Abstract
Environmental silver exposures are increasing due to the use of silver nanoparticles, which exert antimicrobial actions by releasing Ag+, a suspected developmental neurotoxicant. We evaluated the long-term neurochemical and behavioral effects of embryonic Ag+ exposure in zebrafish at concentrations that had no overt effects on morphological development. Exposure to 0.03, 0.1 or 0.3 μM Ag+ during the first five days post-fertilization caused elevations in both dopamine and serotonin turnover in the adult zebrafish brain without affecting basal neurotransmitter levels. Consistent with these synaptic effects, Ag+ -exposed fish showed a faster acquisition of avoidance behavior in a three-chamber test apparatus, without any change in response latency or overall swimming ability. Our results indicate that Ag+ is a developmental neurotoxicant that causes persistent neurobehavioral effects, reinforcing health concerns about Ag+ released from silver nanoparticles.
Keywords: Dopamine, Serotonin, Silver, Zebrafish
Introduction
Silver nanoparticles (AgNPs), which act as antimicrobial agents by releasing monovalent silver ion (Ag+ ), are increasingly used in consumer products, and thus raise concerns about human and environmental Ag+ exposures [30]. The developing brain is especially sensitive to adverse effects of heavy metals [3,5] and silver crosses the placenta and concentrates in the developing rodent brain [17,26]. To our knowledge, there is no information on the potential long-term effects of low-level developmental Ag+ exposures [14], data that are likely to be important in assessing the potential effects of AgNPs. In an in vitro model, we recently showed that Ag+ can interfere with neuronal cell replication and differentiation [24]. We went on to verify that Ag+ exposure in zebrafish during the first five days post-fertilization (dpf), affects survival, embryonic growth and pigmentation at high concentrations, while delaying swim bladder inflation and impairing larval swimming activity at lower concentrations [25]. Here, we extended our studies to determine whether these lower Ag+ concentrations produce synaptic and behavioral changes extending into adulthood. For our evaluations, we focused on two monoamine neurotransmitters, dopamine (DA) and serotonin (5-hydroxytrypamine, 5HT), and used a behavioral test that links anxiety to learning, an approach chosen because developmental exposure to other heavy metals augments anxiety-like behaviors in adult rodents [18,20]. DA and 5HT levels and turnover are readily measurable in the adult zebrafish brain [4,6,7,16] and play a role in tasks reflecting both learning and anxiety [2,6,7], with responses similar to those of mammals in models of spatial memory and aversive stimuli [19,22].
Materials and Methods
All experiments were carried out humanely and with regard for alleviation of suffering. Protocols were in accordance with all federal and state guidelines and underwent approval by the Duke University Institutional Animal Care and Use Committee. Procedures for maintenance of embryonic, larval and adult zebrafish, and for exposures toAgNO3 and NaNO3 were described previously [25]. Briefly, all fish were kept at 28.5°C on a 14/10 h light/dark cycle. Embryos were exposed to AgNO3 or control treatments from 4 hours post-fertilization to 5 dpf and maintained with daily solution renewals. We chose the AgNO3 concentrations based on our larval studies that showed overtly toxic effects on embryonic and larval development at higher concentrations but behavioral effects at lower concentrations that did not induce morphological defects [25]. Adults were housed in tanks with continuous fluid recirculation of 0.013% SeaChem (SeaChem Laboratories Inc., Madison, GA) and 0.05% InstantOcean (InstantOcean, Cincinnati, OH). For neurochemical determinations [7], adult fish (3 months old) were anesthetized in 4°C H2O before decapitation and brain dissection. Tissues were deproteinized with 0.1 N perchloric acid and then analyzed by high performance liquid chromatography with electrochemical detection [7]. A standard was run to enable calculation of the amounts of DA, 3,4-dihydroxyphenylacetic acid (DOPAC), 5HT and 5-hydroxyindoleacetic acid (5HIAA). Neurotransmitter turnovers were then determined from the metabolite ratios, DOPAC/DA and 5HIAA/5HT.
All behavioral testing took place during the light phase, between 8:00 and 17:00 h, using a spatial orientation task triggered by an aversive stimulus [7]. Briefly, the testing apparatus consisted of a tank divided horizontally into three chambers with horizontal stripes along the length of one side of the tank to enable spatial orientation. To begin the test, a single fish was placed in the center chamber and was allowed to acclimate to the tank for 30 s. The dividers between chambers were then opened, allowing movement into either of the two side chambers. After five initial trials to establish which side the fish swam to most consistently (“preferred side”), ten sequential test trials were carried out in the same manner, again with 30 s between trials. If the fish swam to the preferred side, a partition was moved to squeeze the chamber to a smaller volume and the fish was held in the confined space for 60 s. If the fish swam to the non-preferred side, it was allowed to swim freely in the unrestricted chamber for 60 s.
Data were compiled as means and standard errors. Neurochemical data were assessed using ANOVA with treatment as a factor, followed by the post-hoc Fisher’s Protected Least Significant Difference Test for pairwise comparisons of individual treatments. Behavioral data were combined into groups of two trials (trial 1–2, 3–4, 5–6, 7–8, 9–10) and the rate of learning was determined by linear trend analysis (slope of percent choosing the non-squeezed side over number of trials) across treatments, using the same statistical procedures. The sequential values for each fish were used to calculate the slope of learning and the mean and SE were then determined for the linear trend in each treatment group. Significance for all tests was assumed at p < 0.05 (two-tailed). Effects of Ag+ were considered biologically relevant only if the exposed fish were significantly different from both untreated controls (designated as the “H2O” group) and from fish exposed to equivalent concentrations of NaNO3.
Results
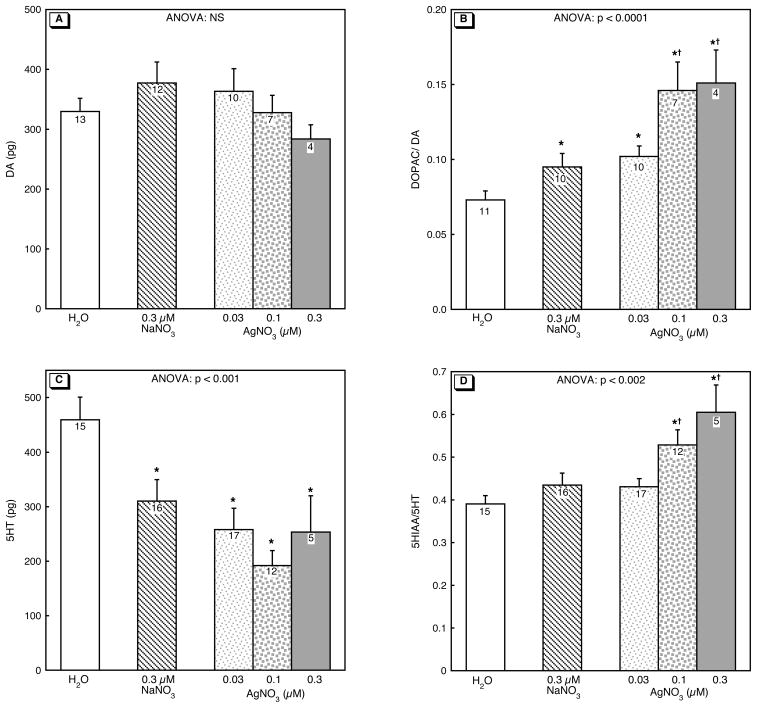
Embryonic exposure to Ag+ had no effect on DA levels in adult zebrafish (Figure 1A). In contrast, DA turnover was markedly increased in a monotonic, dose-related manner (Figure 1B). The 0.3 μM NaNO3 treatment also elevated the DOPAC/DA ratio, but the two higher concentrations of Ag+ had effects that were clearly distinguishable from that of NaNO3. NaNO3 lowered 5HT levels significantly and in this case, there was no discernible effect of Ag+, which reduced 5HT only to the same extent as NaNO3 (Figure 1C). Nevertheless, 5HT turnover showed the same, significant Ag+ -induced increase as DA turnover, with substantial elevations of the 5HIAA/5HT ratio that were distinguishable from both the untreated control group (H2O) and from zebrafish that had been exposed to NaNO3 (Figure 1D).
Figure 1.
Effects of 0–5 dpf Ag+ exposure on neurotransmitter levels and turnover in adultbrain: (A) DA, (B) DOPAC/DA, (C) 5-HT, (D) 5HIAA/5HT. ANOVA across all treatments is shown at the top of the panel. Asterisks denote treatments that differ significantly from the H2O control group and daggers differences between AgNO3 and NaNO3. NS, not significant.
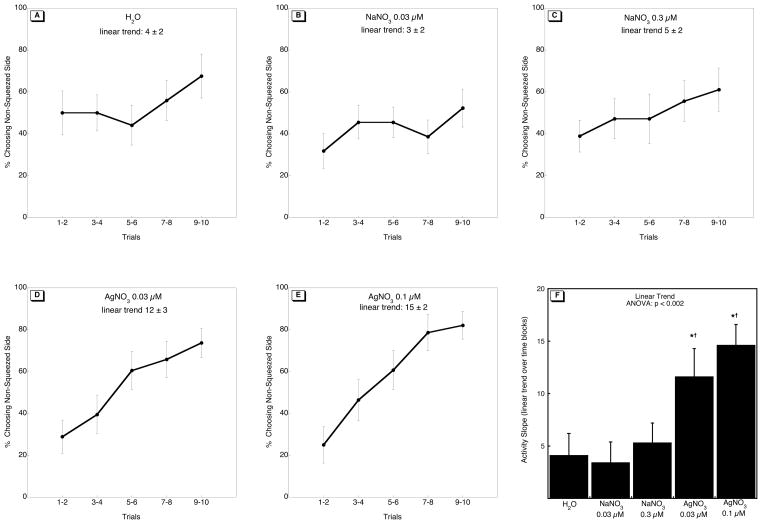
Over the course of ten trials, fish in all groups showed an increase in swimming to the non-squeezed side (Figure 2A–E; p < 0.0001 for the main effect of trial), reflecting successful pairing of the aversive stimulus (squeezing) and spatial orientation (swimming to the non-squeezed side). When compared to controls, fish exposed to Ag+ on 0–5 dpf showed a more rapid behavioral acquisition, indicated by the greater linear increase of fish swimming to the non-aversive side over the ten trials (Figure 2F). This did not reflect overall increases in swimming activity, since there were no differences in response latency (the time that fish took to swim to the chosen chamber; data not shown).
Figure 2.
Effects of 0–5 dpf Ag+ exposure on behavioral performance in adulthood: (A-E) the percentage of fish in each treatment group choosing the non-squeezed side, (F) the linear trend across time blocks. ANOVA appears at the top of panel F; asterisks denote treatments that differ significantly from the H2O control group and daggers differences between AgNO3 and NaNO3. The number of fish used in each group were as follows: 17 for controls; NaNO3, 22 at 0.03 μM, 18 at 0.3 μM; AgNO3, 19 at0.03 μM, 14 at 0.1 μM.
Discussion
Early developmental exposure to Ag+ elevated DA and 5HT turnover in adult zebrafish. These changes cannot be explained by alterations in neurotransmitter levels, and thus are indicative of increased presynaptic activity for these two neurotransmitters. By itself, this finding indicates that embryonic Ag+ exposure leads to long-term changes in synaptic function but of course, it is important to show how these cellular changes relate to neurobehavioral alterations. DA and 5HT play important roles in reward, anxiety and sensorimotor integration [2,6–8,13,21] and many other neurodevelopmental disruptors elicit behavioral changes through effects on neurocircuitry and programming of these particular pathways [9]. To evaluate the functional consequences of Ag+ -induced DA and 5HT hyperactivity, we tested whether fish exposed to Ag+ during development responded differently in a behavioral test that combines avoidance and spatial learning, by using anxiety (squeezing the swim chamber) to trigger spatial choice. In keeping with the neurochemical findings, Ag+ exposed fish demonstrated a more rapid increase in the percentage of fish swimming to the non-aversive environment when compared to controls. Since the same treatments produced deficits in swimming behavior in larval stages of development, our findings point to a persistent pattern of neurobehavioral abnormalities evoked by embryonic exposure to Ag+. To our knowledge, this is the first study to show that Ag+ is a neurobehavioral teratogen.
Taken in isolation, our results do not provide a definitive proof that the changes in monoaminergic synaptic function are causally linked to the behavioral outcomes. However, earlier work in the zebrafish model showed that acute challenge with nicotine, which similarly evokes an increase in synaptic monoamine release, has the same effect on behavior as that seen here [7]; conversely embryonic exposure to chlorpyrifos, which reduces dopamine levels, obtunds behavioral performance [6,15]. The same relationships are apparent for monoaminergic function and similar learning tasks in mammals. Activation of DA circuitry in the hippocampus and midbrain of rodents and non-human primates is critical for visuospatial tasks [28], particularly when learning is triggered by reward or risk [27]. Recent data show that 5HT also plays a critical role in reward-based learning [13], and similar to our findings, elevations in 5HT during development enhance fear-based learning in rodents [12]; importantly, in the rodent studies, learning improvements were tied specifically to fear or avoidance behaviors and thus were not indicative of a general increase in cognitive ability [12]. Our conclusions are further bolstered by findings that developmental exposure to neurotoxicants including other heavy metals, elevates adult anxiety-like behaviors and enhances performance in tasks linked to anxiety [18,20,23]. Importantly, activation of 5HT pathways during development leads to permanent changes in anxiety, while similar changes later in life have no persistent effects [11], thus highlighting the susceptibility of neurodevelopment to toxicants like Ag+.
The novel finding that developmental Ag+ exposure produces lasting behavioral changes in association with altered DA and 5HT synaptic function, highlights the potential impact that elevations in Ag+ exposure could have in human and environmental populations. In that regard, it is important to note that the Ag+ concentrations used here are at or below the limits specified by U.S. government agencies for surface water (2–3 μg/L or 0.02–0.03 μM) [29] and drinking water (100 μg/L or 1 μM) [1]. Further, the mechanisms and outcomes seen in the zebrafish model mimic those known to be linked to the same circuits in mammals, reflecting a continuity spanning different species [7,22]. In humans, long-term effects on DA and 5HT systems are linked not only to changes to learning and anxiety, but also to neurobehavioral disorders such as depression and schizophrenia [8,21]. In light of the rapid incorporation of silver nanoparticles into a variety of products [30], and the growing concern surrounding detrimental neurobehavioral effects from early life exposures to toxicants [10], our findings point to the need for careful scrutiny of the developmental neurotoxicity of AgNPs and Ag+. We are currently conducting parallel studies of Ag+ and AgNP exposure in developing zebrafish to determine whether there are similarities in developmental outcomes that point to long-term effects of AgNPs, analogous to what we describe here for Ag+.
Acknowledgments
Acknowledgments/disclaimers: Research was supported by NIH ES10356 and GM007105. The authors thank Ann Petro and Jerry Yen for helpful technical support and advice. The authors state that they have no conflicts of interest. TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Frost Brown Todd (Charleston WV), Weltchek Mallahan & Weltchek (Lutherville MD), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Frommer Lawrence Haug (Washington DC), Carter Law (Peoria IL), Corneille Law (Madison WI), Angelos Law (Baltimore MD), Kopff, Nardelli & Dopf (New York NY), Gutglass Erickson Bonville & Larson (Madison WI), The Killino Firm (Philadelphia PA) and Alexander Hawes (San Jose, CA).
Abbreviations
- 5HIAA
5-hydroxyindoleacetic acid
- 5HT
serotonin (5-hydroxytryptamine)
- ANOVA
analysis of variance
- DA
dopamine
- dpf
days post-fertilization
- DOPAC
3,4-dihydroxyphenylacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agency for Toxic Substances and Disease Registry. Silver CAS #7440–22–4. 1999. [Google Scholar]
- 2.Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondy SC, Campbell A. Developmental neurotoxicology. J Neurosci Res. 2005;81:605–612. doi: 10.1002/jnr.20589. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee D, Gerlai R. High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure in zebrafish. Behav Brain Res. 2009;200:208–213. doi: 10.1016/j.bbr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa LG, Aschner M, Vitalone A, Syversen T, Soldin OP. Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol. 2004;44:87–110. doi: 10.1146/annurev.pharmtox.44.101802.121424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol Teratol. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eddins D, Petro A, Williams P, Cerutti DT, Levin ED. Nicotine effects on learning in zebrafish: the role of dopaminergic systems. Psychopharmacology (Berl) 2009;202:103–109. doi: 10.1007/s00213-008-1287-4. [DOI] [PubMed] [Google Scholar]
- 8.Egerton A, Mehta MA, Montgomery AJ, Lappin JM, Howes OD, Reeves SJ, Cunningham VJ, Grasby PM. The dopaminergic basis of human behaviors: A review of molecular imaging studies. Neurosci Biobehav Rev. 2009;33:1109–1132. doi: 10.1016/j.neubiorev.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frederick AL, Stanwood GD. Drugs, biogenic amine targets and the developing brain. Dev Neurosci. 2009;31:7–22. doi: 10.1159/000207490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 11.Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 12.Kim JJ, Shih JC, Chen K, Chen L, Bao S, Maren S, Anagnostaras SG, Fanselow MS, De Maeyer E, Seif I, et al. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5929–5933. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2010;166:1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 14.Lansdown ABG. Critical observations on the neurotoxicity of silver. Crit Rev Toxicol. 2007;37:237–250. doi: 10.1080/10408440601177665. [DOI] [PubMed] [Google Scholar]
- 15.Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol Teratol. 2003;25:51–57. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 16.Lopez Patino MA, Yu L, Yamamoto BK, Zhdanova IV. Gender differences in zebrafish responses to cocaine withdrawal. Physiol Behav. 2008;95:36–47. doi: 10.1016/j.physbeh.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyon TD, Patriarca M, Howatson G, Fleming PJ, Blair PS, Fell GS. Age dependence of potentially toxic elements (Sb, Cd, Pb, Ag) in human liver tissue from paediatric subjects. J Environ Monit. 2002;4:1034–1039. doi: 10.1039/b205972j. [DOI] [PubMed] [Google Scholar]
- 18.Maia CSF, Lucena GMRS, Correa PBF, Serra RB, Matos RWM, Menezes FC, Santos SN, Sousa JB, Costa ET, Ferreira VMM. Interference of ethanol and methylmercury in the developing central nervous system. Neurotoxicology. 2009;30:23–30. doi: 10.1016/j.neuro.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Maximino C, de Brito TM, da Silva Batista AW, Herculano AM, Morato S, Gouveia A., Jr Measuring anxiety in zebrafish: A critical review. Behav Brain Res. 2010;214:157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Moreira EG, Vassilieff I, Vassilieff VS. Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol. 2001;23:489–495. doi: 10.1016/s0892-0362(01)00159-3. [DOI] [PubMed] [Google Scholar]
- 21.Nordquist N, Oreland L. Serotonin, genetic variability, behaviour, and psychiatric disorders--a review. Ups J Med Sci. 2010;115:2–10. doi: 10.3109/03009730903573246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norton W, Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010 doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitzer M, Schmidt MH. Neonatal exposure to fenoterol and betamethasone: effects on the behavioral development in the rat. Intl J Neurosci. 2009;119:1548–1571. doi: 10.1080/00207450802323947. [DOI] [PubMed] [Google Scholar]
- 24.Powers CM, Wrench N, Ryde IT, Smith AM, Seidler FJ, Slotkin TA. Silver impairs neurodevelopment: studies in PC12 cells. Environ Health Perspect. 2010;118:73–79. doi: 10.1289/ehp.0901149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powers CM, Yen J, Linney EA, Seidler FJ, Slotkin TA. Silver exposure in developing zebrafish (Danio rerio): persistent effect on larval behavior and survival. Neurotoxicol Teratol. 2010;32:391–397. doi: 10.1016/j.ntt.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rungby J. An experimental study on silver in the nervous system and on aspects of its general cellular toxicity. Danish Med Bull. 1990;37:442–449. [PubMed] [Google Scholar]
- 27.Schultz W. Behav Brain Funct. 2010. Dopamine signals for reward value and risk: basic and recent data. 2010/04/27 Edition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran AH, Uwano T, Kimura T, Hori E, Katsuki M, Nishijo H, Ono T. Dopamine D1 receptor modulates hippocampal representation plasticity to spatial novelty. J Neurosci. 2008;28:13390–13400. doi: 10.1523/JNEUROSCI.2680-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Environmental Protection Agency. Water Quality Criteria. 2009. [Google Scholar]
- 30.Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gosens I, Van De Meent D, et al. Nano-silver -- a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 2009;3:109–138. [Google Scholar]