community using IRB approved advertisements. Written informed consent was obtained from all participants before they were screened for eligibility criteria. Screening included a complete medical history, physical examination and routine laboratory tests (including HIV, Hepatitis B and Hepatitis C testing). All subject study visits occurred at the Tufts Medical Center Clinical Research Center. Subjects were recruited until the end of the influenza season (April 1, 2008).
After meeting eligibility criteria, all participants received nasally administered LAIV according to the manufacturer’s recommendations (FluMist™, Medimmune Vaccines, Inc.) at the baseline study visit. Approximately 0.1 mL (i.e., half of the total sprayer contents) was sprayed into each nostril while the recipient was in the upright position. The 2007–2008 influenza season vaccine contained A/Solomon Islands/3/2006 (H1N1) like (new for the 2007–2008 season), A/Wisconsin/67/2005 (H3N2) like, and B/Malaysia/2506/2004 like antigens.
Randomization
The randomization scheme was generated by the study statistician (Ms Fiorino) using the web randomization site www.randomization.com (GE Dallal). Ms Fiorino had no contact with the study subjects. Randomization assignments (1:1 LGG: placebo) were made in permuted blocks of 2 and 4. Block sizes were also randomly assigned. Once eligibility criteria were met, study participants were randomly assigned to receive capsules of either Lactobacillus GG or matching, identically appearing placebo. Participants were enrolled by the study investigators (PH and LD).
Intervention
The study participants received either Lactobacillus GG (gelatin capsule containing 1×1010 LGG organisms and 295 mg Inulin) or matching, identically appearing placebo (gelatin capsule containing 355 mg Inulin). Capsules were administered twice daily by mouth for 28 days. The first study capsule was administered under observation, immediately after LAIV administration. Subjects received a 28-day supply of study capsules and were instructed to bring their remaining capsules to all study visits.
Blinding
All study participants, physicians, nurses, and clinical staff were blinded to study assignments. By using the randomization scheme detailed above, it was impossible for research personal to adjust randomization or determine what groups participants were assigned. Because the appearance and inactive substances in the placebo and study treatment were identical, neither the subjects nor researchers knew the identity of the subject’s study assignment. All of the above measures let to successful allocation concealment and blinding.
Safety
Subjects were given diary cards and asked to record any symptoms that occurred during the 28 days when they took study capsules. Patients were assessed for adverse events at day 14, day 38 and day 56 study visits. At each study visit, subjects were questioned about specific side effects possibly related to receiving LAIV or probiotics and symptoms consistent with influenza like illnesses. All adverse events elicited during study visits or recorded on the diary cards were graded as mild, moderate or serious. An independent Data Safety Monitoring Board was appointed to monitor this study. The DSMB was charged with monitoring of subject safety and overall conduct and quality of study data and did not monitor vaccine efficacy because antibody titers were batched and run at the end of the study. The DSMB met prior to the beginning of subject recruitment and then yearly until the study was completed. In addition, the DSMB reviewed all serious adverse events with in 72 hours of being reported.
Outcome measures
Serum samples were obtained prior to administration of LAIV at the baseline visit, at day 14, day 28 and day 56 visits. Sera were stored at −80°C and sent on dry ice to the Laboratory for Specialized Clinical Studies at Cincinnati Children’s Hospital Medical center (Cincinnati, Ohio). The laboratory was blinded to subject identifiers or treatment group. Antibody titers to each of the vaccine components was measured by the serum hemagglutinin inhibition (HAI) assay using standard methods (Lu et al., 2009). The primary outcome was a protective HAI titer ≥40 at day 28. Secondary outcomes were geometric mean titers at day 28 and 56, HAI titers after 56 days and seroconversion at day 56. Titers equal to or greater than 1:40 were considered protective. Seroconversion was defined as an increase from <1:40 to ≥1:40 or at least a 4-fold rise in HAI antibody titers at any time after vaccination.
Statistical analysis
Since our study is the first to evaluate LGG as an immune-adjuvant to LAIV, our sample size of 52 subjects (26 per group) had 80% power to detect a large effect size of 0.8 or greater, using a 2-group t-test with a 0.05 two-sided significance level (nQuery Advisor 5.0, Statistical Solutions, Boston, MA). We also based our sample size estimates on data and sample sizes used in other studies of probiotics administered as immune adjuvants (Fang et al., 2000), similar studies evaluating the immunogenicity adjuvants for LAIV and other immune adjuvants including MF59 and CPG 7909 (Frey et al., 2003; Muszkat et al., 2003; Cooper et al., 2004). No adjustment was made for multiple comparisons of response to the three vaccine strains. Analyses were completed on a per protocol basis for this proof of concept study. The proportion of patients with protective antibody response and who seroconverted for each vaccine strain was compared using a Fisher’s exact test. Serum geometric mean antibody titers pre-vaccination and post-vaccination at days 28 and 56 of each vaccine strain were compared using a 2 group t-test. The proportion of subjects who reported adverse events was also evaluated. All statistical analyses were done using SPSS (Version 17.0, Chicago, IL).
RESULTS
Recruitment and enrollment
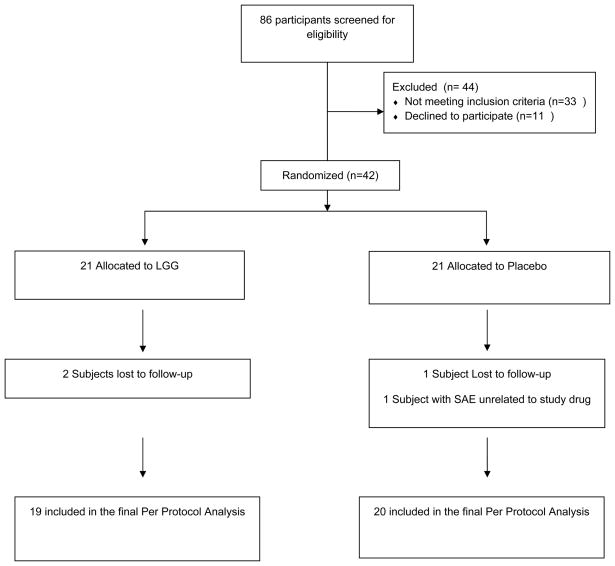
Eighty six subjects were screened between September 26, 2007 and March 28, 2008 (see Figure 1). Forty-four subjects were excluded: 11 declined to participate and 33 did not meet the inclusion criteria. Of the 42 subjects eligible, 42 attended the baseline visit, received LAIV and were randomized to LGG or placebo. We terminated enrollment after 42 subjects because we reached the end of the influenza season and there was no benefit to continued administration of LAIV. Only 3 of the 42 (7%) were lost to follow up, all immediately after the baseline visit. Each of these subjects received 3 phone calls and written notification, but did not respond to repeated attempts to determine why they dropped out from the study.
Figure 1.
Participant Flow Diagram
Baseline demographics and adverse events
Age, gender, and race were similar between groups (Table 1). Approximately 52% of the LGG group and 38% of the placebo group had previously received TIV (P=0.54). No subject had ever received the LAIV vaccine before. Seventeen subjects in the placebo group (85%) and 14 subjects in the LGG group (74%) reported at least one adverse event at any time during the study (Table 2). All were rated as mild. During the course of the study, more subjects in the placebo than LGG group reported muscle aches (P=0.02) and decreased appetite (P=0.047). One placebo recipient reported a serious adverse event (hospitalization for a sinus infection) occurred on day 58. This event was reviewed by the study investigators and the DSMB and considered unrelated to administration of study drug (determination was made prior to knowing that the study drug was placebo) or receipt of vaccine.
Table 1.
Demographic Characteristics of Study Subjects
| LGG (N=21) | PLACEBO (N=21) | P values | |
|---|---|---|---|
| Age ( mean years, range) | 33.5 (18–44) | 33.1 (19–48) | 0.90 |
| Gender: - female (%) | 12 (57%) | 14 (67%) | 0.53 |
| Race: | |||
| - White (%) | 8 (38%) | 10 (48%) | 0.76 |
| - Black or African American (%) | 7 (33%) | 5 (24%) | |
| - Other (%) | 6 (29%) | 6 (29%) | |
| Previously received the influenza vaccine (%) | 11(52%) | 8 (38%) | 0.35 |
Table 2.
Characteristics of Adverse events in the LGG and Placebo Groups and Immune Response to Immunization with Live Attenuated Influenza Vaccine
| ADVERSE EVENTS | LGG (n=19) | PLACEBO (n=20) | P value |
|---|---|---|---|
| Serious Adverse Events | 0 (0%) | 1 (5%) * | 1.00 |
| Any Adverse Event % | 14 (74%) | 17 (85%) | 0.45 |
| - Any Adverse Event by Day 14 | 14 (74%) | 17 (85%) | 0.45 |
| - Any Adverse Event between Day 14 and 28 | 8 (42%) | 11 (55%) | 0.53 |
| - Any Adverse Event between Day 28 and 56 | 7 (37%) | 5 (25%) | 0.50 |
| Non-gastrointestinal Adverse Events | |||
| - Rhinorrhea | 9 (47%) | 9 (45%) | 1.00 |
| - Headache | 6 (32%) | 6 (30%) | 1.00 |
| - Cough | 2 (11%) | 6 (30%) | 0.24 |
| - Muscle Aches | 0 (0%) | 6 (30%) | 0.02 |
| - Sore Throat | 1 (5%) | 4 (20%) | 0.34 |
| - Weakness | 0 (0%) | 3 (15%) | 0.23 |
| - Chills | 0 (0%) | 2 (10%) | 0.49 |
| - Fever | 0 (0%) | 0 (0%) | 1.00 |
| Gastrointestinal Adverse Events | |||
| - Nausea | 6 (32%) | 2 (10%) | 0.13 |
| - Gas | 4 (21%) | 4 (20%) | 1.00 |
| - Rumbling | 2 (11) | 3 (15% ) | 1.00 |
| - Decreased appetite | 0% (0%) | 5 (25%) | 0.047 |
| - Diarrhea | 1 (5%) | 3 (15%) | 0.61 |
| - Bloating | 1 (5%) | 2 (10%) | 1.00 |
| - Abdominal pain | 1 (5%) | 1 (5%) | 1.00 |
| - Constipation | 1 (5%) | 1 (5%) | 1.00 |
| Other Adverse Events Volunteered by Subjects | 1 (5%) ** | 4 (20%) ** | 1.00 |
One study subject in the placebo group had a serious adverse event (hospitalization for a sinus infection on study day 58), considered unrelated to the study protocol.
The other adverse event volunteered by subject in the LGG group were symptoms consistent with conjunctivitis, the other 4 events volunteered by subjects in the placebo group were symptoms consistent with conjunctivitis (1) transient rash (1), ear wax build up (1) and pain after being hit by a car door (1). All these events were rated mild and were considered unrelated to the study protocol.
Outcome
Antibody titers are presented for the 39 subjects who completed the study (per protocol analysis) (see Table 3). Overall, 15% seroconverted for the H1N1 strain, 45% for the H3N2 strain and 41% for the B strain. There was no difference in seroconversion rates between treatment and placebo groups from baseline for the H1N1 and B strains. There was a significant increase in seroprotection in the LGG group vs. placebo for the A/Wisconsin/67/2005 (H3N2) vaccine strain on day 28 (84% vs. 55%, p=0.048). However, at day 56 the rates of seroconversion were not statistically significant (63% in the LGG vs. 33% in the placebo groups, p=0.36).
Table 3.
Immune Response to Immunization with Live Attenuated Influenza Vaccine in the LGG vs. Placebo Groups
| Immunogenicity End Points | LGG N=19 | PLACEBO N=20 | P value |
|---|---|---|---|
| Response to A/Solomon Islands/3/2006 (H1N1)- like | |||
| Baseline | |||
| Subjects with HAI titer ≥40 - % (95% CI) | 37% (16–62%) | 45% (23–68%) | 0.61 |
| Geometric mean titer (95% CI) | 22 (11–44) | 22 (13–39) | 0.94 |
| Day 28 After vaccination | |||
| Subjects with HAI titer ≥40 - % (95% CI) | 42% (20–67%) | 50% (27–73%) | 0.62 |
| Geometric mean titer (95% CI) | 26 (13–50) | 25 (14–48) | 0.98 |
| Seroconversion | |||
| Subjects with seroconversion*- % (95% CI) | 8% (0–38%) | 27% (6–61%) | 0.32 |
| Response to A/Wisconsin/67/2005 (H3N2)- like | |||
| Baseline | |||
| Subjects with HAI titer ≥40 - % (95% CI) | 58% (34–80%) | 40% (19–64%) | 0.26 |
| Geometric mean titer (95% CI) | 45 (23–88) | 28 (14–59) | 0.35 |
| Day 28 After vaccination | |||
| Subjects with HAI titer ≥40 - % (95% CI) | 84% (60–97%) | 55% (32–77%) | 0.048 |
| Geometric mean titer (95% CI) | 72 (39–131) | 51 (26–100) | 0.43 |
| Seroconversion | |||
| Subjects with seroconversion*- % (95% CI) | 63% (24–91%) | 33% (10–65%) | 0.36 |
| Response to B/Malaysia/2506/2004 – like | |||
| Baseline | |||
| Subjects with HAI titer ≥40 - % (95% CI) | 37% (16–61%) | 25% (6–44%) | 0.42 |
| Geometric mean titer (95% CI) | 25 (16–39) | 19 (14–28) | 0.36 |
| Day 28 After vaccination | |||
| Subjects with HAI titer ≥40- % (95% CI) | 53% (29–76%) | 45% (23–68%) | 0.63 |
| Geometric mean titer (95% CI) | 31 (24–41) | 25 (17–36) | 0.31 |
| Seroconversion | |||
| Subjects with seroconversion *- % (95% CI) | 42% (15–72%) | 40% (16–68%) | 1.00 |
Proportion of subjects with baseline titers < 1:40 who had follow-up titers of ≥ 1:40 or a fourfold increase from baseline at 14, 28 or 56 days after vaccination.
DISCUSSION
Influenza remains a major cause of morbidity and mortality in the United States, resulting in approximately 19–36,000 deaths a year, and 200,000 excess hospitalizations (Fiore et al., 2010). Influenza vaccination is the primary means of preventing influenza infection. Two types of vaccine are available - inactivated trivalent influenza vaccine (TIV) and live attenuated influenza vaccine (LAIV). The Advisory Committee on Immunization Practices currently recommends annual universal vaccination of all children and adults greater than age 6 months (Fiore et al., 2010). Although young children and the elderly suffer the greatest morbidity and mortality, influenza vaccination in healthy adults reduces both direct medical costs, such as physician visits and antibiotics uses, and indirect costs such as work absenteeism (Nichol et al., 1999; Wilde et al., 1999; Fiore et al., 2010). The efficacy of influenza vaccine varies from year to year depending on the match between vaccine subtypes and circulating viral strains, patient’s age, and pre-existing immunity. In years in with a good match between vaccine and circulating virus, the efficacy of vaccine in healthy adults ranges between 80–90% for TIV (Wilde et al., 1999; Bridges et al., 2000; Jefferson et al., 2007). Studies of LAIV in healthy adults have demonstrated a wide range of variability, with rates of clinical efficacy ranging between 40–80% (Nichol et al., 1999; Ohmit et al., 2006; Monto et al., 2009). A number of alternatives to improve the efficacy of both LAIV and TIV have been explored in recent years including increasing to the dose of antibody in the vaccine (Centers for Disease Control and Prevention, 2010), using alternative routes of administration such as intradermal injection (Holland et al., 2008) and using immune adjuvants. Currently there is no FDA approved immune adjuvant for the influenza vaccine in the Untied States.
A number of studies in animals and humans have demonstrated the potential of probiotics to act as immune adjuvants. Lactobacillus species and other probiotics stimulate both the humoral and innate immune systems (MacDonald et al., 2010). Healthy volunteers who received LGG prior to oral Salmonella typhi vaccine developed higher IgA antibodies to the vaccine than those who received placebo (Link-Amster et al., 1994; Fang et al., 2000). Infants receiving LGG in conjunction with live oral rotavirus vaccine had higher rates of IgA seroconversion and higher numbers of rotavirus specific IgM secreting cells than infants receiving placebo (Isolauri et al., 1995). Similarly, administration of LGG 1 week prior to an oral polio booster was associated with an increased poliovirus neutralizing antibody titers and poliovirus-specific IgA and IgG (De Vrese et al., 2005). A recently published trial in newborns demonstrated that supplementation with a probiotic formula containing Bifidobacterium longus and Lactobacillus rhamnosus LPR was associated with a trend towards higher hepatitis B titers in infants immunized at birth (Soh et al., 2010).
Studies in humans and animal support the role of Lactobacillus in the prevention of influenza infection. In a mouse model of influenza infection with an H1N1 strain, 3 days of intranasal exposure to LGG was significantly associated with a lower frequency of accumulated symptoms and a higher survival rate than control mice (Harata et al., 2010). Using the same mouse model of influenza infection, oral administration of LGG or Lactobacillus TMC0356 for 19 days was associated with lower clinical symptom scores and pulmonary virus titers as compared to control mice (Kawase et al., 2010). In studies of children in day care centers, administration of LGG was associated with a decrease in upper respiratory infections and length of infections (Hatakka et al., 2001; Hojsak et al., 2010). Probiotics have been investigated as immune adjuvants for TIV. In a placebo controlled trial using a supplement containing Lactobacillus paracasei, elderly patients receiving the influenza and pneumococcal vaccines had an increase in the innate immune response and a decreased number of infections when compared with placebo (Bunout et al., 2004). In another randomized placebo controlled trial, elderly patients who received a yogurt drink containing Lactobacillus casei had higher influenza-specific antibody titers increased after vaccination (Boge et al., 2009). In a trial of healthy adults, Lactobacillus fermentum (CECT5716) improved influenza vaccine immunogenicity (Olivares et al., 2007). Taken together these studies highlight the interaction of Lactobacillus and the respiratory mucosal immune system and the potential of LGG as an immune adjuvant with mucosally administered LAIV
Our study is the first randomized placebo controlled study to examine the effect of LGG on immune response to LAIV in normal healthy adults. The strengths of our study include the 93% completion rate, use of standardized endpoints and completion during a single influenza season. Although subjects receiving probiotics did not achieve greater seroprotection after administration of LAIV for the H1N1 and B strains, there was a significant improvement in those subjects receiving LGG for the H3N2 strain. The seroconversion achieved for any of the vaccine strains using LAIV was suboptimal and was particularly low (17%) for the H1N1 component that was new for the 2007–2008 season. Our suboptimal seroconversion rates are similar to those of DeVilliers et al, who published the results of a large Phase III study of LAIV in the elderly (DeVilliers et al., 2009). Overall seroconversion for all subjects receiving LAIV was 33.9% for the H3N2 strains and seroconversion rates and efficacy against infection with B strains were also low.
The study drug and LAIV were well tolerated in our study subjects. Although there were significantly more myalgias and decreased appetite in the placebo group, adverse events were similar to those observed in other LAIV studies (Ohmit et al., 2009; DeVilliers et al., 2009). One serious adverse event occurred in one placebo recipient who was hospitalized for a sinus infection on study day 58. Prior to this visit, the subject had not reported any sinus or upper respiratory symptoms.
Our study had several limitations. The sample size was small for this proof of concept study and we had insufficient power to detect small and moderate effects on vaccine responsiveness. Subjects previously vaccinated with TIV may have lower antibody responses to subsequent LAIV (Sasaki et al., 2008) and this may have impacted the immune responses in the 49% of our subjects who had previously received TIV. We used traditional hemagglutinin inhibition assay to evaluate antibody titers. Several studies of pandemic H1N1 influenza in 2009 suggested that use of microneutralization assays or virus-free ELISA methods may be more accurate in measuring antibody responses (Clark et al., 2009; Alvarez et al., 2010).
Several important questions remain for future studies. LAIV is administered intra-nasally and it may be more relevant to measure the local mucosal response to evaluate the role of immune adjuvants targeting the mucosal surface. It may also be necessary to administer probiotics prior to LAIV rather than concurrently (Boge et al., 2009). In addition, a more profound immune adjuvant effect may be demonstrated in groups which traditionally have a poor response to the influenza vaccine such as the elderly. The data generated from this study will be of critical importance to power future studies investigating the use of probiotics as immune adjuvants for mucosal vaccines.
Supplementary Material
Acknowledgments
We gratefully acknowledge Irina Andreyeva, Leah Morey, Katie Jors, and Dr Ambarish Athavale for their assistance in patient recruitment and study visits.
Financial Support: NIH grant M01RR00054; Amerifit Brands Inc, Cromwell, CT
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Please note that the Laboratory for Specialized Clinical Studies located at the Cincinnati Children’s Hospital Medical Center was contracted to run the HAI assays on the sera. The lab was blinded to the treatment and vaccine given to study subjects. This lab does contract work with a number of sponsors, but has no conflicts of interest and received no financial gain due to the results of this study.
Reference List
- Alvarez MM, Lopez-Pacheco F, guilar-Yanez JM, Portillo-Lara R, Mendoza-Ochoa GI, Garcia-Echauri S, et al. Specific recognition of influenza A/H1N1/2009 antibodies in human serum: a simple virus-free ELISA method. PLoS One. 2010;5:e10176. doi: 10.1371/journal.pone.0010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer WE, Palache AM, de Jong JC, Osterhaus AD. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002;20:1340–1353. doi: 10.1016/s0264-410x(01)00471-6. [DOI] [PubMed] [Google Scholar]
- Boge T, Remigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R, van der WS. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009;27:5677–5684. doi: 10.1016/j.vaccine.2009.06.094. [DOI] [PubMed] [Google Scholar]
- Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA. 2000;284:1655–1663. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- Bunout D, Barrera G, Hirsch S, Gattas V, de la Maza MP, Haschke F, et al. Effects of a nutritional supplement on the immune response and cytokine production in free-living Chilean elderly. JPEN J Parenter Enteral Nutr. 2004;28:348–354. doi: 10.1177/0148607104028005348. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Licensure of a high-dose inactivated influenza vaccine for persons aged >or=65 years (Fluzone High-Dose) and guidance for use -United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485–486. [PubMed] [Google Scholar]
- Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- Cooper CL, Davis HL, Morris ML, Efler SM, Krieg AM, Li Y, et al. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine. 2004;22:3136–3143. doi: 10.1016/j.vaccine.2004.01.058. [DOI] [PubMed] [Google Scholar]
- De Villiers PJ, Steele AD, Hiemstra LA, Rappaport R, Dunning AJ, Gruber WC, et al. Efficacy and safety of a live attenuated influenza vaccine in adults 60 years of age and older 1. Vaccine. 2009;28:228–234. doi: 10.1016/j.vaccine.2009.09.092. [DOI] [PubMed] [Google Scholar]
- De Vrese M, Rautenberg P, Laue C, Koopmans M, Herremans T, Schrezenmeir J. Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur J Nutr. 2005;44:406–413. doi: 10.1007/s00394-004-0541-8. [DOI] [PubMed] [Google Scholar]
- Fang H, Elina T, Heikki A, Seppo S. Modulation of humoral immune response through probiotic intake. FEMS Immunol Med Microbiol. 2000;29:47–52. doi: 10.1111/j.1574-695X.2000.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- Frey S, Poland G, Percell S, Podda A. Comparison of the safety, tolerability, and immunogenicity of a MF59-adjuvanted influenza vaccine and a non-adjuvanted influenza vaccine in non-elderly adults. Vaccine. 2003;21:4234–4237. doi: 10.1016/s0264-410x(03)00456-0. [DOI] [PubMed] [Google Scholar]
- Harata G, He F, Hiruta N, Kawase M, Kubota A, Hiramatsu M, et al. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses 2. Lett Appl Microbiol. 2010;50:597–602. doi: 10.1111/j.1472-765X.2010.02844.x. [DOI] [PubMed] [Google Scholar]
- Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ. 2001;322:1327. doi: 10.1136/bmj.322.7298.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojsak I, Abdovic S, Szajewska H, Milosevic M, Krznaric Z, Kolacek S. Lactobacillus GG in the prevention of nosocomial gastrointestinal and respiratory tract infections. Pediatrics. 2010;125:e1171–e1177. doi: 10.1542/peds.2009-2568. [DOI] [PubMed] [Google Scholar]
- Holland D, Booy R, De LF, Eizenberg P, McDonald J, Karrasch J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995;13:310–312. doi: 10.1016/0264-410x(95)93319-5. [DOI] [PubMed] [Google Scholar]
- Jefferson TO, Rivetti D, Di PC, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007:CD001269. doi: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- Kawase M, He F, Kubota A, Harata G, Hiramatsu M. Oral administration of lactobacilli from human intestinal tract protects mice against influenza virus infection 3. Lett Appl Microbiol. 2010 doi: 10.1111/j.1472-765X.2010.02849.x. [DOI] [PubMed] [Google Scholar]
- Link-Amster H, Rochat F, Saudan KY, Mignot O, Aeschlimann JM. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol. 1994;10:55–63. doi: 10.1111/j.1574-695X.1994.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Jacobson DL, Ashworth LA, Grand RJ, Meyer AL, McNeal MM, et al. Immune response to influenza vaccine in children with inflammatory bowel disease. Am J Gastroenterol. 2009;104:444–453. doi: 10.1038/ajg.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TT, Bell I. Probiotics and the immune response to vaccines. Proc Nutr Soc. 2010;69:442–446. doi: 10.1017/S0029665110001758. [DOI] [PubMed] [Google Scholar]
- Monto AS, Ohmit SE, Petrie JG, Johnson E, Truscon R, Teich E, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361:1260–1267. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- Muszkat M, Greenbaum E, Ben Yehuda A, Oster M, Yeu’l E, Heimann S, et al. Local and systemic immune response in nursing-home elderly following intranasal or intramuscular immunization with inactivated influenza vaccine. Vaccine. 2003;21:1180–1186. doi: 10.1016/s0264-410x(02)00481-4. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999;282:137–144. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis. 2006;194(Suppl 2):S111–S118. doi: 10.1086/507544. [DOI] [PubMed] [Google Scholar]
- Ohmit SE, Gross J, Victor JC, Monto AS. Reduced reaction frequencies with repeated inactivated or live-attenuated influenza vaccination. Vaccine. 2009;27:1050–1054. doi: 10.1016/j.vaccine.2008.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355:2513–2522. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares M, az-Ropero MP, Sierra S, Lara-Villoslada F, Fonolla J, Navas M, et al. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007;23:254–260. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3:e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh SE, Ong DQ, Gerez I, Zhang X, Chollate P, Shek LP, et al. Effect of probiotic supplementation in the first 6 months of life on specific antibody responses to infant Hepatitis B vaccination. Vaccine. 2010;28:2577–2579. doi: 10.1016/j.vaccine.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Treanor J, Wright PF. Immune correlates of protection against influenza in the human challenge model. Dev Biol (Basel) 2003;115:97–104. [PubMed] [Google Scholar]
- Wang Z, Tobler S, Roayaei J, Eick A. Live Attenuated or Inactivated Influenza Vaccines and Medical Encounters for Respiratory Illnesses Among US Military Personnel. JAMA. 2009;301:945–953. doi: 10.1001/jama.2009.265. [DOI] [PubMed] [Google Scholar]
- Wilde JA, McMillan JA, Serwint J, Butta J, O’Riordan MA, Steinhoff MC. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA. 1999;281:908–913. doi: 10.1001/jama.281.10.908. [DOI] [PubMed] [Google Scholar]
- Yasui H, Kiyoshima J, Hori T. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clin Diagn Lab Immunol. 2004;11:675–679. doi: 10.1128/CDLI.11.4.675-679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.