Abstract
We previously demonstrated that oscillatory fluid flow activates MC3T3-E1 osteoblastic cell calcium signaling pathways via a mechanism involving ATP releases and P2Y2 puringeric receptors. However, the molecular mechanisms by which fluid flow initiates cellular responses are still unclear. Accumulating evidence suggests that lipid rafts, one of the important membrane structural components, may play an important role in transducing extracellular fluid shear stress to intracellular responses. Due to the limitations of current techniques, there is no direct approach to study the role of lipid rafts in transmitting fluid shear stress. In this study, we targeted two important membrane components associated with lipid rafts, cholesterol and glycosylphosphatidylinositol-anchored proteins, to disrupt the integrity of cell membrane structures. We first demonstrated that membrane cholesterol depletion with the treatment of methyl-β-cyclodextrin inhibits oscillatory fluid flow induced intracellular calcium mobilization and ERK1/2 phosphorylation in MC3T3-E1 osteoblastic cells. Secondly, we used a novel approach to decrease the levels of glycosylphosphatidylinositol-anchored proteins on cell membranes by overexpressing glycosylphosphatidylinositol specific phospholipase D in MC3T3-E1 osteoblastic cells. This resulted in significant inhibition of intracellular calcium mobilization and ERK1/2 phosphorylation in response to oscillatory fluid flow. Finally, we demonstrated that cholesterol depletion inhibited oscillatory fluid flow induced ATP releases, which were responsible for the activation of calcium signaling pathways in MC3T3-E1 osteoblastic cells. Our findings suggest that cholesterol and GPI-anchored proteins, two membrane structural components related to lipid rafts, may play an important role in osteoblastic cell mechanotransduction.
Keywords: Osteoblastic mechanotransduction, Oscillatory fluid flow shear stress, Osteoblast, Cholesterol depletion, GPI-anchored proteins
INTRODUCTION
Bone is a unique organ which is continually remodeling to meet its demands for mechanical loading. When increased mechanical loading occurs, net bone mass increases (Sessions et al., 1989), whereas decreased loading results in bone lose (Morey and Baylink, 1978). Previously, we demonstrated that oscillatory fluid flow is a potent mechanical stimulus in vitro, mimicking in vivo biophysical signals experienced by bone cells (osteoblasts and osteocytes) during cyclic mechanical loading (You et al., 2000). In addition, we found that fluid shear stress induced by oscillatory fluid flow activates intracellular calcium (Ca2+i) mobilization and MAKP signaling pathways through ATP release and activation of the P2Y2 receptor (You et al., 2002; You et al., 2001). However, how fluid shear stress initiates this response is unclear.
Many candidates have been suggested as sensors that perceive fluid shear stress, including ion channels, receptors, integrins, and primary cilia (Li et al., 2002; Malone et al., 2007; Pavalko et al., 1998; Shyy and Chien, 2002). Recently, lipid raft, a microdomain in cell membranes, has been suggested to be involved in mechano-sensing (Munro, 2003). For example, caveolae, a special type of lipid rafts, was demonstrated to play an important role in endothelial cell mechanotransduction in vivo (Yu et al., 2006). Lipid rafts consist of cholesterol and glycosphingolipid as well as a variety of transmembrane proteins including some receptor tyrosine kinases and G-protein coupled receptors (Rajendran and Simons, 2005). Lipid rafts have been shown to be involved in many signaling pathways, such as CD39 activity in canine kidney cells and calcium entry in neutrophils (Kannan et al., 2007; Papanikolaou et al., 2005). In addition, lipid rafts were linked to ERK1/2 to activate inhibitor-kappa B kinase in human astrocytoma cells (Kam et al., 2007). More specifically, previous studies have shown that lipid rafts are involved in many cell signal transduction processes including osteoblast proliferation (Tanikawa et al., 2008). In addition, caveolae structure has been shown to be able to regulate endothelial cell response to fluid flow in vitro (Park et al., 2000). These data suggest that lipid rafts may be also involved in initiating cellular responses to fluid flow induced shear stress in bone cells. However, due to the limitations of available techniques, few studies have directly investigated the role of lipid rafts in bone cell mechanotransduction. In this study, we targeted two important membrane components associated with lipid rafts, cholesterol and glycosylphosphatidylinositol-anchored proteins (GPI-anchored proteins), to disrupt the integrity of cell membrane structures. Thus, the goal of this study was to investigate the roles of cholesterol and GPI-anchored proteins in oscillatory fluid flow induced osteoblastic responses.
Fluid flow shear stress has been reported to induce a variety of cellular responses in osteoblastic cells, including intracellular calcium mobilization, ATP, nitric oxide and prostaglandin E2 release, MAPK activation and gene expression changes (Genetos et al., 2005; Johnson et al., 1996; Ponik and Pavalko, 2004; Reich et al., 1997; You et al., 2001). Among these responses, calcium dependent signaling is one of the most important intracellular pathways. Our previous studies demonstrated that osteopontin gene expression is increased through a mechanism involving the calcium and MAPK pathway in osteoblastic MC3T3-E1 cells (You et al., 2001). Calcium is an important second messenger that regulates various intracellular events in osteoblasts. Oscillations in intracellular calcium regulate expression of transcription factors such as NFAT, NFκB, and Oct/OAP, which may be involved in cellular differentiation (Dolmetsch et al., 1998; Li et al., 1998). Similarly, ERK1/2 activation plays a key role in regulating a series of cell activities, including migration, survival, proliferation and differentiation (Marais and Marshall, 1996; Seger and Krebs, 1995). ERK1/2 activation is induced by oscillatory fluid flow and may influence the production of eNOS and RANKL (Rubin et al., 2003). Thus, calcium mobilization and ERK1/2 activation are two important indicators of cellular response to fluid flow induced shear stress in osteoblastic cells.
We have demonstrated that ATP and P2Y2 puringeric receptors are involved in osteoblast mechanotransduction (You et al., 2002). We found that ATP is released from the cytosol to the extracellular space and subsequently binds to P2Y2 receptors and initiates intracellular calcium mobilization. In addition, an earlier report (Genetos et al., 2005) showed that ATP is responsible for fluid flow induced prostaglandin E2 releases in MC3T3-E1 cells. Furthermore, another report (Nakano et al., 2007) demonstrated that ATP is very important for mineralization in MC3T3-E1 cells. Beside osteoblastic cells, ATP has been demonstrated to stimulate endothelial cell and human bone marrow stromal cell proliferation through intracellular calcium mobilization (Boarder and Hourani, 1998; Riddle et al., 2007). Thus, ATP and P2Y are parts of an important signaling pathway in bone biology especially as it related to bone cell mechanotransduction.
In this study we hypothesized that cholesterol and GPI-anchored proteins are involved in transmitting fluid shear stress to activate the calcium signaling pathway in osteoblastic cells. We first examined the effects of cholesterol depletion from cell membranes on oscillatory fluid flow response in MC3T3-E1 cells. We then employed a novel approach to cleave lipid raft-associated GPI-anchored proteins by overexpressing GPI-specific phospholipase D (GPI-PLD), which can specifically cleave GPI-anchored proteins, and examined the effects of decreasing GPI-anchored proteins from cell membranes on fluid flow response in osteoblastic cells. Finally, we demonstrated that cholesterol depletion inhibited fluid flow induced ATP release and subsequent activation of P2Y signaling pathways.
MATERIALS AND METHODS
Cell culture
The mouse osteoblastic cell line MC3T3-E1 was cultured in minimal essential α medium (MEM-α) (Invitrogen) containing 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 1% penicillin and streptomycin (Invitrogen), and maintained in a humidified incubator at 37 °C with 5% CO2. All cells were subcultured on glass slides for 2 days prior to experiments, with the exception of cells cultured for calcium mobilization studies, for which quartz slides were used because of their UV transparency. 1.5×105 cells were seeded on glass slides (75 × 38 × 1.0 mm) for most experiments. 0.85×105 cells were seeded on quartz slides (76 × 26 × 1.6 mm) for two days prior to experiments for calcium imaging. Cells were exposed to oscillatory fluid flow in MEM-α and 2% FBS for calcium imaging experiments, and in MEM-α and 0.5% FBS for other experiments. The different FBS percentages were selected to optimize our fluid flow responses for calcium mobilization and ERK1/2 activation. The fluid flow chamber employed in this study is a parallel plate design and the flow delivery device generated 1Hz sinusoidaly oscillating flow. Both have been described in previous studies (Jacobs et al., 1998; You et al., 2001).
Methyl-β-cyclodextrin (MβCD), Methyl-α-cyclodextrin (ACD) and Hydroxypropyl-beta-cyclodextrin (HβCD) treatment
MC3T3-E1 osteoblastic cells cultured on slides were incubated in flow medium containing 1mM, 5mM or 10mM of MβCD for 60 min at 37°C. MβCD is a small cyclic oligosaccharide with a hydrophobic core that selectively and rapidly extracts cholesterol from plasma membranes thus disrupting lipid rafts (Ohtani et al., 1989). 10mM ACD, which extracts sphingomyelin from sphingolipid-rich lipid rafts (inactive in removing cholesterol) (Motoyama et al., 2009), was used as a control for discarding direct effects of the treatment on membrane proteins. In addition, 30mM HβCD, as an alternative and less potent agent (Motoyama et al., 2009), was employed to confirm our findings. Cells were then subjected to oscillatory fluid flow stimulation. To examine the toxicity of MβCD toward MC3T3-E1 cells, we replaced MβCD medium after 60 minutes treatment with normal flow medium for another 120 minutes. Cells were then either stained with trypan blue to examine their viability or subjected to oscillatory fluid flow to evaluate their response to mechanical stimulation.
Calcium imaging
The details of calcium imaging method were described in our previous studies (You et al., 2001; You et al., 2000). Briefly, intracellular calcium concentration ([Ca2+]i) was quantified with the fluorescent dye fura-2. Fura-2 exhibits a shift in absorption when bound to Ca2+ such that the emission intensity when illuminated with ultraviolet light increases with increasing [Ca2+]i at a wavelength of 340nm, and decreases with increasing [Ca2+]i at 380nm. The ratio of light intensity between the two wavelengths corresponds to [Ca2+]i. Preconfluent (80%) cells were washed with MEM-α and 2% FBS at 37°C, incubated with a 10μM fura-2-acetoxymethyl ester (Molecular Probes, Eugene, OR) solution for 30 min at 37°C and washed again with fresh MEM-α and 2% FBS. The quartz slide with the cells was mounted within a parallel plate flow chamber filled with experimental media. The chamber was placed on an inverted fluorescence microscope (Nikon, Melville, NY) and left undisturbed for 30 min prior to experiments. Cell ensembles were illuminated at wavelengths of 340 and 380nm in turn. Emitted light was passed through a 510nm interference filter and detected with an intensifier charge coupled device camera (International LTD., Sterling, VA). Images were recorded, once every two seconds and analyzed using image analysis software (Universal Imaging, West Chester, PA). We defined a response as an oscillation in at least 2-fold greater than that of the average base-line level of nontreated cells. Basal [Ca2+]i was sampled for 3 minutes and followed by 3 minutes of oscillatory fluid flow.
Western blot
To examine the effects of fluid flow on ERK1/2 phosphorylation, MC3T3-E1 were exposed to oscillatory fluid flow with a shear stress of 1 N/m2 for different periods of time. Immediately after exposure to flow, slides with cells were stored in −80°C. Cells were lysed with RIPA buffer and total protein concentrations were determined by bicinchoninic acid assay (Pierce). 25μg of total protein from each sample was separated by SDS-PAGE and transferred to PVDF membranes. Membranes were incubated with the desired primary antibody overnight at 4°C, and subsequently with the secondary antibody for 1 hour. Visualization of immunoreactive proteins was achieved by employing an ECL detection system and membrane exposure to film.
Measurement of Cholesterol Concentration
To verify the effects of different reagents on efflux of cholesterol from cell membrane, MC3T3-E1 cells were cultured in 10cm dishes for 4 days. After the cells reached 90–100% confluence, the original cell culture media were replaced with fresh 2ml cell culture media containing 0.5% FBS and different concentrations of reagents (MβCD, ACD, or HβCD). Then the media were collected after 45-minute reagent treatments. Finally cholesterol concentrations in the media were measured by using the Cholesterol E kit (Wako Chemicals, Richmond, VA) according to the manufacturer’s protocol.
GPI-PLD transfection
The full-length 3.4-kb cDNA for mouse GPI-PLD (a gift from Dr. Mark A. Deeg, Indiana University) was subcloned into the EcoRI–XhoI sites of a pcDNA3.1 vector (Invitrogen). For stable transfection of MC3T3-E1 cells with GPI-specific phospholipase D (GPI-PLD), cells were transfected with FuGENE 6 (Roche) transfection reagent and DNA, either pcDNA3-GPI-PLD or pcDNA3 empty vector (control) for 24 hours. Geneticin (400 μg/ml) was then added to cell culture medium for selection. Cells were maintained in selection medium for 14 days and then used for experiments.
Fluorescence staining of GPI-anchored proteins
Flourescent aerolysin (FLAER), an AlexaR 488 labeled proaerolysin (Pinewood Scientific Service Inc., Canada), has been demonstrated to be a unique protein that binds tightly and specifically to GPI-anchored proteins on mammalian cells (van Zanten et al., 2009). Thus, we employed FLAER to verify the effects of overexpressing GPI-PLD on decreasing GPI-anchored proteins on cell membrane. To stain cells in 6 well plates, we first washed cells twice with PBS, then added 2.5×10−7 M FLAER solution to cells directly. After 10 minutes, the staining solution was removed and cells were washed with PBS for another two times. Then the living cells in PBS were observed under fluorescence microscope and all fluorescence images from cells with overexpressing GPI-PLD or control vector were acquired at the same exposure time.
ATP detection
After oscillatory fluid flow exposure, samples of conditioned media were collected and immediately stored at −80°C until analyzed. ATP concentration in samples of conditioned media was determined using a commercially available ATP bioluminescence kit (Roche). ATP in 50 μl of each sample serves as a co-factor for luciferase, to convert D-luciferin, in 50 μl of a luciferin-luciferase assay buffer, into oxyluciferin and light. The luminescence from each reaction, as measured by a Monolight 3010 luminometer (BD Pharmingen, San Diego, CA), was compared with a standard curve created by serially diluting an ATP standard. Duplicate measurements were taken from each sample. Control experiments were performed with each pharmacological inhibitor to ensure that they had no detrimental effect on the reaction. Results were normalized to cellular protein concentration using bicinchoninic acid assay (Pierce).
Data Analysis
Data are expressed as mean ± standard error (S.E.). To compare observations from no flow and flow responses, a two-sample Student t-test was used in which sample variance was not assumed to be equal. To compare observations from more than two groups, a one-way analysis of variance was employed followed by a Bonferroni selected-pairs multiple comparisons test (Instat, GraphPad Software Inc., San Diego, CA). p<0.05 was considered statistically significant.
RESULTS
Cholesterol depletion inhibits the calcium response to fluid flow
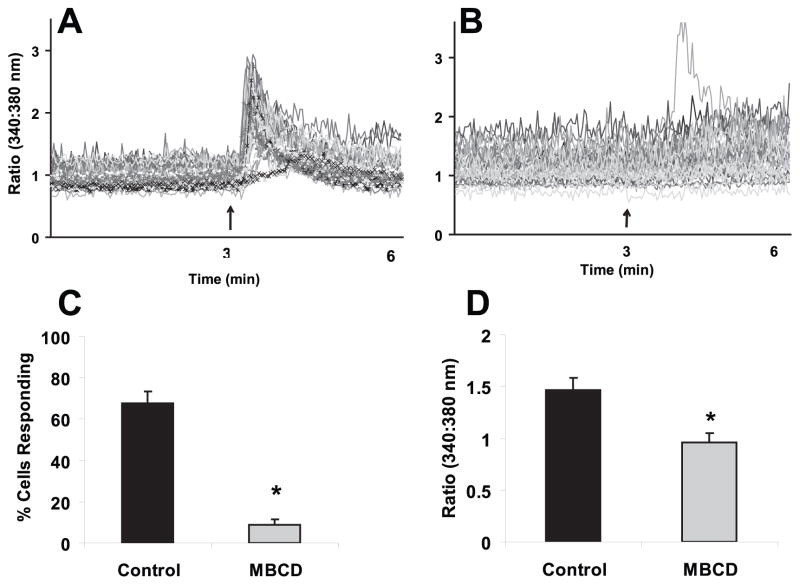
To investigate the role of cholesterol in flow fluid induced calcium signaling, we first employed an often used agent, MβCD, a cyclic oligosaccharide with a hydrophobic core that selectively and rapidly extracts cholesterol from the plasma membrane (Ohtani et al., 1989). Previously we demonstrated that intracellular calcium mobilization is an important initial indicator for osteoblastic response to fluid flow (You et al., 2001). Thus, we first examined the calcium response after cholesterol depletion. MC3T3-E1 osteoblastic cells were cultured on quartz slides and pretreated with MβCD (10mM) or control media for 30 minutes. Cells were then exposed to oscillatory fluid flow (peak shear stress at 2 N/m2, 1 Hz) in the presence of MβCD (10mM) or control media. As we previously reported (You et al., 2002), within 30 seconds of starting fluid flow with the control media, about 67.9±5.6% of cells displayed an increase in [Ca2+]i. In the presence of MβCD (10mM), the percentage of cells responding during the fluid flow with an increased in [Ca2+] was significantly decreased (8.7±2.9%) compared with those cells in the presence of control media (Fig. 1). In addition, the average amplitude of [Ca2+]i in all responding cells during fluid flow following MβCD pre-treatment (10mM) was significantly smaller than that with control media treated.
Figure 1. Treatment of MβCD (10 mM) inhibits oscillatory fluid flow induced increases in [Ca2+]i in MC3T3-E1 osteoblastic cells.
A, a representative example of [Ca2+]i traces obtained from MC3T3-E1 cells exposed to oscillatory flow (2N/m2, 1Hz) in control media. The arrow depicts the onset of flow and each line represents an individual cell response. B, a representative example of [Ca2+]i traces obtained from MC3T3-E1 cells exposed to oscillatory flow (2N/m2, 1Hz) in the presence of MβCD (10 mM). The arrow depicts the onset of flow and each line represents an individual cell response. C, the percentage of MC3T3-E1 cells responding with an increase in [Ca2+]i in the presence or absence of MβCD (10 mM). D, amplitude of increase in [Ca2+]i in MC3T3-E1 cells exposed to oscillatory flow (2N/m2, 1Hz) in the presence or absence of MβCD (10 mM). (*, p<0.05) Each bar represents the mean ± S.E. and each experiment was repeated on 4–6 slides.
Cholesterol depletion inhibits the ERK1/2 response to fluid flow
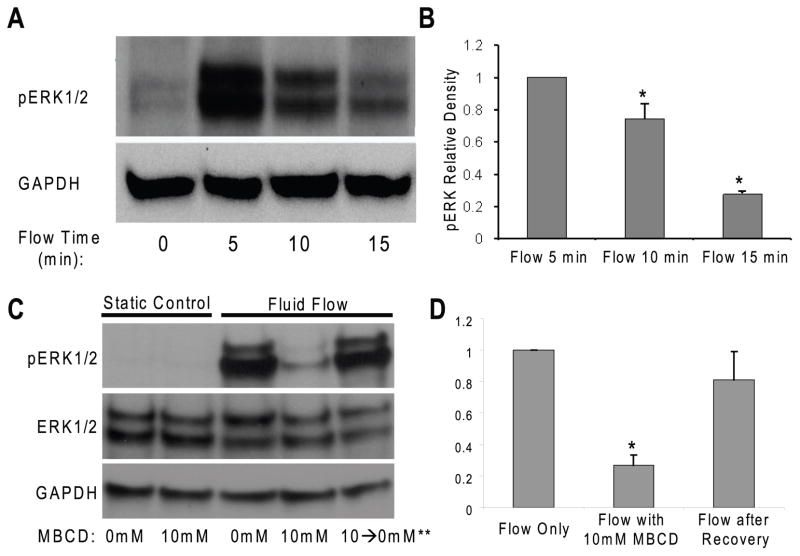
To further confirm the role of cholesterol depletion in fluid flow induced osteoblastic responses, we examined the phosphorylation of ERK1/2, which has been demonstrated to play an important role in proliferation and differentiation in many cell types, in response to oscillatory fluid flow (Riddle et al., 2006). In addition, we and others have demonstrated that ERK1/2 activation is an import signaling pathway in bone cells (You et al., 2001). We first examined, by using western blot techniques, the time course of ERK1/2 phosphorylation in response to oscillatory fluid flow in MC3T3-E1 osteoblastic cells. In the absence of flow there was minimal ERK1/2 phosphorylation (Fig. 2A, B). However, a dramatic increase in ERK1/2 phosphorylation was observed at 5, 10 and 15 minutes in response to oscillatory fluid flow (peak shear stress at 1 N/m2, 1 Hz). To study the role of cholesterol depletion in this response, we pretreated MC3T3-E1 cells with 10 mM MβCD and found that ERK1/2 phosphorylation at 5 min in cells exposed to fluid flow in the presence of MβCD was significantly decreased compared to exposed in the presence of control media (Fig. 2C: Lane 1–4). Additionally, we restored 90% of ERK1/2 phosphorylation in response to fluid flow when we removed the MβCD from the media for 120 minutes (Fig. 2C: Lane 5). Like GAPDH, total ERK1/2 was unchanged in response to fluid flow (Fig, 2C). Thus, we quantified all ERK1/2 phosphorylation normalized to GAPDH.
Figure 2. MβCD inhibits oscillatory fluid flow induced ERK1/2 phosphorylation in MC3T3-E1 osteoblastic cells.
A, a dramatic increase in ERK1/2 phosphorylation was observed at 5, 10 and 15 minutes in response to oscillatory fluid flow (1N/m2, 1Hz). B, bar graph representation of ERK1/2 phosphorylation quantified by scanning densitometry normalized to GAPDH. C, after 10mM MβCD treatment, oscillatory fluid flow induced phosphorylation of ERK1/2 at 5 minutes significantly decreased in MC3T3-E1 osteoblastic cells (lane 1–4). Replacement of MβCD media with control flow media for 120 minutes allowed the recovery of cell responsiveness to fluid flow in terms of ERK1/2 activation (lane 5). D, bar graph representation of ERK1/2 phosphorylation quantified by scanning densitometry normalized to GAPDH. (*, p<0.05) Each bar represents the mean ± S.E. and each experiment was repeated on 3–5 times.
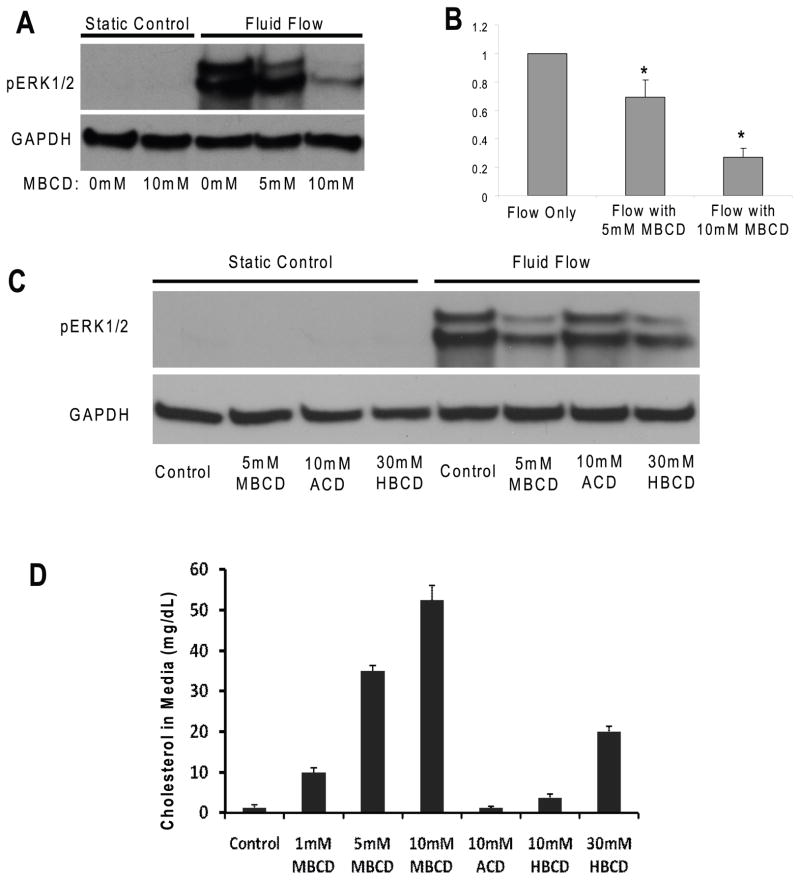
In addition, the inhibition of ERK1/2 phosphorylation in the presence of MβCD was concentration dependent; 10 mM of MβCD inhibited ERK1/2 phosphorylation by 80% and 5 mM only inhibited by 30% (Fig. 3A, B). To confirm the MβCD treatment specifically for cholesterol depletion from membranes, 10 mM ACD, which extracts sphingomyelin from sphingolipid-rich lipid rafts (inactive in removing cholesterol) (Motoyama et al., 2009), was used as a control and our results showed that ACD did not inhibit fluid flow induced ERK1/2 phosphorylation (Fig, 3C: Lane 3, 7). However, a less potent cholesterol depletion agent, 30 mM HβCD still inhibited fluid flow induced ERK1/2 phosphorylation in MC3T3-E1 cells (Fig. 3C Lane: 4, 8).
Figure 3. MβCD inhibits oscillatory fluid flow induced ERK1/2 phosphorylation in MC3T3-E1 osteoblastic cells in a concentration dependent manner.
A, following exposure to MβCD at different concentrations, oscillatory fluid flow induced phosphorylation of ERK1/2 at 5 minutes decreased in MC3T3-E1 osteoblastic cells. B, bar representation of ERK1/2 phosphorylation quantified by scanning densitometry normalized to GAPDH. C, following exposure to MβCD, ACD and HβCD at different concentrations, oscillatory fluid flow induced phosphorylation of ERK1/2 at 5 minutes detected in MC3T3-E1 osteoblastic cells. D, the efflux of cholesterol from cell membranes was quantified for the different treatments of MβCD, ACD and HβCD at different concentrations. (*, p<0.05) Each bar represents the mean ± S.E. and each experiment was repeated on 3–5 times.
To verify the removal of cholesterol by different agent treatments (MβCD, ACD and HβCD), we quantified the efflux of cholesterol from cell membranes in culture media after different treatments. Our results demonstrated that the removal of cholesterol by MβCD was concentration dependent (Fig. 3D: Left 4 bars), which was inversely correlated well with ERK1/2 fluid flow response. 10 mM ACD (control for MβCD) did not remove any cholesterol from membranes, and 10mM or 30mM HβCD removed less cholesterol from membranes compared to MβCD (Fig. 3D: Right 3 bars).
Cleavage of GPI-anchored proteins inhibits the calcium response to fluid flow
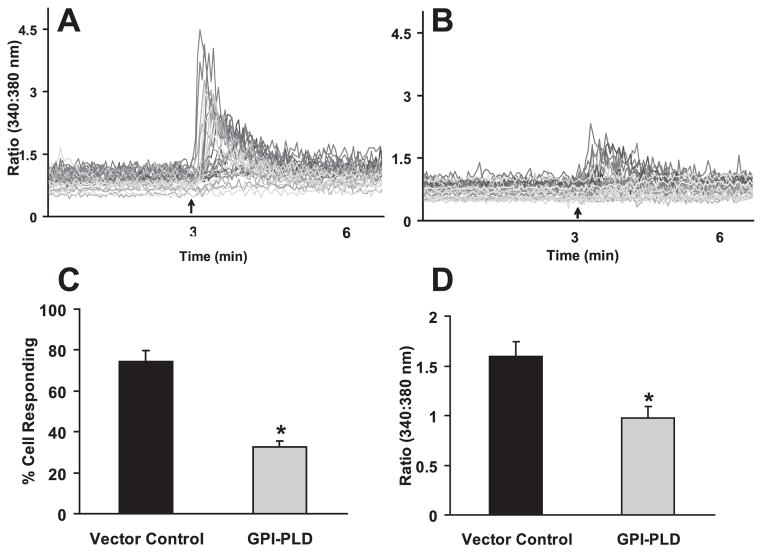
In addition to cholesterol within lipid rafts, there is another class of ecto-proteins, GPI- anchored proteins, which are uniquely associated with lipid rafts. We hypothesized that the GPI-anchored proteins serve as mechanoreceptors on the cell membrane that perceive fluid flow. To investigate the role of GPI-anchored proteins in fluid flow induced osteoblastic responses, we introduced a phospholipase, GPI-PLD, which can specifically cleave GPI-anchored proteins from cell membranes (Metz et al., 1994; Scallon et al., 1991). Overexpressing of GPI-PLD has been demonstrated to significantly decrease the levels of GPI-anchored proteins (Mann et al., 2004). We used western blot analysis to confirm our stable transfection with GPI-PLD in our MC3T3-E1 cells. We then demonstrated that overexpressing of GPI-PLD in MC3T3-E1 cells significantly inhibited the percentage of cells responding to flow with an increase in [Ca2+]i, relative to cells expressing the pcDNA3 vector control (32.8% vs. 74.3% respectively) (Fig. 4A–C). In addition, overexpressing of GPI-PLD significantly decreased the mean [Ca2+]i response amplitude (Fig. 4D).
Figure 4. Overexpressing GPI-PLD inhibits oscillatory fluid flow induced increases in [Ca2+]i in MC3T3-E1 osteoblastic cells.
A, a representative example of MC3T3-E1 cell [Ca2+]i traces obtained after exposure of control cells (expressing pcDNA3 vector control) to oscillatory flow (2N/m2, 1Hz). The arrow depicts the onset of flow and each line represents an individual cell response. B, a representative example of MC3T3-E1 cell [Ca2+]i traces obtained after exposure of pcDNA3-GPI-PLD expressing cells to oscillatory flow (2N/m2, 1Hz). The arrow depicts the onset of flow and each line represents an individual cell response. C, the percentage of MC3T3-E1 cells responding with an increase in [Ca2+ ]i in cells expressing the pcDNA3 vector control or pcDNA3-GPI-PLD (GPI-PLD). D, the mean amplitude of [Ca2+ ]i in MC3T3-E1 cells, expressing the pcDNA3 vector control or pcDNA3-GPI-PLD (GPI-PLD), responding with an increase in [Ca2+]i. (*, p<0.05) Each bar represents the mean ± S.E. and each experiment was repeated on 4–6 slides.
Cleavage of GPI-anchored proteins inhibits the ERK1/2 response to fluid flow
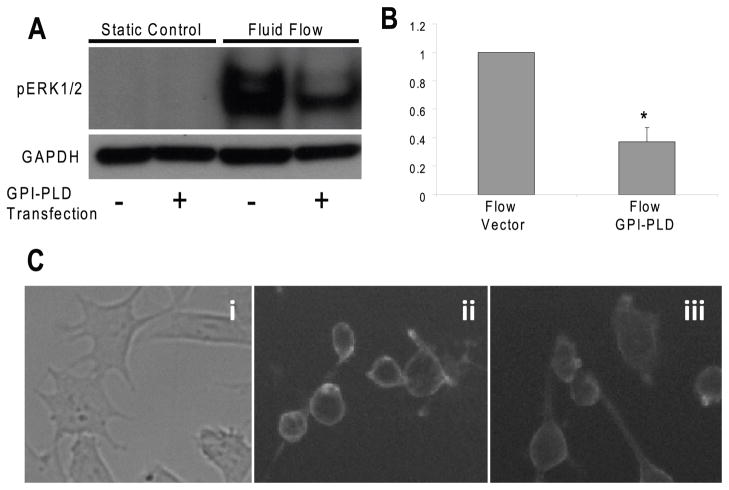
To further examine the role of GPI-anchored proteins in fluid flow induced responses, we examined the effect of GPI-PLD over expression on ERK1/2 activation in response to fluid flow. We found that ERK1/2 phosphorylations in response to fluid flow in cells overexpressing GPI-PLD was significantly lower (63%) than that in cells expressing the pcDNA3 vector control (Fig. 5A, B), suggesting that GPI-anchored proteins are involved in fluid flow induced ERK1/2 activation. To verify the effects of overexpressing GPI-PLD on changing GPI-anchored proteins, we stained both cells overexpressing GPI-PLD and expressing pcDNA3 control vector with FLAER. Our results demonstrated that the fluorescence intensity on membranes in cells overexpressing GPI-PLD (Fig. 5Ciii) was decreased compared to cells with expressing pcDNA3 control vector (Fig. 5Cii), suggesting the decreases of GPI-anchored proteins on cells after overexpressing GPI-PLD.
Figure 5. Overexpression of GPI-PLD inhibits oscillatory fluid flow induced ERK1/2 phosphorylation in MC3T3-E1 osteoblastic cells.
A, oscillatory fluid flow induced ERK1/2 phosphorylation at 5 minutes significantly decreased in GPI-PLD expressing cells (lane 2 and 4) compared with that in control cells (expressing pcDNA3 vector control, lane 1 and 3). B, bar graph representation of ERK1/2 phosphorylation quantified by scanning densitometry normalized to GAPDH. C, bright field image (i) and fluorescence images taken at the same exposure time in cells expressing pcDNA3 control vector (ii) and overexpressing GPI-PLD (iii) in MC3T3-E1 cells. (*, p<0.05) Each bar represents the mean ± S.E. and each experiment was repeated 3–5 times.
ATP release in response to fluid flow is decreased after cholesterol depletion
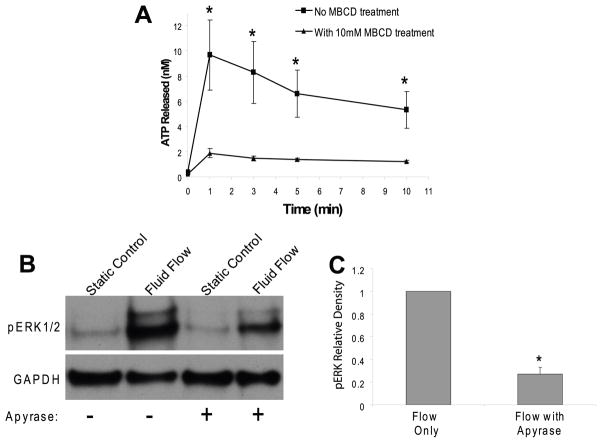
Previously we demonstrated that ATP and one of its receptor subtypes, the P2Y2 puringeric receptor, are involved in flow induced calcium and ERK1/2 responses in osteoblastic cells (You et al., 2002). Here we further investigated whether cholesterol in cell membranes is involved in flow induced ATP release. We removed cholesterol from cell membranes by using MβCD and examined the amount of ATP in conditioned cell culture media during fluid flow at 1, 3, 5, and 10 minutes. Our results demonstrated that MβCD (10mM) significantly inhibited flow induced ATP releases at all time points (Fig. 6A). To elucidate whether ATP release is involved in the ERK1/2 phosphorylation response, we employed an enzyme, apyrase, which rapidly hydrolyzes ATP to AMP and phosphates. In the presence of 10 U/ml of apyrase, fluid flow induced peak ERK1/2 phosphorylation at 5 minute was significantly reduced by 75% (Fig. 6B, C), suggesting that ATP release is required for fluid flow induced ERK1/2 activation.
Figure 6. MβCD (10 mM) inhibits oscillatory fluid flow induced ATP release and blockage of ATP release by apyrase (10 U/ml) inhibits oscillatory fluid flow induced ERK1/2 phosphorylation in MC3T3-E1 osteoblastic cells.
A, oscillatory fluid flow induced ATP release following exposure to MβCD (10 mM) was significantly reduced in MC3T3-E1 osteoblastic cells at 1, 3, 5, and 10 minutes. B, oscillatory fluid flow induced ERK1/2 phosphorylation at 5 minutes is significantly decreased in MC3T3-E1 cells following exposure to apyrase (10 U/ml) (lane 3–4) relative to cells not exposed to apyrase (lane 1–2). C, bar graph representation of ERK1/2 phosphorylation quantified by scanning densitometry normalized to GAPDH. (*, p<0.05) Each bar represents the mean ± S.E. and each experiment was repeated 3–5 times.
Cells become stiffer after cholesterol depletion
We have demonstrated that MβCD inhibits oscillatory fluid flow induced Ca2+i mobilization and ERK1/2 phosphorylation in MC3T3-E1 osteoblastic cells. MβCD may also change the mechanical properties of cells. To address this, we employed AFM to measure cell mechanical properties in the presence of or absence of MβCD. The elastic modulus of cells was determined by indenting the cell membrane with a spherical AFM probe and then analyzing the compression data using the Hertz model (Chizhik et al., 1998). Higher values of elastic modulus represent stiffer cells. The overall cellular elastic modulus distribution functions for MC3T3-E1 cells in the presence or absence of MβCD (10 mM) were plotted in Figure 7. The distribution of cell elastic modulus in the presence of MβCD is shifted toward the higher values of the modulus relative to that in the absence of MβCD, suggesting that cells become stiffer in the presence of MβCD.
Figure 7. MβCD (10 mM) increases stiffness of cells.
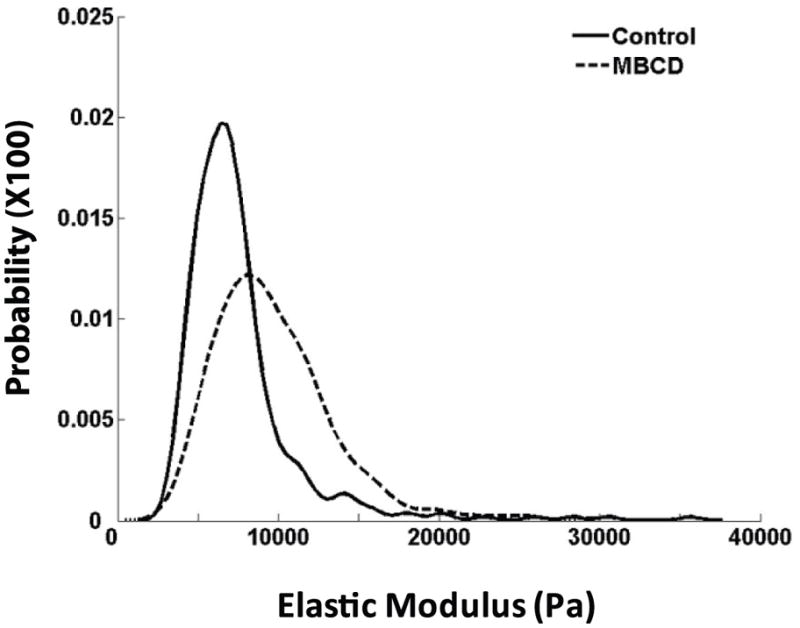
Overall cellular elastic modulus distribution functions for MC3T3-E1 cells in the presence (dashed line) or absence (solid line) of MβCD (10 mM) were plotted using data from 3 experiments for each treatment. The distribution of cell elastic modulus in the presence of MβCD is shifted toward the higher values of the modulus relative to that in the absence of MβCD.
DISCUSSION
While strong evidence suggests mechanical loading regulates bone metabolism, little is known about the molecular mechanisms involved in the process. Osteoblasts and osteocytes are the bone cells best positioned to detect mechanical signals. We and others demonstrated that oscillatory fluid induced shear stress is an important biophysical signal induced in bone by mechanical loading. However, the molecular mechanisms for bone cells to sense mechanical signals and initiate intracellular responses are still unclear. Accumulating evidence suggests that lipid rafts, one of important membrane structural components, may play an important role in transmitting extracellular mechanical force to intracellular responses in endothelial cells (Munro, 2003; Yu et al., 2006). However, due to technical limitations it is rather challenging to directly study the role of lipid rafts in mechanotransduction. In this study, we targeted two important membrane components associated with lipid rafts, cholesterol and GPI-anchored proteins, to disrupt the structural integrity of cell membranes. We first demonstrated that membrane cholesterol depletion reduces oscillatory fluid flow induced Ca2+i and ERK1/2 response in MC3T3-E1 osteoblastic cells. We also found that reduction of GPI-anchored proteins on cell membranes by over expression of GPI-PLD resulted in significant inhibition of Ca2+i mobilization and ERK1/2 phosphorylation in response to oscillatory fluid flow. Finally, we demonstrated that cholesterol depletion inhibited oscillatory fluid flow induced ATP releases, which were responsible for the activation Ca2+i mobilization and ERK1/2 response in MC3T3-E1 osteoblastic cells. Our findings suggest that cholesterol and GPI-anchored proteins, as two structural components related to lipid rafts, may play an important role in osteoblastic cell mechanotransduction.
Lipid rafts have been suggested to be involved in mechanotransduction in endothelial cells (Munro, 2003). For instance, caveolae, specialized lipid rafts, have been demonstrated to play an important role in endothelial mechanotransduction in vivo (Yu et al., 2006). A few studies have reported that caveolae may be involved in bone cell proliferation and differentiation (Tanikawa et al., 2008). However, the role of lipid rafts in bone cell mechanotransduction is unknown. We first employed MβCD to deplete cholesterol from membranes. MβCD is often used to investigate the role of cholesterol or lipid rafts (Awasthi-Kalia et al., 2001). Our data demonstrated that cholesterol depletion from membranes inhibits fluid flow induced Ca2+i mobilization, suggesting cholesterol in cell membranes may be involved in fluid flow induced calcium signaling. These results are consistent with previous studies, in which cholesterol depletion inhibits hyaluronan-mediated and caldribine-induced Ca2+i mobilization in endothelial cells (Singleton and Bourguignon, 2004) and leukemia cells (Takahashi et al., 2006), respectively. However, it is the first report that mechanical stimulation (oscillatory fluid flow) induced Ca2+i was inhibited by MβCD. Ca2+i is a ubiquitous second messenger, which controls a number of cellular responses (Dolmetsch et al., 1998; Li et al., 1998), and is one of the earliest intracellular signals activated by extrarcellular signals. Thus, our Ca2+i results suggest that cholesterol in cell membranes may be an important structural component for osteoblastic cells to perceive mechanical signaling and initiate responses.
In addition to early Ca2+i responses, we demonstrated that cholesterol depletion from cell membranes inhibits fluid flow induced ERK1/2 phosphorylation, suggesting cholesterol in cell membranes are also involved in fluid flow induced ERK1/2 signaling. Our finding is consistent with the previous studies in which MβCD inhibits H2O2 induced ERK1/2 phosphorylation in endothelial cells (Yang et al., 2006) and hydrostatic pressure induced ERK1/2 phosphorylation in human osteoblasts (Ferraro et al., 2004). However, cholesterol depletion in cell membranes by MβCD does not always have an effect on ERK1/2 signaling pathways. For instance, MβCD has no effect on angiopoietin-1 induced ERK1/2 phosphorylation in endothelial cells (Katoh et al., 2009). This suggests that the involvement of cholesterol in cell membranes in signal transduction is not universal but restricted to certain cells and certain extracellular signals. In any case, our data strongly suggest that cholesterol in cell membranes are involved in oscillatory fluid flow induced ERK1/2 activation in osteoblastic cells. It is known that ERK1/2 phosphorylation plays a key role in regulating a series of cell activities, such as migration, survival, proliferation and differentiation (Marais and Marshall, 1996; Seger and Krebs, 1995). Therefore, our findings support that cholesterol in cell membranes plays an important role in bone cell mechanotransduction.
MβCD has often been used to study the role of cholesterol in different cell types. In the current study, we not only tested different-dose effects of MβCD on the viability of MC3T3-E1 osteoblastic cells, but also examined fluid flow induced ERK1/2 phosphorylation at two different concentrations (5 and 10 mM). Our measurement of the efflux of cholesterol in the presence of MβCD confirmed that the dose-dependent fluid flow induced ERK1/2 response was due to cholesterol depletion from cell membranes. Our results demonstrating ERK1/2 inhibition at 10 mM MβCD are consistent with other studies (Awasthi-Kalia et al., 2001; Yang et al., 2006), and the results from ACH and HβCD justify the concentration of MβCD we used in other experiments. Our recovery experiments demonstrated the reversible effects of cholesterol depletion by MβCD, suggesting that 10 mM MβCD is not toxic to the cells. More importantly, we demonstrated that the amount of efflux of cholesterol was inversely correlated with flow induced ERK1/2 responses in osteoblasts (Fig. 3), suggesting again cholesterol in cell membranes may play an important role in bone cell mechanotransduction.
To further verify the role of membrane integrity in bone cell mechanotransduction, we targeted GPI-anchored proteins to disrupt the integrity of cell membranes. The unique configuration of GPI-anchored proteins in cell membranes makes them a logical choice to investigate in this context. First, the ecto-structure of these anchored proteins on cell membranes is an ideal candidate for perceiving extracellular mechanical signals, such as fluid flow induced shear stress. Indeed, similar structures, the endothelial glycocalyx layer and bone primary cilia, have been reported to transduce fluid flow induced shear stress to the intracellular responses in endothelial cells (Weinbaum et al., 2007) and bone cells (Malone et al., 2007). Secondly, GPI-anchored proteins are rich in lipid rafts, and lipid rafts have been reported to link to cytoskeletal actin filaments via raft-associated proteins, such as supervillin, myosin-IIA, myosin IG and ezrin–radixin–moesin proteins (Chichili and Rodgers, 2009). In addition, the linkage of GPI-anchored proteins, lipid rafts and cytoskeleton provides an ideal structure to efficiently transmit fluid shear stress to the cytosol. We first demonstrated that overexpressing GPI-PLD decreased the GPI-anchored proteins on cell surfaces (Fig. 5C). Additionally, our data from GPI-PLD overexpressing cells demonstrate that GPI-anchored proteins in cell membranes are involved in fluid flow induced Ca2+i mobilization and ERK1/2 activation, suggesting that GPI-anchored proteins may be involved in transmitting fluid flow induced shear stress into intracellular responses. To our knowledge, this is the first study to investigate the role of GPI-anchored proteins in mechanotransduction.
Previously, we and others demonstrated that ATP release and subsequent activation of P2Y2 receptors are responsible for fluid flow induced Ca2+i mobilization (Genetos et al., 2005; You et al., 2002). Vesicular release, connexin hemichannels, ATP-binding cassette transporters and conductive channels have been proposed to be involved in mechanically induced ATP release from different cells (Praetorius and Leipziger, 2009). Specifically, recent studies in MC3T3-E1 cells suggest that vesicular release most likely is involved in fluid flow induced ATP releases in osteoblastic cells (Genetos et al., 2005). In this study, we demonstrated that cholesterol in cell membranes are involved in fluid flow induced ATP releases from MC3T3-E1 cells. Thus, our data suggest that cholesterol in cell membranes may be important for fluid flow induced ATP-vesicular release in osteoblastic cells.
In addition, we demonstrated that the fluid flow induced ERK1/2 response was inhibited by blockage of ATP releases (Fig. 6B) from cells, suggesting that ATP parecrine/autocrine signaling are involved in fluid flow induced ERK1/2 activation. Previously, we also demonstrated that P2Y2 puringeric receptors are critical for the fluid flow induced intracellular calcium response (You et al., 2002). Thus, our results suggest P2Y2 may be involved in the fluid flow induced ERK1/2 response. Indeed, some studies have demonstrated that ATP activates P2Y2 receptors and subsequently activates ERK1/2 via PLC/PKC PLC signaling pathway in human endometrial stromal cells (Chang et al., 2008).
Several limitations should be considered when interpreting our results. For instance, MβCD may change the mechanical properties of membranes, which may complicate our explanation of cellular response under mechanical stimulation. To address this, we employed AFM to measure local cell mechanical properties in the presence of or absence of MβCD. We found that cells became stiffer in the presence of MβCD compared with those in the absence of MβCD, which is consistent with previous studies (Byfield et al., 2004). Recent studies suggest that stiffer cells may be less responsive to mechanical signals (Chowdhury et al.), which may partially explain the decreased response to fluid flow we observed in the presence of MβCD. However, other studies demonstrate stiffer cells are more responsive to mechanical signals (Salvi et al., 2009). In addition, we did not detect a decreased cyclooxygenase-2 response to fluid flow (data not shown here) in the presence of MβCD, which is independent of the calcium signaling (Saunders et al., 2003). Thus, we believe that changes in the mechanical properties of cells in the presence of MβCD do not fully explain the decreased mechanosensitivity we observed in the presence of MβCD. The most likely explanation for the decreased mechanosensitivity is that MβCD disrupts membrane integrity, disconnects membrane and cytoskeleton, reduces the strength of fluid shear stress transmitted from GPI-anchored proteins to cytoskeleton, and decreases vesicular ATP releases. More detailed mechanistic studies are under investigation in our laboratory to further address this issue.
In summary, we demonstrated that cholesterol depletion inhibits fluid flow induced ATP releases from MC3T3-E1 osteoblastic cells, and subsequently reduces fluid flow induced Ca2+i mobilization and ERK1/2 phosphorylation, suggesting cholesterol in cell membranes may be involved in fluid flow induced calcium signaling pathways. In addition, we showed that decreasing GPI-anchored proteins by overexpressing GPI-PLD in osteoblastic cells inhibits fluid flow induced Ca2+i mobilization and ERK1/2 phosphorylation, suggesting GPI-anchored proteins in cell membranes may serve as an transducer to transmit fluid shear stress to biochemical responses (e.g. activating calcium signaling pathways). Taken together, our studies demonstrate a possible molecular mechanism related to cell membrane integrity by which fluid flow induced shear stress initiates osteoblastic responses. The understanding of the molecular mechanisms underlying fluid flow induced calcium signaling pathways in osteoblastic cells will provide guidance in developing novel therapeutic approaches to various bone diseases, such as disuse osteopenia and age related osteoporosis.
Acknowledgments
This work was supported by National Institutes of Health Grant AR054851 (to J.Y.) and the Pennsylvania Department of Health Tobacco Settlement Funds. We greatly appreciate the gift of the full-length 3.4-kb cDNA for mouse GPI-PLD in pKS+ plasmid from Dr. Mark A. Deeg (Indiana University).
References
- Awasthi-Kalia M, Schnetkamp PP, Deans JP. Differential effects of filipin and methyl-beta-cyclodextrin on B cell receptor signaling. Biochem Biophys Res Commun. 2001;287(1):77–82. doi: 10.1006/bbrc.2001.5536. [DOI] [PubMed] [Google Scholar]
- Boarder MR, Hourani SM. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol Sci. 1998;19(3):99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- Byfield FJ, Aranda-Espinoza H, Romanenko VG, Rothblat GH, Levitan I. Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophys J. 2004;87(5):3336–3343. doi: 10.1529/biophysj.104.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SJ, Tzeng CR, Lee YH, Tai CJ. Extracellular ATP activates the PLC/PKC/ERK signaling pathway through the P2Y2 purinergic receptor leading to the induction of early growth response 1 expression and the inhibition of viability in human endometrial stromal cells. Cell Signal. 2008;20(7):1248–1255. doi: 10.1016/j.cellsig.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Chichili GR, Rodgers W. Cytoskeleton-membrane interactions in membrane raft structure. Cell Mol Life Sci. 2009;66(14):2319–2328. doi: 10.1007/s00018-009-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhik SA, Huang Z, Gorbunov VV, Myshkin NK, Tsukruk VV. Micromechanical properties of elastic polymeric materials as probed by scanning force microscopy. Langmuir. 1998;14(10):2606–2609. [Google Scholar]
- Chowdhury F, Na S, Li D, Poh YC, Tanaka TS, Wang F, Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 9(1):82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392(6679):933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Ferraro JT, Daneshmand M, Bizios R, Rizzo V. Depletion of plasma membrane cholesterol dampens hydrostatic pressure and shear stress-induced mechanotransduction pathways in osteoblast cultures. Am J Physiol Cell Physiol. 2004;286(4):C831–839. doi: 10.1152/ajpcell.00224.2003. [DOI] [PubMed] [Google Scholar]
- Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20(1):41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31(11):969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DL, McAllister TN, Frangos JA. Fluid Flow Stimulates Rapid and Continuous Release Of Nitric Oxide In Osteoblasts. American Journal of Physiology - Endocrinology & Metabolism. 1996;34(1):E 205–E 208. doi: 10.1152/ajpendo.1996.271.1.E205. [DOI] [PubMed] [Google Scholar]
- Kam AY, Liu AM, Wong YH. Formyl peptide-receptor like-1 requires lipid raft and extracellular signal-regulated protein kinase to activate inhibitor-kappa B kinase in human U87 astrocytoma cells. J Neurochem. 2007;103(4):1553–1566. doi: 10.1111/j.1471-4159.2007.04876.x. [DOI] [PubMed] [Google Scholar]
- Kannan KB, Barlos D, Hauser CJ. Free cholesterol alters lipid raft structure and function regulating neutrophil Ca2+ entry and respiratory burst: correlations with calcium channel raft trafficking. J Immunol. 2007;178(8):5253–5261. doi: 10.4049/jimmunol.178.8.5253. [DOI] [PubMed] [Google Scholar]
- Katoh SY, Kamimoto T, Yamakawa D, Takakura N. Lipid rafts serve as signaling platforms for Tie2 receptor tyrosine kinase in vascular endothelial cells. Exp Cell Res. 2009;315(16):2818–2823. doi: 10.1016/j.yexcr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Li J, Duncan RL, Burr DB, Turner CH. L-type calcium channels mediate mechanically induced bone formation in vivo. J Bone Miner Res. 2002;17(10):1795–1800. doi: 10.1359/jbmr.2002.17.10.1795. [DOI] [PubMed] [Google Scholar]
- Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392(6679):936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104(33):13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KJ, Hepworth MR, Raikwar NS, Deeg MA, Sevlever D. Effect of glycosylphosphatidylinositol (GPI)-phospholipase D overexpression on GPI metabolism. Biochem J. 2004;378(Pt 2):641–648. doi: 10.1042/BJ20031326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R, Marshall CJ. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]
- Metz CN, Brunner G, Choi-Muira NH, Nguyen H, Gabrilove J, Caras IW, Altszuler N, Rifkin DB, Wilson EL, Davitz MA. Release of GPI-anchored membrane proteins by a cell-associated GPI-specific phospholipase D. EMBO J. 1994;13(7):1741–1751. doi: 10.1002/j.1460-2075.1994.tb06438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey ER, Baylink DJ. Inhibition of bone formation during space flight. Science. 1978;201(4361):1138–1141. doi: 10.1126/science.150643. [DOI] [PubMed] [Google Scholar]
- Motoyama K, Toyodome H, Onodera R, Irie T, Hirayama F, Uekama K, Arima H. Involvement of lipid rafts of rabbit red blood cells in morphological changes induced by methylated beta-cyclodextrins. Biol Pharm Bull. 2009;32(4):700–705. doi: 10.1248/bpb.32.700. [DOI] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115(4):377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Addison WN, Kaartinen MT. ATP-mediated mineralization of MC3T3-E1 osteoblast cultures. Bone. 2007;41(4):549–561. doi: 10.1016/j.bone.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur J Biochem. 1989;186(1–2):17–22. doi: 10.1111/j.1432-1033.1989.tb15171.x. [DOI] [PubMed] [Google Scholar]
- Papanikolaou A, Papafotika A, Murphy C, Papamarcaki T, Tsolas O, Drab M, Kurzchalia TV, Kasper M, Christoforidis S. Cholesterol-dependent lipid assemblies regulate the activity of the ecto-nucleotidase CD39. J Biol Chem. 2005;280(28):26406–26414. doi: 10.1074/jbc.M413927200. [DOI] [PubMed] [Google Scholar]
- Park H, Go YM, Darji R, Choi JW, Lisanti MP, Maland MC, Jo H. Caveolin-1 regulates shear stress-dependent activation of extracellular signal-regulated kinase. Am J Physiol Heart Circ Physiol. 2000;278(4):H1285–1293. doi: 10.1152/ajpheart.2000.278.4.H1285. [DOI] [PubMed] [Google Scholar]
- Pavalko FM, Chen NX, Turner CH, Burr DB, Atkinson S, Hsieh YF, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. American Journal of Physiology. 1998;275(6 Pt 1):C1591–1601. [PubMed] [Google Scholar]
- Ponik SM, Pavalko FM. Formation of focal adhesions on fibronectin promotes fluid shear stress induction of COX-2 and PGE2 release in MC3T3-E1 osteoblasts. J Appl Physiol. 2004;97(1):135–142. doi: 10.1152/japplphysiol.01260.2003. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal. 2009;5(4):433–446. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci. 2005;118(Pt 6):1099–1102. doi: 10.1242/jcs.01681. [DOI] [PubMed] [Google Scholar]
- Reich KM, McAllister TN, Gudi S, Frangos JA. Activation of G proteins mediates flow-induced prostaglandin E2 production in osteoblasts. Endocrinology. 1997;138(3):1014–1018. doi: 10.1210/endo.138.3.4999. [DOI] [PubMed] [Google Scholar]
- Riddle RC, Taylor AF, Genetos DC, Donahue HJ. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Physiol Cell Physiol. 2006;290(3):C776–784. doi: 10.1152/ajpcell.00082.2005. [DOI] [PubMed] [Google Scholar]
- Riddle RC, Taylor AF, Rogers JR, Donahue HJ. ATP Release Mediates Fluid Flow-Induced Proliferation of Human Bone Marrow Stromal Cells. J Bone Miner Res. 2007 doi: 10.1359/jbmr.070113. [DOI] [PubMed] [Google Scholar]
- Rubin J, Murphy TC, Zhu L, Roy E, Nanes MS, Fan X. Mechanical strain differentially regulates endothelial nitric-oxide synthase and receptor activator of nuclear kappa B ligand expression via ERK1/2 MAPK. J Biol Chem. 2003;278(36):34018–34025. doi: 10.1074/jbc.M302822200. [DOI] [PubMed] [Google Scholar]
- Salvi JD, Lim JY, Donahue HJ. Finite Element Analysis of Fluid Flow Conditions in Cell Culture. Tissue Eng Part C Methods. 2009 doi: 10.1089/ten.tec.2009.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders MM, You J, Zhou Z, Li Z, Yellowley CE, Kunze EL, Jacobs CR, Donahue HJ. Fluid flow-induced prostaglandin E(2) response of osteoblastic ROS 17/2.8 cells is gap junction-mediated and independent of cytosolic calcium. Bone. 2003;32(4):350–356. doi: 10.1016/s8756-3282(03)00025-5. [DOI] [PubMed] [Google Scholar]
- Scallon BJ, Fung WJ, Tsang TC, Li S, Kado-Fong H, Huang KS, Kochan JP. Primary structure and functional activity of a phosphatidylinositol-glycan-specific phospholipase D. Science. 1991;252(5004):446–448. doi: 10.1126/science.2017684. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9(9):726–735. [PubMed] [Google Scholar]
- Sessions ND, Halloran BP, Bikle DD, Wronski TJ, Cone CM, Morey-Holton E. Bone response to normal weight bearing after a period of skeletal unloading. Am J Physiol. 1989;257(4 Pt 1):E606–610. doi: 10.1152/ajpendo.1989.257.4.E606. [DOI] [PubMed] [Google Scholar]
- Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91(9):769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Bourguignon LY. CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp Cell Res. 2004;295(1):102–118. doi: 10.1016/j.yexcr.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Inanami O, Ohta T, Matsuda A, Kuwabara M. Lipid raft disruption prevents apoptosis induced by 2-chloro-2'-deoxyadenosine (Cladribine) in leukemia cell lines. Leuk Res. 2006;30(12):1555–1561. doi: 10.1016/j.leukres.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Tanikawa R, Tanikawa T, Okada Y, Nakano K, Hirashima M, Yamauchi A, Hosokawa R, Tanaka Y. Interaction of galectin-9 with lipid rafts induces osteoblast proliferation through the c-Src/ERK signaling pathway. J Bone Miner Res. 2008;23(2):278–286. doi: 10.1359/jbmr.071008. [DOI] [PubMed] [Google Scholar]
- van Zanten TS, Cambi A, Koopman M, Joosten B, Figdor CG, Garcia-Parajo MF. Hotspots of GPI-anchored proteins and integrin nanoclusters function as nucleation sites for cell adhesion. Proc Natl Acad Sci U S A. 2009;106(44):18557–18562. doi: 10.1073/pnas.0905217106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- Yang B, Oo TN, Rizzo V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. FASEB J. 2006;20(9):1501–1503. doi: 10.1096/fj.05-5359fje. [DOI] [PubMed] [Google Scholar]
- You J, Jacobs CR, Steinberg TH, Donahue HJ. P2Y purinoceptors are responsible for oscillatory fluid flow-induced intracellular calcium mobilization in osteoblastic cells. J Biol Chem. 2002;277(50):48724–48729. doi: 10.1074/jbc.M209245200. [DOI] [PubMed] [Google Scholar]
- You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. Osteopontin Gene Regulation by Oscillatory Fluid Flow via Intracellular Calcium Mobilization and Activation of Mitogen-activated Protein Kinase in MC3T3-E1 Osteoblasts. J Biol Chem. 2001;276(16):13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- You J, Yellowley CE, Donahue HJ, Zhang Y, Chen Q, Jacobs CR. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng. 2000;122(4):387–393. doi: 10.1115/1.1287161. [DOI] [PubMed] [Google Scholar]
- Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116(5):1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]