Abstract
Members of the extracellular signal-regulated kinase (ERK) family may have distinct roles in the development of cell injury and repair, differentiation and carcinogenesis. Here we show, using a synthetic small molecule MEK1/2 inhibitor (U0126) and RNA silencing of ERK1 and 2, comparatively, that ERK2 is critical to transformation and homeostasis of human epithelioid malignant mesotheliomas (MMs), asbestos-induced tumors with a poor prognosis. Whereas MM cell (HMESO) lines stably transfected with shERK1 or shERK2 both exhibited significant decreases in cell proliferation in vitro, injection of shERK2 cells, and not shERK1 cells, into immunocompromised SCID mice showed significant attenuated tumor growth in comparison to shControl cells. Inhibition of migration, invasion, and colony formation occurred in shERK2 MM cells in vitro, suggesting multiple roles of ERK2 in neoplasia. Microarray and qRT-PCR analyses revealed gene expression that was significantly increased (CASP1, TRAF1, FAS) or decreased (SEMA3E, RPS6KA2, EGF, BCL2L1) in shERK2-transfected MM cells in contrast to shControl-transfected MM cells. Most striking decreases were observed in mRNA levels of Semaphorin 3 (SEMA3E), a candidate tumor suppressor gene linked to inhibition of angiogenesis. These studies demonstrate a key role of ERK2 in novel gene expression critical to the development of epithelioid MMs. After injection of sarcomatoid human MM (PPMMill) cells into SCID mice, both shERK1 and shERK2 lines showed significant decreased tumor growth, suggesting heterogeneous effects of ERKs in individual MMs.
Keywords: Asbestos, mesothelioma, extracellular signal regulated kinase (ERK1/2), Mitogen activated protein kinases, gene expression
Introduction
Extracellular signal-regulated kinases (ERKs) are under intense scrutiny because of their association with a number of neuropathies, cancers, and nonmalignant lung diseases1. In addition, they mediate a number of fundamental cell processes including injury and survival, differentiation, cytoskeletal dynamics, and responses to oncogenes and growth factors2, 3. Although at least 8 members of the ERK family have been reported, ERK1 and ERK2 are the most abundant mammalian ERKs and have been studied in combination largely because both commercial antibodies for histochemistry and synthetic inhibitors fail to discriminate between ERK1 and ERK2. Many studies suggest that both ERK1 and ERK2 compete for upstream mitogen-activated protein kinase kinases (MEK1/2) and a multiplicity of substrates 4. Specific substrates, as well as the intensity and duration of ERK signaling, dictate intracellular compartmentalization 5 and downstream consequences of ERK signaling 6.
Increased ERK1/2 phosphorylation (activation) occurs in human malignant mesotheliomas (MMs) 7 and a variety of other cancers. In rodent mesothelial cells in vitro, ERK1/2 is chronically activated after exposure to carcinogenic asbestos fibers, but not after exposure to a number of chemically similar and nonpathogenic particles 8. ERK1/2 phosphorylation also occurs in distal bronchioles, a site of impaction after inhalation of chrysotile asbestos fibers in a murine model of fibrogenesis 9. Recently, we have shown, using a dominant-negative MEK1 construct selectively targeted to the airway epithelium of transgenic mice, that crocidolite asbestos-induced bronchiolar epithelial cell proliferation and gene expression (procollagen 3-a-1, procollagen 1-a-1, and Il-6) linked to lung remodeling are curtailed 10. In concert, these studies suggest that ERK1/2 activation may be linked to the development of asbestos-induced inflammation, cancers (lung cancer and MM) and fibrosis (asbestosis).
ERK activation has been identified as a potential survival pathway in several tumor type, and recent studies suggest that ERKs may also be activated in response to chemotherapeutic drugs 11 or mTOR inhibitors 12. Since ERK1 and ERK2 may have functionally different capacities in cell cycle arrest 13 and replication 14, 15, we focused here on whether ERK1 and ERK2 played critical and distinct roles in the development of MM, a generally incurable cancer exhibiting marked chemoresistance. We show, using human epithelioid MM (HMESO) lines expressing shERK constructs that both ERK1 and ERK2 contribute to the proliferation of human MMs in vitro. However, ERK2 is unique in governing increased migration, invasion and autonomous growth of MM cells in soft agar. Microarray and qRT-PCR analyses of these cell lines revealed that ERK2 inhibition was linked to decreases in mRNA levels of genes related to proliferation, survival and angiogenesis as well as increased expression of apoptosis-related genes. Most importantly, we demonstrate that ERK2 is essential to growth of HMESO MMs in a mouse xenograft model using severe combined immunodeficiency (SCID) mice. In contrast to this epithelioid MM line, a sarcomatoid MM line (PPMMill) showed that neither shERK1 nor shERK2 lines exhibited tumor growth in vivo. Our results suggest that targeting ERK1/2 may be beneficial in therapy of MMs and possibly other asbestos-associated cancers and fibrosis.
Materials and Methods
Human malignant mesothelioma (MM) cells
A sarcomatoid (MO) and 3 epithelioid (ME-12, ME-26 and ME-27) human pleural MM cell lines were obtained from Drs. Luciano Mutti (Maugeri Foundation, Pavia, Italy) and Maurizio Bocchetta (Loyola University, Mayfield, IL), respectively. The epithelioid HMESO cell line was originally characterized by Reale et al 16. The sarcomatoid MM cell line (PPMMill) was obtained from Dr Pass (NYU). Human mesothelial cells, i.e., LP9/TERT-1 (LP9) an hTERT-immortalized cell line that phenotypically and functionally resembles normal human mesothelial cells 17, were obtained from Dr. James Rheinwald (Brigham and Women’s Hospital, Harvard University, Boston MA). All cells were incubated at 37°C in 5% CO2 and grown to approximately 80–90% confluency as described previously 18, 19. The synthetic MEK1/2 inhibitor, U0126, and its inactive analog, U0124, were obtained from Calbiochem (La Jolla, CA) and added to cells at 10 and 20 μM in medium containing ≤0.1% DMSO. Control cultures received medium without compounds but with vehicle (≤0.1% DMSO) alone and were treated identically.
Transfection of ME-26 cells with siRNA
On-Target plus Non-targeting siRNA #1 (scrambled control), and On Target plus SMART pool human ERK1 and ERK2 siRNA (100 nM; Dharmacon, Lafayette, CO) were transfected into ME-26 cells at near confluence using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), following the manufacturer’s protocol. The efficiency of ERK knockdown was determined by qRT-PCR.
Growth curves
Proliferation of MM cells was studied by plating cells at 1 × 105 in 35 mm dishes, followed by trypsinization and determination of viable cell numbers (by Trypan blue exclusion test) using a hemocytometer. In siRNA transfection experiments, cells were plated at equal density and counted after 24, 48 and 72 h of transfection.
Migration assays
Migration of MM cells was assessed using 6-well Transwell polycarbonate filters (Corning Costar Corp., Corning, NY) with an 8-μm pore size. Approximately 2.5 × 104 cells were seeded in the upper chamber of the Transwell inserts before incubation for 72 h at 37°C in serum-free medium. DMEM/F12 medium containing 10% FBS was used as a chemoattractant in the bottom chamber. Cells that did not migrate through the pores of the Transwell inserts were manually removed with a cotton swab. Cells that migrated to the bottom of the membrane were fixed in cold MeOH for 10 min and then stained with 0.01% crystal violet in 20% EtOH for 10 min. Filters then were washed thoroughly in H2O and suspended in 200 μl of 5% acetic acid and 5% MeOH before readings were taken at OD595. siRNA transfected ME-26 cells were seeded in the upper chamber of the Transwell insert after 24 h of transfection.
Invasion assays
Modified Boyden chamber Transwell polycarbonate filters (6.5 mm in diameter, 8 μm pore size, Costar) were coated with 100 μl of Matrigel (BD Biosciences, Bedford, MA) at a 1:20 dilution in serum-free DMEM/F12 medium and were air dried for 24 h. Cells (1 × 105 cells) were then plated on coated inserts in serum-free medium. Medium containing 10% FBS was placed in the bottom wells. After 48–72 h, invading cells adherent to the undersurface of the inserts were fixed and stained as described above before readings were taken at OD595.
Growth in soft agar
Anchorage-independent growth of MM cells was assessed by a colony formation assay in soft agar. The CytoSelect™ Cell Transformation Assay (Cell Biolabs, Inc. San Diego, CA) was used following the manufacturer’s instructions. Briefly, cells were incubated for 6–8 days in a proprietary semi-solid agar medium before colony formation was detected and quantitated microscopically or by MTS assay (at 570 nm) as described by the manufacturer.
Creation of shERK1 and shERK2 stable MM lines
Confluent HMESO or PPMMill cells were transfected with either ERK1 or ERK2, or scrambled control Sure Silencing Plasmids (4 shConstructs per gene per cell line were used) from SA Biosciences (Frederick, MD), using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After selection for 14 days in G418 (400 μg/ml)-containing medium, clones were screened by qRT-PCR for inhibition of ERK mRNA levels as compared to scrambled control (shCon) transfected clones. Two clones from each shERK1 and shERK2 groups were processed by limited dilution to obtain clones in which individual ERKs were inhibited by more than 70% in comparison to shCon clones. Following this procedure, shERK1 and shERK2 clones exhibiting inhibition of >80 % ERK expression were obtained. The experimentally verified shRNA design algorithm (SA Biosciences) assures gene-specificity and efficacy. An advanced specificity search in addition to BLAST built into the algorithm helped to reduce potential off-target effects.
Western blots
To verify reduced ERK protein levels in MM cells transfected with shERK1 or shERK2, Western blots were performed as described previously 8 using antibodies specific to total ERK1/2 (rabbit polyclonal anti-ERK1/2, 1:1000, Cell Signaling Technology, Danvers, MA), and total β-Actin 1:2000 (Abcam, Cambridge, MA). Western blots were quantitated as described previously and normalized to β-actin.
Affymetrix gene profiling
Microarrays were performed on samples from 3 independent experiments as described previously 20. Each of the samples was analyzed on a separate array, i.e., N=3 arrays per MM line (3 independent biological replicates). A Human U133A 2.0 array (Affymetrix, Santa Clara, CA) was scanned twice (Hewlett-Packard GeneArray Scanner), the images overlaid, and the average intensities of each probe cell compiled. Microarray data were analyzed using GeneSifter software (VizX Labs, Seattle, WA). This program used a “t test” for pairwise comparison and a Benjamini-Hochberg test for false discovery rate (FDR 5%) to adjust for multiple comparisons. A 2-fold cut-off limit was used for significance.
Quantitative real time PCR (qRT-PCR)
To confirm decreased RNA expression of selective ERKs in tumors arising from MM cells stably transfected with shERK1 or shERK2 constructs, and to validate microarray profiles of 13 genes, qRT-PCR (TaqMan) was performed as described previously 20. Triplicate assays were performed with RNA samples isolated from at least 3 independent experiments. Fold changes in gene expression were calculated using the delta-delta Ct method using hypoxanthine phosphoribosyl transferase (HPRT1) as the normalization control. The Assay on Demand primers and probes used were purchased from Applied Biosystems (Foster City, CA).
Assessment of human MM tumor development in a mouse xenograft model
HMESO or PPMMill cells stably transfected with either shERK1, shERK2 or shCon were injected into 2 or 4 subcutaneous sites (5 × 106 cells per injection site) on the dorsa of 6 wk-old Fox Chase SCID mice (Jackson Laboratories, Bar Harbor, ME). All mice (N=5–6/group in duplicate experiments) were weighed weekly and examined every other day for morbidity and for tumor growth (measured using a digital caliper). When the largest axis of the tumors in the shCon mice reached 1 cm, all mice were weighed and euthanized as described above, necropsied to determine possible gross metastases, and major organs removed and stored in 4% paraformaldehyde before processing for histopathology. Tumor volumes were calculated using formula (π × long axis × short axis × short axis)/6. Tumors were characterized by one of us (M.C.), using previously described histochemical criteria 21, and hematoxylin and eosin-stained sections examined by K.J.B. Other portions of tumors were frozen in liquid nitrogen or used to establish cell lines. Karyotyping was performed to prove that the tumors appearing in mice were human in origin. All experiments using mice were approved by the Institutional Animal Care and Use Committee at the University of Vermont College of Medicine.
Ki-67 staining and the TUNEL technique
Tumor sections were stained using an antibody to Ki-67, a marker present during all active phases of the cell cycle (G1, S, G2, and mitosis), but absent from resting cells (G0), and the TUNEL technique to demonstrate necrosis or apoptosis as described previously 22.
Statistical analyses
In all in vitro assays, at least 3 independent samples were examined at each time point per group in duplicate experiments. Data were evaluated by ANOVA using the Student Neuman-Keul’s procedure for adjustment of multiple pairwise comparisons between treatment groups or using the non-parametric Kruskal-Wallis and Mann-Whitney tests. Differences with p values ≤0.05 were considered statistically significant. The difference in tumor growth rates between different groups was assessed using a hierarchical regression model to take into account the correlation between repeated measurements on the same tumor and multiple tumors in the same animal. In this analysis, the regression coefficient describing tumor growth is modeled as a function of treatment group as well as random variation due to differences between animals and tumors on the same animal.
Results
The MEK1/2 inhibitor, U0126, inhibits growth of human MM lines in vitro
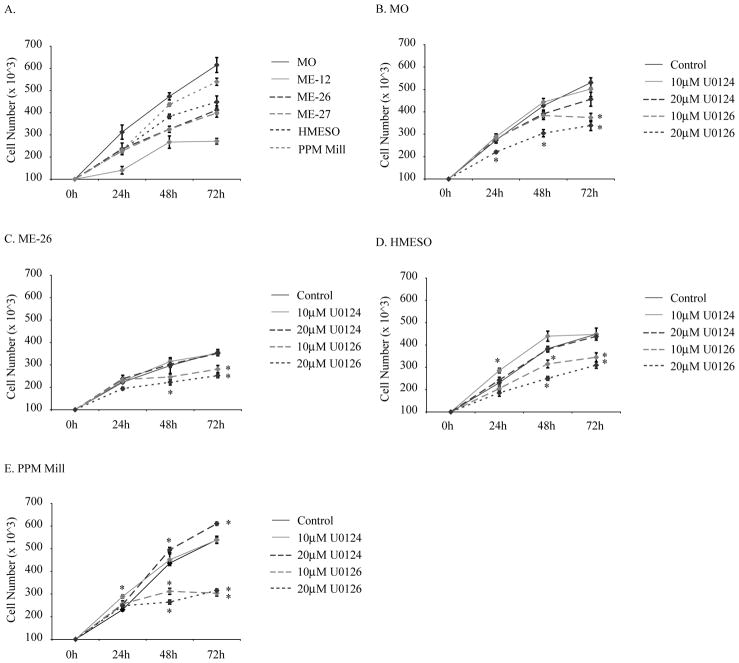
To test whether the ERK pathway plays a causal role in the proliferation of human MM cells, we evaluated the growth of 4 epithelioid MM cell lines (ME-12, ME-26, ME-27, HMESO) and 2 sarcomatoid MM lines (MO, PPMMill) over a 72 h period (Figure 1A). These studies revealed that the sarcomatoid lines had faster growth rates than the epithelioid MM cell lines, an observation that might relate to the overall poorer prognosis of sarcomatoid vs. epithelioid MMs in humans 23. We then focused on inhibition of cell proliferation using U0126, a synthetic, small molecule MEK1/2 inhibitor that blocks the phosphorylation of both ERK1 and ERK2, in contrast to the inactive structurally similar analog, U01246. As shown in Figure 1B-E, only U0126 inhibited growth of MM cell lines in a dose-responsive fashion.
Figure 1.
A small molecule MEK1/2 inhibitor, U0126, inhibits the growth of human MM cells in vitro. (A) Growth curves of 5 human MM lines. (B) Growth of MO line. (C) ME-26 line. (D) HMESO line. (E) PPMMill line in the presence of U0126 or U0124, its inactive analog. Results are expressed as Mean +/− SEM. *=p<0.05 in comparison to untreated solvent control group at the same time point.
U0126 inhibits migration and invasion of human MM lines in vitro
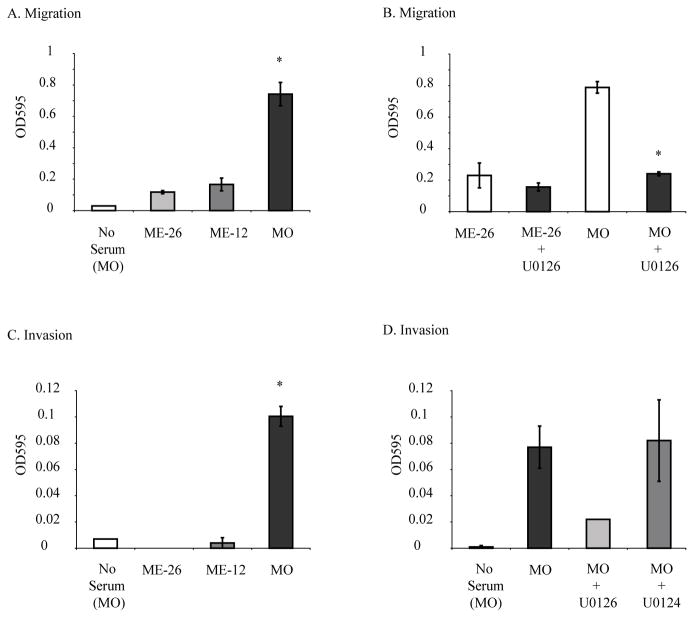
We next evaluated the effects of U0126 on migration and invasion of MM cells in vitro to determine the role of the ERK1/2 pathway in these processes. These studies revealed that the MO sarcomatoid line exhibited the most striking increases in migration and invasion (p<0.05) (Figure 2A, C). Studies showed that both migration and invasion were diminished (p<0.05) (Figure 2B, D) by U0126 (20 μM) whereas the inactive U0124 compound (20 μM) had no effects (Figure 2D).
Figure 2.
A small molecule MEK1/2 inhibitor U0126 inhibits migration and invasion of human MM cells in vitro. (A) A transwell insert model shows significantly increased migration of the sarcomatoid MO line in comparison to other MM lines. (B) The MEK1/2 inhibitor, U0126, inhibits migration of the MO line. (C) Increased invasion of the MO line using a transwell insert coated with Matrigel. (D) The MEK1/2 inhibitor, U0126 (20 μM) inhibits invasion of MO cells, whereas its inactive analog, U0124 (20 μM) is ineffective. Results are expressed as Mean +/− SEM. *=p<0.05 in comparison to untreated solvent control group.
Selective inhibition of ERK1 or ERK2 expression is observed in human MM cell lines stably transfected with shERK constructs
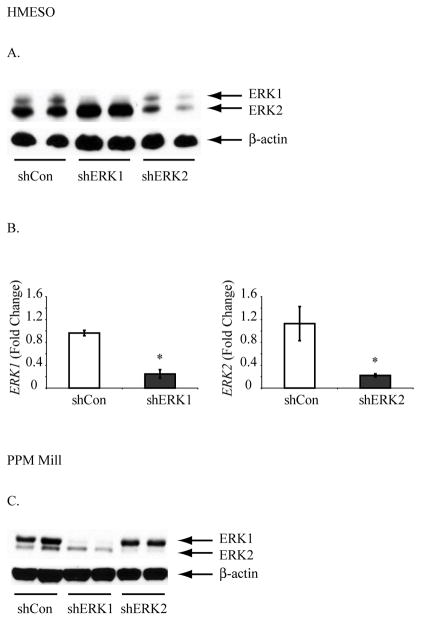
Having established that the ERK1/2 pathway is causally related to proliferation, migration and invasion of MM cells, we then used RNA interference approaches to knock down expression of ERK1 or ERK2 selectively in an epithelioid (HMESO) 16, and sarcomatoid (PPMMill) line, both forming tumors after injection into SCID mice. Stably transfected clones were selected in the presence of G418 medium and characterized with shControl (shCon) cells for expression of ERKs. Western blots (Figure 3A, C) illustrate that ratios of ERK1 and ERK2 protein levels to β-actin, a loading control, were decreased in shERK1 and shERK2 lines in contrast to shControl cells. In addition, we show by qRT-PCR that fold-changes in total amounts of ERK1 and ERK2 mRNA levels remained decreased (p<0.05) in HMESO MM tumors after injecting these respective lines into SCID mice (Figure 3B). ERK expression was reduced by approximately 80% in both shERK1 and shERK2 lines and remain inhibited after xenografting and tumor development. In PPMMill-injected mice, no tumors were obtained at the end of the experimental period in shERK1 and shERK2 groups, in contrast ot shControls. Therefore, there is no assessment of ERK1/2 RNA levels in these tumors.
Figure 3.
Selective inhibition of ERK1 or ERK2 in human MM lines and tumors generated from them. (A) A Western blot showing selective inhibition of either ERK1 or ERK2 in respective sh stable HMESO line. (B) shERK1 or shERK2 Hmeso cells were injected subcutaneously in to SCID mice and tumors thus obtained were assessed for ERK1/ERK2 mRNA levels by qRT-PCR. Results are expressed as Mean +/− SEM of N≥3 per group *=p<0.05 as compared to shCon. (C) A Western blot showing selective inhibition of either ERK1 or ERK2 in respective sh stable PPMMill line.
Inhibition of ERK2 causes decreased growth and parameters of preneoplasia in a human epithelioid (HMESO) MM line in vitro
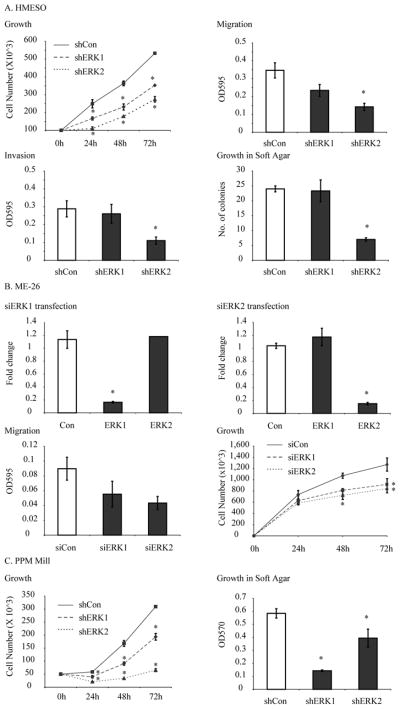
We first characterized the proliferation of shERK1-, shERK2-, and shCon-transfected lines in vitro. As shown in Figure 4A, both shERK1 and shERK2 HMESO MM lines exhibited significant decreases in cell proliferation at all time points evaluated (24, 48 and 72 h) in comparison to the shCon cell line. Subsequently, we examined shERK1, shERK2, and shCon cell lines using established in vitro assays for migration, invasion, and anchorage independence. In these studies, only the shERK2 cell line demonstrated inhibition (p<0.05) of cell migration, invasion, and growth in soft agar (Figure 4A). ME-26, another epithelioid MM line, showed inhibition of growth and migration after inhibition of both ERK1 or ERK2, by siRNA (Figure 4B). Both sarcomatoid PPMMill shERK1 and shERK2 lines exhibited significant decreases in cell proliferation and growth in soft agar (Figure 4C). No inhibition of migration or invasion was observed using either shERK1 or shERK2 PPMMill lines (data not shown).
Figure 4.
Human MM lines stably transfected with shERK1 or shERK2 show decreases in cell proliferation and other tumorigenic properties in vitro. (A) HMESO cells: Growth curves, both shERK1 and shERK2 showing significantly (p≤0.05) reduced proliferation as compared to shCon. Migration assay: shERK2 MM cells exhibit significantly diminished migration. Invasion assay: shERK2 MM cells exhibit significantly diminished invasion. Growth in soft agar: shERK2 MM cells exhibit significantly diminished growth in soft agar. (B) ME-26 cells: Transfection with either siERK1 or siERK2 resulted in > 80% reduction of ERK1 or ERK2 mRNA levels respectively as compared to siCon. Growth curves: inhibition of either ERK1 or 2 inhibited growth of cells significantly as compared to siCon. Migration assay: inhibition of either ERK1 or ERK2 inhibited migration of ME-26 cells as compared to siCon, however, the inhibition was not significant. (C) PPMMill cells: Growth curves: both shERK1 and shERK2 lines show significantly reduced proliferation as compared to shCon. Growth in soft agar: both shERK1 and shERK2 lines showed reduced growth in soft agar. Results are expressed as Mean +/− SEM of N≥3 per group in duplicate experiments. *=p<0.05 in comparison to shControl (shCon) MM cells.
Inhibition of ERK1 and ERK2 pathways in human HMESO MM cells causes unique changes in gene expression
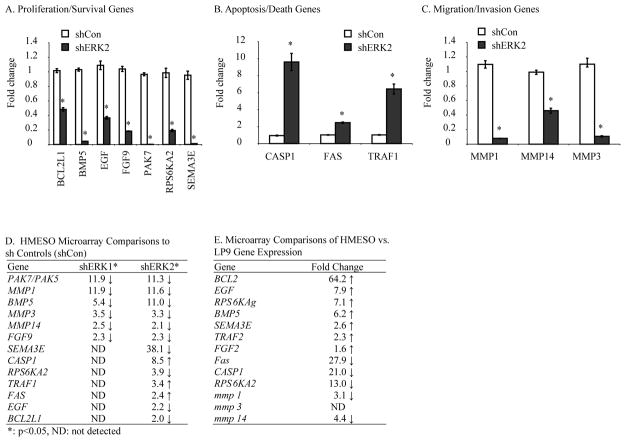
To reveal changes in gene expression resulting from ERK1 or ERK2 inhibition, we performed Affymetrix microarray analysis on shERK1, shERK2, and shCon MM cell lines at confluency. In comparison to the shCon line, a total of 875 and 789 alterations in gene expression (>2 fold, p<0.05) were observed in shERK1 and shERK2 MM (HMESO) cell lines, respectively. Moreover, when comparing alterations in gene expression between shERK1 and shERK2 MMs, a total of 1,067 genes, including 602 that were increased and 465 that were decreased, were observed. These included genes linked to cell death, cell adhesion and motion, as well as cell cycle, growth, and proliferation (Table S1). The greatest numbers of gene expression changes were related to signal transduction that included both intracellular and intercellular (cell-cell) pathways (Table S1). We next validated by qRT-PCR the fold changes in the mRNA of 7 genes (BCL2L1, BMP5, EGF, FGF9, PAK7 (PAK5), RPS6KA2, SEMA3E) important in tumor proliferation and survival, and 3 matrix metalloproteinases (MMP1, MMP3, MMP14), all of which were decreased (p<0.05) in shERK2 cells in comparison to shCon cells (Figure 5A, C). In contrast, increased (p<0.05) levels of the apoptosis-related genes, CASP1, FAS and TRAF1 were observed in shERK2 vs. shCon MM cells as validated by qRT-PCR (Figure 5B). Microarray results (Figure 5D) showed that patterns in mRNA levels of p21 (CDKN1A)-Activated Kinase 7 (PAK7), Bone Morphogenic Protein 5 (BMP5), Fibroblast Growth Factor 9 (FGF9), MMP1, MMP3 and MMP14 were similar in both shERK1 and shERK2 lines in comparison to the shControl. Conversely, increases in Caspase 1 (CASP1), FAS, TNF Receptor Superfamily 6 (TRAF1), and decreases in Semaphorin3E (SEMA3E), Ribosomal Protein S6 Kinase (RPS6KA2), Epidermal Growth Factor (EGF) and BCL2-Like1 (BCL2L1) gene expression were unique to shERK2 MMs (p<0.05). These studies suggest that inhibition of ERK2 causes decreases in expression of genes essential for cell survival as well as increases in gene expression associated with the development of apoptosis. In order to assess the levels of these selected genes in HMESO cells as compared to non-transformed human mesothelial cells (LP9/hTERT), we compared microarray data from LP9 and HMESO cells. We observed (Figure 5E) that EGF, BMP5, SEMA3E, and FGF9 were significantly (p≤0.05) increased and FAS, CASP1, RPS6KA2, MMP1 and MMP14 were significantly (p≤0.05) decreased in MM cells as compared to non-transformed mesothelial cells.
Figure 5.
ERK2 inhibition causes decreases in expression of genes essential for proliferation/survival and increases in expression of genes associated with the development of apoptosis. (A–C) qRT-PCR results on 13 selected genes shown by microarray analysis (D) to show altered expression in mRNA levels in shERK1- and shERK2-transfected MM lines as compared to shCon-transfected cells. Results are expressed as Mean +/− SEM of N=3 determinations per group. *=p<0.05 in comparison to shControl (shCon) MM cells. (E) Expression of selected (as in D) genes in HMESO cells as compared to mesothelial cells (LP9) from microarray analysis. Results are expressed as Mean +/− SEM of N=3 determinations per group. *=p<0.05 in comparison to LP9 cells.
ERK2 is indispensible to the development of human epithelioid HMESO MMs in a SCID mouse xenograft model
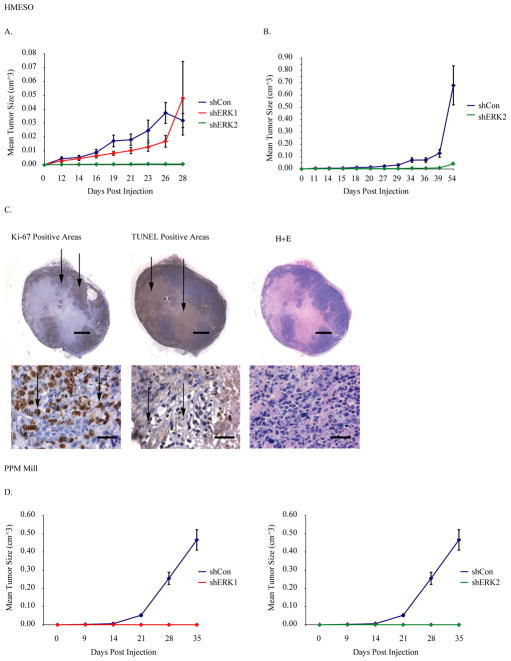
Duplicate experiments using a SCID mouse xenograft model were performed to determine if the size and rate of MM tumor growth was altered in shERK vs. shCon HMESO lines. As shown in Figure 6A, injection of shERK2 HMESO lines did not result in formation of tumors over a 28 day period in contrast to shCon and shERK1 HMESO lines. Although the mean tumor volume (p≤0.05) differed between the shERK1 and shCon groups on days 19, 21, and 23, the rates of tumor growth did not differ significantly between these two groups at any time point. In a second experiment which included only the shERK2 and shCon HMESO lines and was carried out over a longer time period (54 days), tumor sizes and growth again were significantly lower in the shERK2 animals at all time points (Figure 6B). Thus, both experiments substantiate a key role of ERK2 in epithelioid MM tumor development. In Figure 6C, the appearance of HMESO tumors developing in the mouse xenograft model is depicted using Ki-67 staining to show proliferation of cells on the tumor periphery and a necrotic/apoptotic core as verified by TUNEL-positive staining. The right panel of H&E stained sections shows representative morphology of shCon MMs appearing in SCID mice. These tumors exhibited a biphasic or epithelioid morphology as is most frequently observed in patients with MM 21. The small size and few numbers of shERK2 HMESO tumors precluded comparative assessment of proliferation, apoptosis and angiogenesis with shCon or shERK1 MMs.
Figure 6.
ERK2 inhibition significantly attenuates human epithelioid MM (HMESO) growth in SCID mice. (A) In the first experiment, shERK1, shERK2 and shControl stable MM lines were injected subcutaneously at 4 sites per mouse (N=4 mice or 16 tumors per group), and tumor growth was measured over 28 days. shERK1 significantly (p≤0.05) different from shCon at 19, 21, and 23, days; shERK2 significantly (p<0.05) different from shCon at all time points except 14 and 28 days. Rate of tumor growth for shERK2 group differed significantly from shCon group, whereas rate of tumor growth for shERK1 group did not differ significantly from shCon group (B) In the second experiment, shERK2 and shCon MM lines were injected subcutaneously as described above (N=5 mice or 20 tumors per group), and tumor growth was evaluated over 54 days (p≤0.05 at all time points). (C) Tumor sections were stained using an antibody to Ki-67, a marker of G1–G2 phase entry 22 and TUNEL staining for necrosis/apoptosis 22. Upper row shows dissecting microscope pictures and lower row shows higher magnification (40X) pictures of the same tumor by light microscopy. Hematoxylin and eosin (H&E) staining shows the characteristic biphasic and epithelioid patterns of human MMs. Black bar = 1 mm (upper row); Black bar = 50 μm (lower row). (D) ERK1 or ERK2 inhibition attenuates human fibrosarcomatoid MM (PPMMill) growth in SCID mice as compared to shCon. Data for two separate graphs shown in (D) were generated in a single experiment, to avoid overlap between shERK1 and shERK2 two separate graphs are provided. The difference in tumor growth rates between different groups in in vivo experiments (A, B, D) was assessed using a hierarchical regression model to take into account the correlation between repeated measurements on the same tumor and multiple tumors in the same animal. In this analysis, the regression coefficient describing tumor growth is modeled as a function of treatment group as well as random variation due to differences between animals and tumors on the same animal.
In comparative studies to determine effects of ERK inhibition on tumor growth rates in the sarcomatoid PPMMill MM line, stably transfected shERK1 or shERK2 cells also were injected subcutaneously into SCID mice, and tumor growth was followed for 7 weeks. As shown in Figure 6D, no tumor growth was observed in either shERK1 or shERK2 transfected PPMMill cells in contrast to shCon transfected cells. The absence of tumors in shERKgroups prohibited further mechanistic studies on these MM lines.
Discussion
We show here that both ERK1 and ERK2 contribute to the proliferation of human MM cells in vitro, as well as tumor growth of a sarcomatoid MM line, whereas ERK2 is necessary for the development of a human epithelioid MM in vivo. Most studies have focused on the role of ERKs, MEKs, and related kinases in protein phosphorylation and dephosphorylation or other feedback mechanisms in these pathways 24, However, our results in HMESO cells illustrate unique roles of ERK1 and ERK2 in gene transcription and function, revealing several ERK2-specific genes that may govern MM cell survival, inhibition of apoptosis, and essential processes (migration, invasion, angiogenesis) involved in the maintenance of tumor homeostasis. Data in Figure 4A showing that inhibition of ERK1 or ERK2 inhibits MM cell proliferation, support some reports suggesting that ERK1 and ERK2 (or MEK1 and MEK2) are interchangeable and functionally redundant, especially in the epidermis 25. However, blocking ERK2 selectively inhibited parameters of tumor function in vitro and growth of epithelioid MMs. This conclusion is also supported by recent RNA interference studies in hepatocytes where ERK2, but not ERK1, plays a key role in both in vitro and in vivo growth of hepatocytes 14, 15. In contrast to other reports 26 showing increased PI3K/AKT proteins in MEK inhibited tumor tissues, we did not see different AKT levels in the shERK2 HMESO line (data not shown).
A unique contribution of our studies is the elucidation of several shERK2-linked genes that may be critical to the development of epithelioid tumors which constitute the vast majority of human MMs. As shown in Table S1, mRNA levels of a number of genes were significantly increased or decreased in microarray analyses after silencing of either ERK1 or ERK2, and these patterns were confirmed in 13 selected genes using qRT-PCR (Figure 5). Several of these genes, including PAK7 or other members of this serine/threonine protein kinase family can directly activate MEK1 and inhibit cell transformation by preventing Ras-induced activation of ERKs 27, 28. Others such as BMP5 are activated via synergistic activation of Smad and p38 kinases and are differentially regulated during limb and tumor development 29. FGF family members have been identified as secreted factors exhibiting growth stimulatory effects in human MMs, and silencing of FGF2 curtails MM migration, invasion, and anchorage independence of MMs in vitro 30. Although silencing of ERK2 decreased MM migration selectively, patterns of diminished expression of MMPs 1, 3 and 14 (membrane type MT1-MMP) were similar in both shERK1 and shERK2 MMs (Figure 5D). MMP1 and MMP14 are regulated by Activator-Protein-1 (AP-1) 31 and ERK1/232, respectively, and MMP14, which has been found in MM pleural effusions, may be linked to transcription or activation of pro-MMP2 in the facilitation of migration 33.
In contrast to shERK1 HMESO cells, expression of RPSK6KA2 and EGF were selectively down-regulated in shERK2 MMs. RPSK6KA2 encodes a serine/threonine kinase of the RSK ribosomal S6 family associated with phosphorylation of the MAPK signaling pathway, and phosphorylation of the EGFR, as well as its increased biosynthesis, occurs in rodent mesothelial cells exposed to asbestos 8. Thus, ERK2 appears to cause increased transcription of genes encoding proteins that perpetuate ERK signaling in a positive feedback pathway.
Expression of 3 apoptosis-related genes (CASP1, FAS, and TRAF1) were increased selectively in shERK2 HMESOs suggesting that ERK2, in contrast to ERK1, inhibits gene expression linked to several critical pro-apoptotic pathways. In addition to initiating apoptosis, activated caspase-1 proteolytically cleaves pro-IL-1β to active IL-1β that elicits inflammation by asbestos 34 and promotes the transformation of human immortalized mesothelial cells 35. FASL has been detected in MMs by immunohistochemistry 18, 36, and cisplatin synergistically interacts with soluble FASL to potentiate apoptosis in MM cells 37. Moreover, hemizygous loss of FAS-associated factor 1 (Faf1) and down-regulation of Faf1 protein occurs in a mouse model of MMs 18. TRAF1 and TRAF2 form a heterodimer required for TNF-α mediated activation of JNKs, NF-κB and inhibitor of apoptosis proteins (IAPs) 38. These events may be linked to TNF-α-associated cytokine production by asbestos and transformation of human MMs 19, 39. Our data also show that BCL2L1 gene expression is decreased selectively in shERK2 cells (Figure 5) in accordance with knowledge that protein levels of this anti-apoptotic gene is regulated by ERK1/240. MMs express BCL-xL, but less than 5% express BCL-2, thus, attenuation of BCL-xL gene and protein has been a focus of MM chemotherapy 41. Our data suggest BCL2-L1 as another potential pro-survival candidate to be targeted in MM.
Recent reports suggest that MMs are angiogenesis-dependent tumors, and highly vascularized tumors are more aggressive 42, 43. A unique observation here is that gene expression of SEMA3E, a member of the secreted and membrane-bound semaphorin family linked to angiogenesis, was significantly decreased (~38 fold) in shERK2 MMs. SEMA3E is essential for structural and functional abnormalities in tumor vascularization and increases vascular permeability 44, 45. Interestingly, the related SEMA3F is a candidate tumor suppressor gene located at chromosome band 3p21.3 and is often deleted in lung cancers 46. Frequent loss of heterozygosity (LOH) at 3p21 also has been reported in MMs 47. The multi-functional role of ERK2 in enhancing survival gene expression while inhibiting pro-apoptotic and pro-angiogenic gene expression makes it an attractive candidate for therapeutic intervention in cancers and fibroproliferative diseases.
In contrast to epithelioid HMESO cells, injection of sarcomatoid PPMMill lines showed involvement of both ERK1 and 2 in tumorigenesis of these tumors that are highly aggressive in nature.
A recent study 48 supports our findings that both ERK1 and 2 play redundant roles in fibroblast cell proliferation. The observed differences in ERKs in growth and proliferation of different MM cell lines might be attributed to different relative expression levels of ERK1 and 2 in these MM lines (Figure 3). Although no significant endogenous activation of ERK1/2 was observed in MM lines, the inhibitory effect of U0126 on cell proliferation (Fig 1) could be attributed to other possible targets, including ERK549. Our group has reported previously the involvement of ERK5 in proliferation of lung epithelial cells50. In conclusion, our data show key roles of ERK1 and ERK2 in growth and tumorigenesis of human MMs which may be related to distinct patterns of gene expression by individual ERKs in MMs. Our results advocate targeting ERKs alone or in combination with chemotherapeutic drugs in MM therapy.
Supplementary Material
Acknowledgments
We thank Jennifer Diaz for manuscript preparation, the Vermont Cancer Center (VCC) DNA Analysis Facility for qRT-PCR, and the Vermont Genetics Network Microarray Facility for performing microarray experiments. We acknowledge grant support from, National Cancer Institute grant P01CA114047 (MC, BTM, JRT, HIP, NHH, AS), Mesothelioma Applied Research Foundation grants (BTM and AS), National Institute of Environmental Health Sciences grant T32ES07122 (BTM and JMH), National Institutes of Health grant P30CA006927 (JRT), an appropriation from the Commonwealth of Pennsylvania (JRT), and a Vermont Cancer Center/Lake Champlain Cancer Research Organization grant (AS).
Abbreviations used
- AP-1
Activator-Protein-1
- BCL2LI
BCL2-Like 1
- BMP5
Bone Morphogenic Protein 5
- CASP1
Caspase 1
- EGF
Epidermal Growth Factor
- ERK
Extracellular signal-regulated kinase
- FAF1
FAS-associated factor 1
- FBS
fetal bovine serum
- FGF9
Fibroblast Growth Factor 9
- HPRT1
hypoxanthine phosphoribosyl transferase 1
- IAPs
inhibitor of apoptosis proteins
- LOH
loss of heterozygosity
- MM
malignant mesotheliomas
- MEK
mitogen-activated protein kinase kinase
- PAK7
P21 (CDKNIA)-Activated Kinase 7
- qRT-PCR
quantitative Real Time PCR
- RPS6KA2
Ribosomal Protein S6 Kinase
- SEMA3E
Semaphorin 3E
- SCID
severe combined immunodeficiency
- shCon
shControl
- TRAF1
TNF Receptor Superfamily 6
Footnotes
Statement: This is first report showing that ERK2 is indispensible to the development of human epithelioid malignant mesotheliomas. Outcome of this study will have major impact on development of MM treatment strategies, by combining ERK1/2 inhibitors with chemotherapeutic drugs.
References
- 1.Krab LC, Goorden SM, Elgersma Y. Oncogenes on my mind: ERK and MTOR signaling in cognitive diseases. Trends Genet. 2008;24:498–510. doi: 10.1016/j.tig.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Pullikuth AK, Catling AD. Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective. Cellular signalling. 2007;19:1621–32. doi: 10.1016/j.cellsig.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–39. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 4.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth factors (Chur, Switzerland) 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 5.Skarpen E, Flinder LI, Rosseland CM, Orstavik S, Wierod L, Oksvold MP, Skalhegg BS, Huitfeldt HS. MEK1 and MEK2 regulate distinct functions by sorting ERK2 to different intracellular compartments. Faseb J. 2008;22:466–76. doi: 10.1096/fj.07-8650com. [DOI] [PubMed] [Google Scholar]
- 6.Buder-Hoffmann SA, Shukla A, Barrett TF, MacPherson MB, Lounsbury KM, Mossman BT. A protein kinase Cdelta-dependent protein kinase D pathway modulates ERK1/2 and JNK1/2 phosphorylation and Bim-associated apoptosis by asbestos. Am J Pathol. 2009;174:449–59. doi: 10.2353/ajpath.2009.080180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Melo M, Gerbase MW, Curran J, Pache JC. Phosphorylated extracellular signal-regulated kinases are significantly increased in malignant mesothelioma. J Histochem Cytochem. 2006;54:855–61. doi: 10.1369/jhc.5A6807.2006. [DOI] [PubMed] [Google Scholar]
- 8.Zanella CL, Posada J, Tritton TR, Mossman BT. Asbestos causes stimulation of the extracellular signal-regulated kinase 1 mitogen-activated protein kinase cascade after phosphorylation of the epidermal growth factor receptor. Cancer Res. 1996;56:5334–8. [PubMed] [Google Scholar]
- 9.Cummins AB, Palmer C, Mossman BT, Taatjes DJ. Persistent localization of activated extracellular signal-regulated kinases (ERK1/2) is epithelial cell-specific in an inhalation model of asbestosis. Am J Pathol. 2003;162:713–20. doi: 10.1016/S0002-9440(10)63867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manning CB, Sabo-Attwood T, Robledo RF, Macpherson MB, Rincon M, Vacek P, Hemenway D, Taatjes DJ, Lee PJ, Mossman BT. Targeting the MEK1 cascade in lung epithelium inhibits proliferation and fibrogenesis by asbestos. American journal of respiratory cell and molecular biology. 2008;38:618–26. doi: 10.1165/rcmb.2007-0382OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Xia S, Li R, Liu H, Huang Y, Qian X, Xiao X, Xu X, Lin X, Tian Y, Zong Y, He D, et al. Doxycycline enhances the Ras-MAPK signaling and proliferation of mouse thymic epithelial cells. Journal of cellular biochemistry. 2009;107:494–503. doi: 10.1002/jcb.22147. [DOI] [PubMed] [Google Scholar]
- 12.Grant S. Cotargeting survival signaling pathways in cancer. The Journal of clinical investigation. 2008;118:3003–6. doi: 10.1172/JCI36898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sturgill TW. MAP kinase: it’s been longer than fifteen minutes. Biochemical and biophysical research communications. 2008;371:1–4. doi: 10.1016/j.bbrc.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Fremin C, Ezan F, Boisselier P, Bessard A, Pages G, Pouyssegur J, Baffet G. ERK2 but not ERK1 plays a key role in hepatocyte replication: an RNAi-mediated ERK2 knockdown approach in wild-type and ERK1 null hepatocytes. Hepatology (Baltimore, Md. 2007;45:1035–45. doi: 10.1002/hep.21551. [DOI] [PubMed] [Google Scholar]
- 15.Bessard A, Fremin C, Ezan F, Fautrel A, Gailhouste L, Baffet G. RNAi-mediated ERK2 knockdown inhibits growth of tumor cells in vitro and in vivo. Oncogene. 2008;27:5315–25. doi: 10.1038/onc.2008.163. [DOI] [PubMed] [Google Scholar]
- 16.Reale FR, Griffin TW, Compton JM, Graham S, Townes PL, Bogden A. Characterization of a human malignant mesothelioma cell line (H-MESO-1): a biphasic solid and ascitic tumor model. Cancer Res. 1987;47:3199–205. [PubMed] [Google Scholar]
- 17.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Molecular and cellular biology. 2000;20:1436–47. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altomare DA, Menges CW, Pei J, Zhang L, Skele-Stump KL, Carbone M, Kane AB, Testa JR. Activated TNF-alpha/NF-kappaB signaling via down-regulation of Fas-associated factor 1 in asbestos-induced mesotheliomas from Arf knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3420–5. doi: 10.1073/pnas.0808816106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, Bocchetta M, Kroczynska B, Elmishad AG, Chen Y, Liu Z, Bubici C, Mossman BT, Pass HI, Testa JR, Franzoso G, Carbone M. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10397–402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shukla A, MacPherson MB, Hillegass J, Ramos-Nino ME, Alexeeva V, Vacek PM, Bond JP, Pass HI, Steele C, Mossman BT. Alterations in gene expression in human mesothelial cells correlate with mineral pathogenicity. American journal of respiratory cell and molecular biology. 2009;41:114–23. doi: 10.1165/rcmb.2008-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King JE, Galateau-Salle F, Haselton PS. Histopathology of malignant pleural mesothelioma. In: O’Byrne K, Rusch V, editors. Malignant Pleural Mesotheliomaed. New York: Oxford University Press; 2006. pp. 61–103. [Google Scholar]
- 22.Jung M, Davis WP, Taatjes DJ, Churg A, Mossman BT. Asbestos and cigarette smoke cause increased DNA strand breaks and necrosis in bronchiolar epithelial cells in vivo. Free Radic Biol Med. 2000;28:1295–9. doi: 10.1016/s0891-5849(00)00211-2. [DOI] [PubMed] [Google Scholar]
- 23.Burgers JA, Hegmans JP. Clinicopathpathological prognostic factors and scoring systems in malignant pleural mesothelioma. In: O’Byrne K, Rusch V, editors. Malignant Pleural Mesotheliomaed. New York: Oxford University Press; 2006. pp. 105–28. [Google Scholar]
- 24.Catalanotti F, Reyes G, Jesenberger V, Galabova-Kovacs G, de Matos Simoes R, Carugo O, Baccarini M. A Mek1-Mek2 heterodimer determines the strength and duration of the Erk signal. Nature structural & molecular biology. 2009;16:294–303. doi: 10.1038/nsmb.1564. [DOI] [PubMed] [Google Scholar]
- 25.Scholl FA, Dumesic PA, Barragan DI, Charron J, Khavari PA. Mek1/2 gene dosage determines tissue response to oncogenic Ras signaling in the skin. Oncogene. 2009;28:1485–95. doi: 10.1038/onc.2008.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rexer BN, Ghosh R, Arteaga CL. Inhibition of PI3K and MEK: it is all about combinations and biomarkers. Clin Cancer Res. 2009;15:4518–20. doi: 10.1158/1078-0432.CCR-09-0872. [DOI] [PubMed] [Google Scholar]
- 27.Park ER, Eblen ST, Catling AD. MEK1 activation by PAK: a novel mechanism. Cellular signalling. 2007;19:1488–96. doi: 10.1016/j.cellsig.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giroux V, Iovanna JL, Garcia S, Dagorn JC. Combined inhibition of PAK7, MAP3K7 and CK2alpha kinases inhibits the growth of MiaPaCa2 pancreatic cancer cell xenografts. Cancer gene therapy. 2009;16:731–40. doi: 10.1038/cgt.2009.22. [DOI] [PubMed] [Google Scholar]
- 29.Zuzarte-Luis V, Montero JA, Rodriguez-Leon J, Merino R, Rodriguez-Rey JC, Hurle JM. A new role for BMP5 during limb development acting through the synergic activation of Smad and MAPK pathways. Developmental biology. 2004;272:39–52. doi: 10.1016/j.ydbio.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Stapelberg M, Gellert N, Swettenham E, Tomasetti M, Witting PK, Procopio A, Neuzil J. Alpha-tocopheryl succinate inhibits malignant mesothelioma by disrupting the fibroblast growth factor autocrine loop: mechanism and the role of oxidative stress. The Journal of biological chemistry. 2005;280:25369–76. doi: 10.1074/jbc.M414498200. [DOI] [PubMed] [Google Scholar]
- 31.Kroczynska B, Cutrone R, Bocchetta M, Yang H, Elmishad AG, Vacek P, Ramos-Nino M, Mossman BT, Pass HI, Carbone M. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14128–33. doi: 10.1073/pnas.0604544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong J, Gencay MM, Bubendorf L, Burgess JK, Parson H, Robinson BW, Tamm M, Black JL, Roth M. ERK1/2 and p38 MAP kinase control MMP-2, MT1-MMP, and TIMP action and affect cell migration: a comparison between mesothelioma and mesothelial cells. Journal of cellular physiology. 2006;207:540–52. doi: 10.1002/jcp.20605. [DOI] [PubMed] [Google Scholar]
- 33.Sivertsen S, Hadar R, Elloul S, Vintman L, Bedrossian C, Reich R, Davidson B. Expression of Snail, Slug and Sip1 in malignant mesothelioma effusions is associated with matrix metalloproteinase, but not with cadherin expression. Lung cancer (Amsterdam, Netherlands) 2006;54:309–17. doi: 10.1016/j.lungcan.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science (New York, NY) 2008;320:674–7. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Faux SP, Hallden G, Kirn DH, Houghton CE, Lemoine NR, Patrick G. Interleukin-1beta and tumour necrosis factor-alpha promote the transformation of human immortalised mesothelial cells by erionite. International journal of oncology. 2004;25:173–8. [PubMed] [Google Scholar]
- 36.Kokturk N, Firat P, Akay H, Kadilar C, Ozturk C, Zorlu F, Gungen Y, Emri S. Prognostic significance of Bax and Fas ligand in erionite and asbestos induced Turkish malignant pleural mesothelioma. Lung cancer (Amsterdam, Netherlands) 2005;50:189–98. doi: 10.1016/j.lungcan.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Stewart JHt, Tran TL, Levi N, Tsai WS, Schrump DS, Nguyen DM. The essential role of the mitochondria and reactive oxygen species in Cisplatin-mediated enhancement of fas ligand-induced apoptosis in malignant pleural mesothelioma. The Journal of surgical research. 2007;141:120–31. doi: 10.1016/j.jss.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 38.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–52. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 39.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. American journal of respiratory and critical care medicine. 1998;157:1666–80. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- 40.Lee MW, Bach JH, Lee HJ, Lee DY, Joo WS, Kim YS, Park SC, Kim KY, Lee WB, Kim SS. The activation of ERK1/2 via a tyrosine kinase pathway attenuates trail-induced apoptosis in HeLa cells. Cancer investigation. 2005;23:586–92. doi: 10.1080/07357900500283036. [DOI] [PubMed] [Google Scholar]
- 41.Cao XX, Mohuiddin I, Ece F, McConkey DJ, Smythe WR. Histone deacetylase inhibitor downregulation of bcl-xl gene expression leads to apoptotic cell death in mesothelioma. American journal of respiratory cell and molecular biology. 2001;25:562–8. doi: 10.1165/ajrcmb.25.5.4539. [DOI] [PubMed] [Google Scholar]
- 42.Ranieri G, Ruggieri E, Falco G, Zizzo N, Mattioli E, Zito AF, Patruno R, Gasparini G. Drug targets to pro-angiogenetic factors with special reference to primary peritoneal mesothelioma. Endocrine, metabolic & immune disorders drug targets. 2006;6:271–7. doi: 10.2174/187153006778250028. [DOI] [PubMed] [Google Scholar]
- 43.Roe OD, Anderssen E, Sandeck H, Christensen T, Larsson E, Lundgren S. Malignant pleural mesothelioma: genome-wide expression patterns reflecting general resistance mechanisms and a proposal of novel targets. Lung cancer (Amsterdam, Netherlands) 2010;67:57–68. doi: 10.1016/j.lungcan.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Serini G, Maione F, Giraudo E, Bussolino F. Semaphorins and tumor angiogenesis. Angiogenesis. 2009;12:187–93. doi: 10.1007/s10456-009-9138-4. [DOI] [PubMed] [Google Scholar]
- 45.Acevedo LM, Barillas S, Weis SM, Gothert JR, Cheresh DA. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood. 2008;111:2674–80. doi: 10.1182/blood-2007-08-110205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Futamura M, Kamino H, Miyamoto Y, Kitamura N, Nakamura Y, Ohnishi S, Masuda Y, Arakawa H. Possible role of semaphorin 3F, a candidate tumor suppressor gene at 3p21.3, in p53-regulated tumor angiogenesis suppression. Cancer Res. 2007;67:1451–60. doi: 10.1158/0008-5472.CAN-06-2485. [DOI] [PubMed] [Google Scholar]
- 47.Lu YY, Jhanwar SC, Cheng JQ, Testa JR. Deletion mapping of the short arm of chromosome 3 in human malignant mesothelioma. Genes, chromosomes & cancer. 1994;9:76–80. doi: 10.1002/gcc.2870090114. [DOI] [PubMed] [Google Scholar]
- 48.Voisin L, Saba-El-Leil MK, Julien C, Fremin C, Meloche S. Genetic demonstration of a redundant role of ERK1 and ERK2 MAP kinases in promoting fibroblast proliferation. Molecular and cellular biology. 2010 Apr 5; doi: 10.1128/MCB.00131-10. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishimoto S, Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO reports. 2006;7:782–6. doi: 10.1038/sj.embor.7400755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scapoli L, Ramos-Nino ME, Martinelli M, Mossman BT. Src-dependent ERK5 and Src/EGFR-dependent ERK1/2 activation is required for cell proliferation by asbestos. Oncogene. 2004;23:805–13. doi: 10.1038/sj.onc.1207163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.