Abstract
Objective
To characterize prenatal and delivery care in an urban African setting.
Methods
The Zambia Electronic Perinatal Record System (ZEPRS) was implemented to record demographic characteristics, past medical and obstetric history, prenatal care, and delivery and newborn care for pregnant women across 25 facilities in the Lusaka public health sector.
Results
From June 1, 2007, to January 31, 2010, 115 552 pregnant women had prenatal and delivery information recorded in ZEPRS. Median gestation age at first prenatal visit was 23 weeks (interquartile range [IQR] 19–26). Syphilis screening was documented in 95 663 (83%) pregnancies: 2449 (2.6%) women tested positive, of whom 1589 (64.9%) were treated appropriately. 111 108 (96%) women agreed to HIV testing, of whom 22% were diagnosed with HIV. Overall, 112 813 (98%) of recorded pregnancies resulted in a live birth, and 2739 (2%) in a stillbirth. The median gestational age was 38 weeks (IQR 35–40) at delivery; the median birth weight of newborns was 3000 g (IQR 2700–3300 g).
Conclusion
The results demonstrate the feasibility of using a comprehensive electronic medical record in an urban African setting, and highlight its important role in ongoing efforts to improve clinical care.
Keywords: Electronic medical record, Newborn care, Perinatal audit, Prenatal care, Sub-Saharan Africa
1. Introduction
Pregnancy, delivery, and the early postpartum period are associated with high health risks for many women in low-income countries [1]. Perinatal audit—the cyclic process in which causes of adverse outcomes are identified and targeted interventions are implemented to address them—has been shown to improve outcomes across various settings, including those in Sub-Saharan Africa [2,3]. Although favorable outcomes have been achieved with only modest investments in auditing systems (e.g. weekly hospital review panels), such approaches may be insufficient in more complex medical systems. In many African settings, for example, patients carry their own medical records—an arrangement that challenges the reliable measurement of facility-based outcomes. At the facility level, visits are often documented only via registers, which can be burdensome to review systematically and might contain only basic medical information [4]. The data collation process can also be inaccurate and misrepresent actual site performance [5].
In these situations, the use of electronic medical records (EMRs) holds promise [6,7]. Routinely collected data can be queried to generate reports about site or provider performance, and to identify common characteristics of adverse perinatal events. Such systems can also enhance the standardization, quality, and completeness of medical documentation, which is essential to ongoing quality care [8]. Unfortunately, such sophisticated resources are rarely available in resource-constrained settings [9], where the occurrence of most adverse obstetric outcomes is highest.
Like most Sub-Saharan countries, Zambia is affected by shortages in healthcare providers [10]. The population of Lusaka, the capital city of Zambia, was estimated at 1.1 million in the 2000 census [11]; however, there has been considerable growth and expansion in the past decade. HIV prevalence in the prenatal population is estimated at 21%, a figure that has declined in recent years [12]. Estimates of maternal mortality rates in Lusaka are high and consistent with other parts of the country [13,14]. In such settings, innovative approaches to perinatal audit are vital for addressing the multitude of challenges associated with high disease burden and resource constraints [13,14].
The aim of the present study was to test the design and implementation of an EMR system, the Zambia Electronic Perinatal Record System (ZEPRS), in Lusaka, an urban African setting.
2. Materials and methods
In the present cross-sectional study, data collected from pregnant women seeking prenatal care and delivery services in the Lusaka public health sector were entered in the ZEPRS database. The analysis cohort included pregnancies from June 1, 2007, to January 31, 2010, for which medical information was collected during prenatal care (for at least one visit) and around the time of delivery. The start date of the cohort was set to June 1, 2007, to maximize data capture for enrolled patients; before this date, not all sites had fully implemented the ZEPRS data system. Use of these routinely collected data was approved by the ethical review committees at the University of Zambia (Lusaka, Zambia) and the University of Alabama at Birmingham (Birmingham, AL, ACCEPTED MANUSCRIPT USA).
The design of ZEPRS began in 2004 with funding from the Bill and Melinda Gates Foundation. A team of Zambian and expatriate experts was formed from the University Teaching Hospital, Lusaka Urban District Health Management Team, Centre for Infectious Disease Research in Zambia, University of Alabama at Birmingham, and RTI International. A careful field assessment of Lusaka’s public sector was performed and, on the basis of this evaluation, the software framework for ZEPRS was developed.
The ZEPRS application employs “real-time” data entry, whereby registered users (e.g. nurses, midwives, and clerical staff) directly enter information from each patient visit at the point of care (Table 1). Pre-programmed “checks” prevent the entry of nonsense values; abnormal (but plausible) findings trigger alerts, so that providers can either correct the data entry (if a mistake was made) or intervene as needed. To ensure that ZEPRS was fully integrated into the Zambian Ministry of Health’s nationwide reporting system (the “Health Management Information System”), automated programs were developed to generate and print pre-populated prenatal records, registers, and reports. This feature allows personnel to dedicate more time to the care of individual patients and less time to the lengthy process of weekly and monthly tally counts. To ensure continuity of care between pregnancies, we worked closely with the Ministry of Health to implement standard identification numbers linked to the patient, rather than to each individual pregnancy. ZEPRS has the provision for entering subsequent pregnancies; data on past pregnancies are archived but accessible. At time of birth, the system generates unique identification numbers specifically for infants, and these are linked to the mother’s record. This ensures a continuity of care for these children and links infant outcomes to the mother’s prenatal course.
Table 1.
Medical information captured by ZEPRS
| Category | Sample variables collected |
|---|---|
| Current pregnancy | Sample variables collected Estimated due date (calculated from last menstrual period, fundal height, and/or ultrasound); singleton or multiple gestation; pregnancy complications; prior history of HIV testing; maternal immunizations received; use of insecticide-treated bed net |
| Past obstetrical history | Gravidity; parity; date and site of past delivery; mode of delivery; duration of labor; pregnancy complications (e.g. infection, eclampsia, hemorrhage); sex of infant; infant birth weight; infant’s current vital status |
| Past medical history | Review of symptoms; previous medical conditions, including hypertension, diabetes, asthma, heart disease, kidney disease, liver disease, thyroid disease, tuberculosis, HIV, and recent malaria; history of surgery, particularly pelvic surgery; drug allergies; past contraception use |
| Routine prenatal visit | Estimated gestational age; fundal height measurement; fetal lie, presentation, and descent; fetal heart rate; blood pressure measurement; maternal weight; full physical examination; HIV counseling and testing results (CD4 screening and result for those with HIV diagnosis); antiretroviral drug regimen prescribed; syphilis screening and test result; penicillin treatment administered; partner screening and treatment for HIV and syphilis; administration/dispensation of routinely used drugs in pregnancy (e.g. iron, folate, mebendazole, sulfadoxine/pyrimethamine); results from routinely performed laboratory tests (e.g. hemoglobin, urine analysis, malaria blood smear) |
| Labor and delivery | Estimated gestational age; review of symptoms including contractions, fetal movement, vaginal bleeding, and ruptured membranes; fundal height measurement; fetal lie, presentation, and descent; blood pressure measurement; full physical examination; fetal heart rate; vaginal examination, including cervical dilation, effacement, and position; clinical pelvimetry measurements; medications used to induce or augment labor; mode of delivery; complications of labor (e.g. chorioamnionitis, prenatal or postpartum hemorrhage, pre-eclampsia or eclampsia, dystocia); date and time of birth; infant sex; infant birth weight; Apgar scores at 1, 5, and 10 minutes; neonatal resuscitation measurement used; neonatal physical examination; infant dosing for HIV prophylaxis |
Because patients frequently move from site to site during prenatal care and for delivery, a network-based system with central data storage was developed [15,16]. A city-wide wireless network was implemented across Lusaka, so that all medical information can be maintained from a central server and retrieved regardless of location. Individual patient data are secured via Lightweight Extensible Authentication Protocol (LEAP; Cisco Systems; San Jose, CA, USA), coupled with a Radius server and WEP 128-bit encryption on the wireless links. Other infrastructure investments include computers for entering and accessing patient record information; internet telephony for inter-facility communication; back-up battery supplies for short-term power outages; and a back-up “mirrored” server in case of system failure.
To maximize data security, all users undergo training in patient confidentiality and must sign confidentiality agreements before they are registered with individualized logins and passwords. The platform requires password confirmation at certain points in data entry and has automatic logout provisions when the application is idle for more than 10 minutes. System security is based on commercially available anti-virus software that is updated regularly at the facility level. All staff are trained in the regular use of ZEPRS. Workshops range from 2 to 10 days and focus on basic (e.g. hardware orientation, word processing) to application-specific skills (e.g. data entry, record retrieval).
Data quality is assessed regularly. On a monthly basis, duplicate entries and internal data inconsistencies are flagged. Where possible, these issues are resolved centrally by a data coordinator; issues that require further investigation are reviewed at the site by clerical staff and district nurses. Site staff also perform monthly data audits comparing aggregated ZEPRS data to statistics collected by government clinic registers. When significant discrepancies arise, more comprehensive audits are performed to resolve discrepancies between the 2 data sources.
Field testing of ZEPRS began in November 2005 at 3 sites in Lusaka. On the basis of user feedback, several innovations were made before the broader implementation of the system. At larger prenatal clinics, for example, the high patient-to-provider ratio prohibited real-time entry of medical information by health providers alone. Instead, dedicated clerical staff were hired to support data entry at specific points within the clinic (e.g. during patient registration). A back-up system using paper documentation was developed for periods of prolonged power and/or network outages; these records were then entered into ZEPRS when the system was restored. With these modifications, ZEPRS was expanded in a phased approach. By June 2007, it had been implemented across 25 Lusaka prenatal care clinics (23 government primary care clinics, 1 private facility, and the University Teaching Hospital), 13 of which are delivery centers (11 government sites, 1 private facility, and the University Teaching Hospital).
To assess the utility of ZEPRS, routinely collected demographic and obstetric characteristics of the study cohort were analyzed, including aggregate details regarding prenatal care, labor, and delivery. Continuous variables were reported by median and interquartile range (IQR) and then categorized. Estimated gestation age was calculated by last menstrual period for pregnancies that were less than 20 weeks at time of enrollment. For pregnancies at or more than 20 weeks at enrollment, both last menstrual period and physical examination (i.e., fundal height) were used; if these 2 methods yielded gestational ages that were within 3 weeks of each other, the date based on the last menstrual period was used. If not, then the fundal height-derived gestational age was used. Histograms were developed to show the distribution of gestational age at enrollment and delivery, and to describe birth weight. Pregnancy losses were considered spontaneous abortions if they were 28 weeks or less, and stillbirths if they were more than 28 weeks [17]. Descriptive analyses were performed with SAS version 9.13 (SAS Institute, Cary, NC, USA).
3. Results
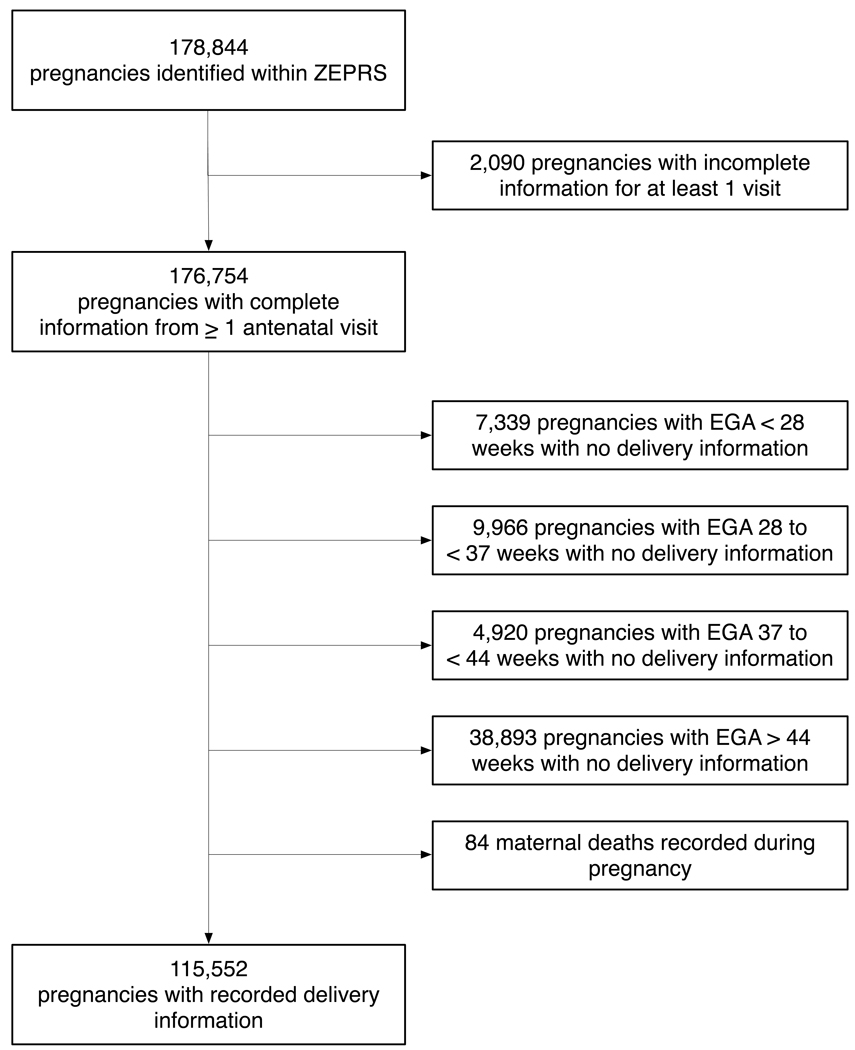
The expansion of ZEPRS across the Lusaka district began in February 2006 via a phased approach and reached full implementation by June 2007. From June 1, 2007, to January 31, 2010, a total of 178 844 pregnancies had been recorded during prenatal care, and 116 675 deliveries had been entered into the database. In the present study, the descriptive analysis is limited to pregnancies with complete medical information recorded for delivery and at least 1 prenatal visit.
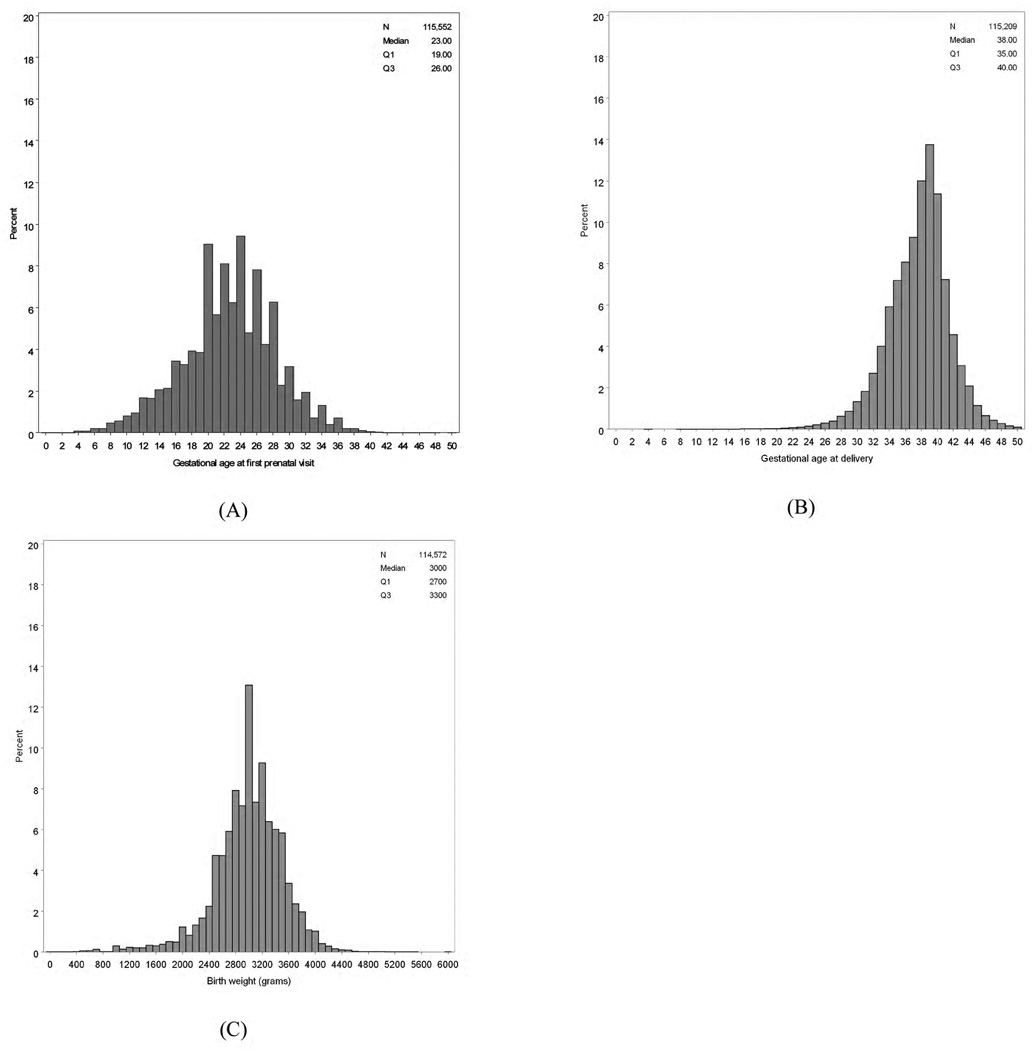
Of the 176 754 pregnancies with complete information for at least 1 prenatal visit, 61 202 (34.6%) had no delivery information—many of them were still in early pregnancy—and were thus excluded from the analysis. Of the 116 675 deliveries, 1123 (1.0%) had no record of prenatal care in ZEPRS and were similarly excluded (Figure 1). Among the 115 552 pregnancies in the analysis cohort, 1088 (1.0%) represented repeat pregnancies entered into ZEPRS—that is, either the second (n=1086) or the third (n=2) pregnancy for the same woman. Median gestation age at the first prenatal visit was 23 weeks (IQR 19–26; Figure 2A). Other demographic and medical characteristics at time of enrollment are given in Table 2.
Figure 1.
Description of the study cohort with prenatal care and delivery information entered into the ZEPRS from June 1, 2007, to January 31, 2010. * The 38 893 pregnancies with no delivery information after 44 weeks represent non-institutional delivery, institutional delivery outside the ZEPRS-supported sites, and losses to follow-up. All gestational ages are based on dating information at the enrollment visit. EGA, estimated gestational age.
Figure 2.
Distribution of estimated gestation age and birth weight among pregnancies entered into the ZEPRS from June 1, 2007, to January 31, 2010. (A) Estimated gestation age at enrollment into prenatal care. (B) Estimated gestation age at time of delivery. (C) Birth weight at delivery.
Table 2.
Maternal and obstetric characteristics at enrollment for 115,552 pregnancies at sites supported by ZEPRS from June 1, 2007, to January 31, 2010
| Characteristic | No. of pregnancies with data recorded |
Value a |
|---|---|---|
| Age, years | 115 552 | 25 (21–29) |
| <15 years | 515 (0.4) | |
| 15–19 years | 19 827 (17.2) | |
| 20–24 years | 37 113 (32.1) | |
| 25–29 years | 30 892 (26.7) | |
| 30–34 years | 18 607 (16.1) | |
| 35–39 years | 7227 (6.3) | |
| ≥40 years | 1371 (1.2) | |
| Education | 99 655 | |
| None | 4070 (4.1) | |
| Primary | 43 580 (43.7) | |
| Secondary | 47 883 (48.0) | |
| Tertiary | 4122 (4.1) | |
| Marital status | 111 140 | |
| Single | 10 053 (9.0) | |
| Married or cohabitating | 100 542 (90.5) | |
| Divorced or widowed | 545 (0.5) | |
| Number of prior pregnancies | 115 552 | 1 (0–3) |
| None | 33 669 (29.1) | |
| 1–2 | 29 221 (25.3) | |
| 3 or more | 30 377 (26.3) | |
| Prior history of cesarean delivery | 81 883 | 3079 (3.8) |
| Prior history of spontaneous abortion | 115 552 | 2738 (2.4) |
| Prior history of terminated pregnancy | 115 552 | 88 (0.1) |
| Prior history of stillbirth | 81 883 | 2181 (2.7) |
| Previous diagnosis of hypertension | 98 584 | 1407 (1.4) |
| Previous diagnosis of diabetes mellitus | 98 980 | 92 (0.1) |
| Previous diagnosis of heart disease | 98 050 | 134 (0.1) |
| Previous diagnosis of HIV | 95 825 | 4226 (4.4) |
| Gestational age at enrollment visit, weeks | 115 552 | 23 (19–26) |
| <20 weeks | 29 390 (25.4) | |
| 20–27 weeks | 64 081 (55.5) | |
| 28–31 weeks | 15 416 (13.3) | |
| 32–35 weeks | 5048 (4.4) | |
| ≥36 weeks | 1617 (1.4) | |
| Weight, kg | 96 772 | 59 (54–66) |
| BMI | 68 631 | 24 (22–26) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters).
Values are given as number (percentage) or median (IQR) unless otherwise indicated.
There were several notable findings across ZEPRS-supported sites (Table 3). Among the 60 657 pregnancies with data for 2 or more clinical visits, 5779 (9.5%) had documented prenatal visits at 2 facilities, whereas only 68 (0.1%) had documented visits at 3 or more facilities. Syphilis screening was documented in 95 663 of 115 552 (82.8%) pregnancies; 2449 (2.6%) of the women tested positive by rapid plasma reagin assay. Only 1589 of these 2449 (64.9%) women had single-dose penicillin treatment—the local standard of care—documented in ZEPRS. The Lusaka district’s policy of “opt-out” HIV testing [18] accomplished high rates of screening: more than 95% agreed to HIV testing, of whom approximately 22% were diagnosed with HIV infection. Nearly 80% (18 928 of 23 932) of women diagnosed with HIV during pregnancy underwent CD4-positive screening for antiretroviral therapy eligibility. Half (9419 of 18 928) of these women met the CD4-positive criteria of less than 350 cells/µL to initiate HIV treatment.
Table 3.
Characteristics of prenatal and delivery care for 115,552 pregnancies at sites supported by the ZEPRS from June 1, 2007, to January 31, 2010
| Characteristic | No. of pregnancies with data recorded |
Value a |
|---|---|---|
| Reported sleeping under insecticide-treated bed net | 98 242 | 40 044 (40.8) |
| Screened for syphilis by RPR assay | 95 663 | |
| Non-reactive | 93 214 (97.4) | |
| Reactive | 2449 (2.6) | |
| Documented treatment for syphilis among those testing RPR-positive | 2449 | 1589 (64.9) |
| Agreed to HIV testing | 115 552 | 111 108 (96.2) |
| HIV test result missing | 111 108 | 313 (0.3) |
| HIV test result reported to patient | 111 108 | 110 795 (99.7) |
| HIV-positive result | 23 932 (21.6) | |
| HIV-negative result | 86 863 (78.6) | |
| Declined HIV testing, known HIV infection | 4444 | 1296 (29.2) |
| CD4 screening among women diagnosed with HIV during pregnancy | 23 932 | 18 928 (79.1) |
| CD4 cell count among HIV-infected women tested | 18 928 | 352 (234–498) |
| ≤200 cells/µL | 3514 (18.6) | |
| 201–350 cells/µL | 5905 (31.2) | |
| 351–500 cells/µL | 4839 (25.6) | |
| >500 cells/µL | 4670 (24.7) | |
| Antiretroviral prophylaxis prescribed among those diagnosed with HIV | 23 850 | |
| None | 3209 (13.5) | |
| Intrapartum nevirapine only | 7988 (33.5) | |
| Short-course zidvoduine and intrapartum nevirapine | 8269 (34.7) | |
| Three-drug combination therapy | 4384 (18.4) | |
| Gestational age in weeks at delivery, median (IQR) | 112 051 | 38 (35–40) |
| <28 weeks | 1558 (1.4) | |
| 28 to <32 weeks | 5344 (4.8) | |
| 32 to <37 weeks | 32 150 (28.7) | |
| 37 to <42 weeks | 61 786 (55.1) | |
| 42 weeks or more | 11 213 (10.0) | |
| Single versus multiple gestation | 115 552 | |
| Singleton | 113 424 (98.2) | |
| Twin gestation | 2092 (1.8) | |
| Triplet or quadruplet gestation | 36 (<0.1) | |
| Timing of ruptured membranes prior to delivery | 65 303 | |
| Same calendar day | 60 635 (92.9) | |
| Earlier calendar day | 4668 (7.1) | |
| Labor induced | 91 663 | 438 (0.5) |
| Mode of delivery | 113 947 | |
| Spontaneous vaginal delivery | 108 255 (95.0) | |
| Cesarean delivery | 4697 (4.1) | |
| Forceps or vacuum extraction | 186 (0.2) | |
| Breech | 809 (0.7) | |
| Pregnancy outcome | 115 552 | |
| Live birth | 112 813 (97.6) | |
| Fresh stillbirth | 1325 (1.1) | |
| Macerated stillbirth | 1414 (1.2) | |
| Apgar score at 1 minute | 106 524 | 9 (8–9) |
| Score <7 | 4416 (4.1) | |
| Apgar score at 5 minute | 103 198 | 9 (9–9) |
| Score <7 | 3409 (3.3) | |
| Birth weight, g | 114 572 | 3000 (2700–3300) |
| <1500 g | 1622 (1.4) | |
| 1500–2499 g | 10 779 (9.4) | |
| 2500–3499 g | 83 314 (72.7) | |
| 3500–3999 g | 16 442 (14.4) | |
| ≥4000 g | 2415 (2.1) |
Abbreviation: RPR, rapid plasma reagin.
Values are given as number (percentage) or median (IQR) unless otherwise indicated.
Overall, 112 813 of 115 552 (97.6%) deliveries resulted in a live birth, and 2739 deliveries were categorized as stillbirth, giving a rate of 24 stillbirths per 1000 deliveries (95% confidence interval, 23–25). Documentation of induced labor was exceedingly rare (n=438, 0.5%). Caesarean delivery was performed in 4697 (4.1%) of women with mode of delivery documented. Median birth weight of the newborn was 3000 g (IQR 2700–3300 g; Figure 2C). Other characteristics of labor and delivery are given in Table 3.
4. Discussion
The present study has demonstrated the successful design and implementation of a comprehensive electronic perinatal record system in an urban African setting. Now fully utilized across the Lusaka public sector, ZEPRS has the capacity for enhanced monitoring and evaluation of prenatal and newborn care. Many notable findings from this descriptive analysis could not be quantified by the previous system of patient-held prenatal records and clinic-based registers.
Much has been written about the challenges inherent to register-based information systems for program monitoring and management. Work by Mate et al. [5] in South Africa, for example, demonstrated gross inconsistencies between monthly tally reports and data audits designed to verify program statistics. In a review of 6 core indicators for prevention of mother-to-child HIV transmission services, independent teams found that monthly reports were complete only 50% of the time and accurate (i.e. within 10% of reconstructed values) only 13% of the time, features that they attributed to poor data collation on site [5]. According to focus groups in South Africa, data collation was viewed as highly burdensome among healthcare providers; few providers routinely used these statistics to inform management decisions at the site level [4]. Similar trends have been noted in other areas of health provision, including adult HIV testing and childhood immunizations [19,20].
By standardizing data collection and linking antecedent medical information to later outcomes, ZEPRS has the capacity for sophisticated queries far out of the reach of previous paper-based systems. Although greater investments in time are needed at the time of data entry, reports can be automated to provide quick updates of site performance. The framework of ZEPRS also permits careful evaluation of health services. Problem areas such as syphilis treatment—estimated at only 65% in the present analysis—are easily identified through regular performance audits and can be used to guide further investigation into potential causes at the health system (e.g. supply interruptions), facility (e.g. staff shortages), or even provider (e.g. clinical oversight) levels. By regularly auditing these clinical indicators, strategies to correct specific deficiencies can be carefully assessed and, where needed, modified. Although promising, such strategies require rigorous and formal evaluation; such work is beyond the scope of the current analysis.
In the present study, the ZEPRS database has been analyzed to provide a well-characterized description of pregnant women and their newborn infants seeking care in Lusaka government clinics. An unexpectedly high proportion of women receiving prenatal care in Lusaka public health clinics appeared to deliver outside the government system, either in private hospitals or at home, or outside the city itself. Of the 176 754 pregnancies with at least 1 prenatal visit entered in ZEPRS, roughly a fifth were at 44 weeks gestation or more and yet had no record of delivery. This is concerning from a clinical perspective, given the known association between non-institutional delivery and poor obstetric outcomes [21]. Such attrition also negatively affects our ability to monitor outcomes at a population level, particularly for rare conditions such as stillbirth and maternal death.
In the present evaluation, digit preferences among health providers were noted in 2 key fields, namely, estimated gestational age at enrollment (for even-numbered weeks; Figure 2A) and birth weight (at 2000 grams and 3000 grams; Figure 2C). This clustering is unlikely to have a significant impact on individual clinical care; however, further investigation is needed to understand the extent of potential entry bias and the quality of ascertained data.
Although the implementation of ZEPRS has been successful, it has not been without challenges. It is possible to cope with short-term power interruptions, but seasonal scheduled “black-outs” by power companies have led to greater reliance on a less efficient paper back-up system. To maximize network stability, a dedicated team has been employed to trouble-shoot problems arising from power interruptions, weather, and hardware malfunctions. An “off-line” mode is planned for the next ZEPRS upgrade, so that data entered during network outages are stored locally and uploaded automatically once the wireless signal is restored. Efficient patient and information flow has required ongoing review and optimization. Full-time data clerks have been stationed at large facilities, where they support entry of medical information. We are also working to link ZEPRS to a web-based laboratory platform, so that users can quickly and easily retrieve missing test results.
The issue of long-term sustainability must also be addressed. At present, ZEPRS is maintained through funding from the Zambian government and international donors, primarily within the context of service provision. A demonstration of the effectiveness and cost-effectiveness of ZEPRS as part of a comprehensive program-monitoring package would be a compelling justification for long-term support. In the meantime, we are working to enhance provider efficiency and to reduce hardware and/or infrastructure costs. The promising operations research framework provided by ZEPRS has also been successfully leveraged to fund ongoing development and supplement maintenance costs. Ultimately, a key factor to the long-term success of EMRs is the engagement of local policy-makers. We are encouraged by the Zambian Ministry of Health’s substantial track record with technology-based health innovations.
In summary, the feasibility of a comprehensive EMR in an urban African setting has been demonstrated by the implementation of ZEPRS. In the present study, the platform has been used to characterize the prenatal and newborn populations in Lusaka and to provide basic data regarding patient outcomes. With continued optimization, we see a greater role for this data system in improving healthcare. However, the impact and cost-effectiveness of ZEPRS require formal evaluation to understand better its role in improving maternal and child outcomes.
Acknowledgments
The ZEPRS was designed, developed, and implemented through support from the Bill and Melinda Gates Foundation. Additional funding was provided by Novartis Pharma AG to evaluate outcomes ascertainment. Investigator salary or trainee support was provided by the National Institutes of Health (K01-TW06670; D43-TW001035; P30-AI027767) and a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (2007061). The funding agencies had no involvement in study design, data collection, data analysis, or manuscript writing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicts of interest.
References
- 1.Rosenfield A, Maine D. Maternal mortality—a neglected tragedy. Where is the M in MCH? Lancet. 1985;2(8446):83–85. doi: 10.1016/s0140-6736(85)90188-6. [DOI] [PubMed] [Google Scholar]
- 2.Bugalho A, Bergstrom S. Value of perinatal audit in obstetric care in the developing world: a ten-year experience of the Maputo model. Gynecol Obstet Invest. 1993;36(4):239–243. doi: 10.1159/000292637. [DOI] [PubMed] [Google Scholar]
- 3.Pattinson R, Kerber K, Waiswa P, LDay LT, Mussell F, Asiruddin SK, et al. Perinatal mortality audits: counting, accountability, and overcoming challenges in scaling up low- and middle-income countries. Int J Gynaecol Obstet. 2009;107(Suppl 1):S113–S121. S121–S122. doi: 10.1016/j.ijgo.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Garrib A, Stoops N, McKenzie A, Dlamini L, Govender T, Rohde J, et al. An evaluation of the District Health Information System in rural South Africa. S Afr Med J. 2008;98(7):549–552. [PubMed] [Google Scholar]
- 5.Mate KS, Bennett B, Mphatswe W, Barker P, Rollins N. Challenges for routine health system data management in a large public programme to prevent mother-to-child HIV transmission in South Africa. PLoS One. 2009;4(5):e5483. doi: 10.1371/journal.pone.0005483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slagle TA. Perinatal information systems for quality improvement: visions for today. Pediatrics. 1999;103 1 Suppl E:266–277. [PubMed] [Google Scholar]
- 7.Phelan ST. The prenatal medical record: purpose, organization and the debate of print versus electronic. Obstet Gynecol Clin North Am. 2008;35(3):355–368. doi: 10.1016/j.ogc.2008.06.001. vii. [DOI] [PubMed] [Google Scholar]
- 8.Haberman S, Feldman J, Merhi ZO, Markenson G, Cohen W, Minkoff H. Effect of clinical-decision support on documentation compliance in an electronic medical record. Obstet Gynecol. 2009;114(2 Pt 1):311–317. doi: 10.1097/AOG.0b013e3181af2cb0. [DOI] [PubMed] [Google Scholar]
- 9.Williams F, Boren SA. The role of the electronic medical record (EMR) in care delivery development in developing countries: a systematic review. Inform Prim Care. 2008;16(2):139–145. doi: 10.14236/jhi.v16i2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schatz JJ. Zambia's health-worker crisis. Lancet. 2008;371(9613):638–639. doi: 10.1016/s0140-6736(08)60287-1. [DOI] [PubMed] [Google Scholar]
- 11.Zambia Central Statistical Office. [Accessed 2010];2000 Census of Population and Housing: November 2003. http://www.zamstats.gov.zm. http://www.zamstats.gov.zm/media/sum_rpt.pdf. Published 2000.
- 12.Stringer EM, Chintu NT, Levy JW, Sinkala M, Chi BH, Muyanga J, et al. Declining HIV prevalence among young pregnant women in Lusaka, Zambia. Bull World Health Organ. 2008;86(9):697–702. doi: 10.2471/BLT.07.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Central Statistical Office [Zambia], Central Board of Health [Zambia], ORC Macro. Zambia Demographic and Health Survey 2001–2002. www.afro.who.int. http://www.afro.who.int/index.php?option=com_docman&task=doc_download&gid=2539. Published 2005.
- 14.Ahmed Y, Mwaba P, Chintu C, Grange JM, Ustianowski A, Zumla A. A study of maternal mortality at the University Teaching Hospital, Lusaka, Zambia: the emergence of tuberculosis as a major non-obstetric cause of maternal death. Int J Tuberc Lung Dis. 1999;3(8):675–680. [PubMed] [Google Scholar]
- 15.Berkowicz DA, Chueh HC, Barnett GO. Design considerations in migrating an obstetrics clinical record to the Web. Proc AMIA Annu Fall Symp. 1997:754–758. [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser HS, Biondich P, Moodley D, Choi S, Mamlin BW, Szolovits P. Implementing electronic medical record systems in developing countries. Inform Prim Care. 2005;13(2):83–95. doi: 10.14236/jhi.v13i2.585. [DOI] [PubMed] [Google Scholar]
- 17.Stanton C, Lawn JE, Rahman H, Wilczynska-Ketende K, Hill K. Stillbirth rates: delivering estimates in 190 countries. Lancet. 2006;367(9521):1487–1494. doi: 10.1016/S0140-6736(06)68586-3. [DOI] [PubMed] [Google Scholar]
- 18.Stringer EM, Sinkala M, Stringer JS, Mzyece E, Makuka I, Goldenberg RL, et al. Prevention of mother-to-child transmission of HIV in Africa: successes and challenges in scaling-up a nevirapine-based program in Lusaka, Zambia. AIDS. 2003;17(9):1377–1382. doi: 10.1097/01.aids.0000060395.18106.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otwombe KN, Wanyungu J, Nduku K, Taegtmeyer M. Improving national data collection systems from voluntary counselling and testing centres in Kenya. Bull World Health Organ. 2007;85(4):315–318. doi: 10.2471/BLT.06.033712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronveaux O, Rickert D, Hadler S, Groom H, Lloyd J, Bchir A, et al. The immunization data quality audit: verifying the quality and consistency of immunization monitoring systems. Bull World Health Organ. 2005;83(7):503–510. [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell OM, Graham WJ. Strategies for reducing maternal mortality: getting on with what works. Lancet. 2006;368(9543):1284–1299. doi: 10.1016/S0140-6736(06)69381-1. [DOI] [PubMed] [Google Scholar]