Abstract
Werner's Syndrome (WS) or adult-onset progeria is an autosomal recessive disorder of accelerated aging caused by mutations of the DNA RecQ helicase/exonuclease (WRN). WRN is an ATP-dependent helicase with 3′ to 5′ DNA exonuclease activity that regulates the replicative potential of dividing cells, and WRN loss-of-function mutations promote cellular senescence and neoplastic transformation. These molecular findings translate clinically into adult-onset progeria manifested by premature hair graying, dermal atrophy, cardiovascular disease, and cancer predilection along with a markedly reduced life expectancy. Recently, a patient with WS who developed pancreatic adenocarcinoma was identified in Honolulu suggesting a significant prevalence of loss-of-function WRN mutations in Hawai‘i's Japanese-American population. Based upon the indigenous Japanese WRN loss-of-function mutation heterozygote rate of 6 per 1,000, we speculate the possibility of approximately 1,200 heterozygotes in Hawai‘i. Our ongoing studies aim to evaluate Hawai‘i's true allelic prevalence of WRN loss-of-function mutations in the Japanese-American population, and the role of WRN silencing in sporadic cancers. In summary, WRN plays a nexus-like role in the complex interplay of cellular events that regulate aging, and analysis of WRN polymorphisms in Hawai‘i's population will generate novel insights to advance care for age-related pathologies.
Background
In 1904, the medical student Otto Werner described adult-onset progeria as a genetic disorder inherited in a Mendelian autosomal recessive fashion that is manifested by features of pathologic aging. Werner reported patients in his doctoral thesis who developed changes that mimicked “accelerated aging” at puberty, causing them to appear grossly older than their stated age by the second decade of life. These findings included premature hair graying, skin and subcutaneous fat atrophy, and classic “bird-like” facies.1 This pathologic aging was accompanied by premature cardiovascular and cancer-related mortality resulting in a markedly reduced life expectancy. Since the initial description of Werner's Syndrome (WS), less than 1,500 cases of WS have been reported to date.2
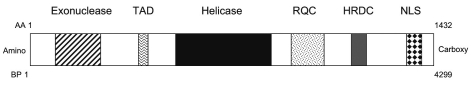
With the advent of modern molecular biology, WRN was identified in the early 1990's as the gene involved in WS through positional cloning,3 and mutant forms of WRN as well as WRN siRNA knockdown have been demonstrated to reduce the replicative potential of cells in mammalian models.4 Further characterization of WRN revealed that it encodes a 165 kDA conserved DNA RecQ helicase located on chromosome 8p12, possessing both a helicase domain and 3′ to 5′ DNA exonuclease domain (Figure 1). Thus, wild-type WRN is considered to be a “caretaker of the genome,” that protects genomic integrity, maintains telomere length, and prevents chromosomal aberrations, by promoting cell cycle progression and acting as a DNA repair enzyme.5 Epigenetic silencing of WRN expression through CpG-promoter methylation has recently been implicated as a cellular mechanism of aging and tumorigenesis,6 suggesting that WRN serves a nexus-like function at the complex interplay of cellular events regulating aging and neoplastic transformation.7,8
Figure 1.
Functional domains of the the Werner's Syndrome WRN RecQ helicase/exonuclease. HRDC, helicase RNase D C-terminus domain; NLS, nuclear localization sequence; RQC, RecQ conserved domain; TAD, transactivation domain.
Cases of WS have important implications for Hawai‘i's population as WS has been recognized in Honolulu, Hawai‘i. Recently, Chun et al. (2010) reported a case of WS associated with metastatic pancreatic adenocarcinoma in a 5th generation Japanese-American man from Hawai‘i, and has proposed that WS represents a novel hereditary pancreatic cancer predisposition syndrome.9,10 Given the real possibility of other cases as well and the potential presence of more than 1,200 heterozygotes carrying loss-of-function mutations of WRN in Hawai‘i, we provide a review of WS including pertinent clinical findings, population genetics, therapeutic approach, and translational insights gained from this fascinating genetic condition.
Clinical Findings and Diagnosis
Like Hutchinson-Gilford Syndrome, xeroderma pigmentosum, and Cockayne's Syndrome, WS is classified as a segmental progeria in that it resembles some, but not all aspects of aging.11 Unlike other progerioid syndromes, WS is not clinically apparent during childhood and infancy, and is therefore defined as an adult-onset progeria. The first signs of WS occur during adolescence with the absence of growth spurt at puberty. Subsequently, afflicted individuals develop premature graying of the hair and begin to appear decades older than their stated age by their second decade of life with the development classic “bird-like” facies due to subcutaneous fat atrophy (Figure 2).1,2 Along with the gross appearance of accelerated aging, patients acquire age-associated morbidities including type II diabetes mellitus, bilateral cataracts, cardiovascular disease, osteoporosis, and dermal atrophy. On physical examination, voice changes that are characterized as hoarse and high-pitched are observed in WS that are similar to the voice changes occurring in the geriatric population. These classic findings have lead to the proposal of clinical diagnostic criteria by the Werner International Registry in order to select appropriate patients for further genetic testing (Table 1).2 While a diagnosis of WS may be made clinically, confirmatory genetic analyses for WRN loss-of-function mutations are available from the Werner International Registry using polymerase chain reaction based gene analysis and immunologic protein assays.2
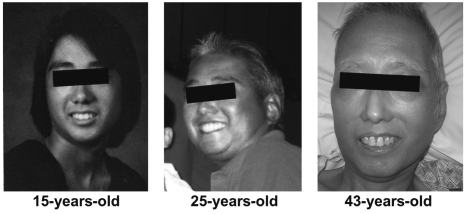
Figure 2.
Patients with Werner's Syndrome classically develop pathologic aging and “bird-like facies” with onset during the 2nd decade of life. Images reproduced with permission from Chun, et al.: Gastrointest Cancer Res 4: xx-xx (in press); © 2011 by International Society of Gastrointestinal Oncology.
Table 1.
The clinical manifestations and diagnostic criteria for Werner's Syndrome adapted from the International Registry of Werner Syndrome.
| Major Criteria | Minor Criteria | Diagnostic Criteria |
| Bilateral cataracts Dermatologic pathology (atrophy, tight skin, ulceration bird-like facies, hyperkeratosis, subcutaneous atrophy) Short stature Parental consanguinity or affected sibling Premature hair graying or thinning Positive 24-hour urine hyaluronic acid test |
Diabetes Mellitus Hypogonadism Osteoporosis Osteosclerosis (radiographic finding) Soft Tissue Calcification Premature Atherosclerosis Cancer (Rare, Mesenchymal, Multiple) Voice changes (Hoarse/High Pitched) Flat Feet |
Definite: All Major + 2 Minor Probable: First 3 Major + Any 2 additional Possible: Either 1st two Major + Any 4 other Exclusion: Onset before adolescence |
The average life expectancy is reduced to 45–47 years in WS due to the development of early cancer and cardiovascular disease.12 Myocardial infarctions and cerebrovascular accidents are frequently reported as a cause of morbidity and mortality in patients with WS.13 While a number of malignancies including colorectal, skin, thyroid, and pancreatic cancers have been reported to occur in association with WS, soft tissue sarcomas have a particularly high incidence in WS.14,15 As WRN primarily plays a role in cell cycle progression and the genomic integrity of dividing cells, non-dividing tissues are thought to be spared in WS. Therefore, dementias such as Alzheimer's Disease are not observed in WS, and histologic analysis of neuronal tissue reveals the absence of atrophic changes.9,10
The accelerated development of multiple age-associated pathologies caused by the loss-of-function of a single gene makes WS an attractive model system to study cancer, cardiovascular disease, and senescence. While WS is rare, ongoing efforts seek to document new cases through the Werner International Registry at the University of Washington as further study of WS has potential to generate insight for the purpose of formulating novel interventions to advance care for age-related pathologies.
Population Genetics
WS is a rare autosomal recessive disorder caused by homozygous loss-of-function mutations of the WRN RecQ helicase, and less than 1,500 cases of WS have been reported since its initial characterization in 1904. In the general population, the rate of heterozygous loss-of-function mutations is approximately one per million. However, the indigenous ethnic Japanese population carries a much higher heterozygote rate and over 75% of all cases of WS have occurred in ethnic Japanese.15,16 Analysis of the most common WRN loss-of-function mutations in 1,000 random healthy ethnic Japanese from the southern prefecture of Kanagawa, Japan revealed a heterozygote rate for WRN loss-of-function mutations to be greater than 6 per 1,000.16 The high prevalence in Japan is attributed to multiple founder mutations in mountainous regions of Japan where geography has historically limited gene flow, thereby allowing amplification of mutant WRN alleles. A similar geographic situation is present in Hawai‘i providing a potential geographic mechanism for amplification of mutant loss-of-function alleles from a founder effect suggested by the case reported by Chun et al (2010).9
Based upon epidemiologic data from Japan as well as the case reported by Chun et al (2010), we propose a significant prevalence of WRN loss-of-function mutations in Hawai‘i's Japanese-American population. Given approximately 200,000 Japanese-Americans in Hawai‘i (United States Census, 2000), the ethnic Japanese heterozygote rate of 6 per 1,000,16 and negligible contributions from other ethnic groups, we estimate the presence of 1,200 Japanese-Americans carrying heterozygous WRN loss-of-function mutations in Hawai‘i. We also propose that the rate of WRN loss-of-function mutations in the southern prefecture of Kanagawa, Japan reported by Satoh et al. (1996) is applicable to Hawai‘i's population, especially as the majority of Japanese who immigrated to Hawai‘i originated from southern prefectures.17 Furthermore, for the majority of the 20th century the Japanese-Americans in Hawai‘i have almost exclusively married within their ethnic group, providing another potential mechanism for amplification of WRN loss-of-function mutations within this population.18 Immigration has also been limited in this population due to the geographic isolation of Hawai‘i coupled with the Asian Exclusion Act of 1924, effectively blocking gene flow into this population for the majority of the 20th century. The case reported by Chun et al (2010) of a 5th generation Japanese-American man who was a compound heterozygote with two different causative loss-of-function mutations confirms the presence of at least two founder effects in the late 1800's.9 Additional inquiry has revealed that other cases of WS have been observed in Hawai‘i without genetic confirmation (personal correspondence with Dr. Lee Buenconsejo-Lum). While we estimate the presence of 1,200 Japanese-American individuals carrying heterozygous loss-of-function mutations in Hawai‘i, factors including multiple founder effects, geographic isolation, and historical circumstances suggest that the true rate could be much higher.
A potentially high heterozygote prevalence rate in Hawai‘i underlies the importance of recognizing the clinical manifestations of WS, and opens the door for population genetic studies of WRN loss-of-function mutations in Hawai‘i. Similar to other DNA repair disorders such as ataxia telengiectasia, Lynch Syndrome (HNPCC), hereditary breast and ovarian cancer syndrome (BRCA2), and xeroderma pigmentosum that predispose tumorogenesis in heterozygotes,10, 19–21 we hypothesize that heterozygous WRN loss-of-function mutations predispose cancer in Hawai‘i's Japanese-American population. As the Japanese-American population of Hawai‘i has the highest rate of cancer and cardiovascular disease among their ethnic cohorts worldwide,22 determination of the rate of WRN loss-of-function mutations of Japanese-Americans in Hawai‘i will help to address this cancer disparity by determining whether these mutations predispose tumorigenesis.
Therapeutic Approach
At present, there is no specific treatment for WS although gene therapy might be feasible in the future with further development of gene technologies. Treatment for WS is supportive although novel molecularly-targeted therapeutic approaches have recently been proposed.23 Although an evidence-based approach to WS is precluded due to its rarity, its diagnosis potentially changes clinical management by changing pre-test probabilities for age-related pathologies. Early cardiovascular disease and cancer are a hallmark of WS, and we propose that any patient with WS should have screening for hyperlipidemia, breast cancer, colorectal cancer, cataracts, diabetes mellitus, hypothyroidism and osteoporosis from the 2nd decade of life regardless of clinical symptoms. The development of these morbidities should be treated aggressively both pharmacologically and through lifestyle modification.13 Additionally, given the Mendelian inheritance of WS, genetic testing of other family members at risk of developing WS may be considered. Chemotherapeutic toxicity has been reported in WS consistent with the cellular roles of WRN as a DNA repair enzyme, suggesting that patients are highly sensitive to the mutagenic effects of clastogenic agents.24–27 We suggest the judicious use of therapeutic radiation and reduced-dose chemotherapy at least initially to avoid complications in patients with WS who develop cancer. Surgical interventions should also be pursued cautiously as patients with WS have been reported to exhibit impaired wound healing, likely as a result of impaired fibroblast proliferation.28,29 Such impairment of fibroblast proliferation may also predispose patients with WS to anastomotic breakdown in gastrointestinal surgeries. In summary, while an evidence-based approach for WS is not possible, we suggest that genetic testing, early screening, and the use of translational insights gained from experimental models of WS may provide a reasonable clinical approach to WS on an ad hoc basis.
Discussion
It has been more than a century since the initial characterization of WS and it continues to provide us with a fascinating model system to investigate the genetic basis for aging, tumorigenesis, and cardiovascular disease. Multiple investigations are underway to interrogate and clarify the complex roles that WRN plays in the nexus of cellular events contributing to senescence and age-related pathologies. However, relatively little is known about the role that heterozygous loss-of-function mutations of WRN play in cancer predisposition in humans. Thus, the Japanese-American population of Hawai‘i provides a novel source of translational data to understand the role of WRN in humans given the recent reports of a founder effect in Hawai‘i,9 and the likelihood of a high rate of heterozygous loss-of-function mutations of Japanese-Americans in Hawai‘i.
While WS is fascinating from the standpoint of genetics, pathology, and aging, it also provides us with valuable clinical lessons. Although WS is rare, it teaches us the importance of correlating the patient's age with their general appearance on physical examination. It is notable that the case reported by Chun et al. (2010) went undiagnosed until age 43, in spite of having obvious signs of pathologic aging and having seen multiple medical specialists upon numerous admissions to the hospital.9 In this age of technology, sophisticated diagnostic and prognostic tests are now becoming increasingly available. However, the value of evaluating the general appearance of the patient on physical examination cannot be overstated.
The Japanese-American population in Hawai‘i has long been recognized as having one of the highest rates of colorectal cancer in the world as well as significantly higher rates of other cancers compared to their other cohorts worldwide.22 Multiple studies have sought to address this cancer disparity in the Japanese-American population of Hawai‘i by correlating this cancer predisposition with diet, obesity, and other lifestyle variables.18 Despite these attempts to understand the cancer predisposition of the Japanese-American population of Hawai‘i, no definitive answer explaining this cancer disparity has been forthcoming. We hypothesize that Japanese-Americans in Hawai‘i carrying heterozygous loss-of-function mutations of WRN may at least partially explain this cancer predisposition, especially as multiple DNA repair disorders such as Lynch Syndrome (HNPCC) similarly predispose tumorigenesis in heterozygotes.30 Our ongoing studies will determine the true rate of WRN loss-of-function mutations in Hawai‘i's Japanese-American population for the purpose of determining whether WRN gene analysis represents a feasible marker to screen for cancer risk in Japanese individuals.
The recent development of the biomedical infrastructure in Hawai‘i will facilitate the analysis of WRN loss-of-function mutations in Hawai‘i's population. Several serum, tissue, and DNA repositories have been established at the University of Hawai‘i at Manoa through the John A. Burns School of Medicine and the Cancer Research Center of Hawai‘i. The data generated from tissue banks and repositories correlated with clinicopathologic features of patients harboring WRN loss-of-function mutations will provide a valuable translational resource to advance knowledge of cancer epidemiology, mechanisms of oncogenesis, and the genetics of aging. Additionally, the establishment of a pancreatic cancer cell line from a patient with WS will serve as a novel translational tool to understand the mileu of cellular events surrounding the association of cancer and aging.9 Using these tools, we aim to further clarify the mechanistic role of WRN at the cellular level and translate these findings from bench-to-bedside into novel screening tools and interventions at the population level.
In summary, we provide a review of WS along with implications for Hawai‘i's population in light of a recent case of WS reported by Chun et al (2010).9 Further study of WS and the WRN RecQ helicase will provide novel insight in order to develop screening and interventional strategies for aging and age-related pathologies. Given our estimates of more than 1,200 heterozygous individuals harboring loss-of-function mutations of WRN in Hawai‘i's Japanese-American population, further study will be necessary to delineate whether these individuals embody a hereditary cancer predisposition. It has long been established that Japanese-Americans in Hawai‘i have among the highest rates of colorectal and other types of cancers in the world, and analysis of WRN may provide answers to this phenomenon.
Acknowledgements
This manuscript was supported by research grant 123-6260-4 from the Cancer Research Center of Hawai‘i and the University of Hawai‘i Foundation (S.G.C.). D.S. is a Research Fellow of the Department of Surgery at the John A. Burns School of Medicine of the University of Hawai‘i at Manoa.
References
- 1.Murata K, Nakashima H. Werner's Syndrome: 24 cases and review of the medical literature. J Am Geriatr Soc. 1982;30(5):303–308. doi: 10.1111/j.1532-5415.1982.tb05617.x. [DOI] [PubMed] [Google Scholar]
- 2.Muftuoglu M, Oshima J, von Kobbe C, et al. The clinical characteristics of Werner Syndrome: molecular and biochemical diagnosis. Hum Genet. 2008;124(4):369–377. doi: 10.1007/s00439-008-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu CE, Oshima J, Fu YH, Wijsman EM, et al. Positional cloning of the Wener's Syndrome gene. Science. 1996;272(5259):258–262. doi: 10.1126/science.272.5259.258. [DOI] [PubMed] [Google Scholar]
- 4.Opresko PL. Telomere ResQue and preservation - roles for the Werner Syndrome protein and other RecQ helicases. Mech Ageing Dev. 2008 Jan–Feb;129(1–2):79–90. doi: 10.1016/j.mad.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Chu WK, Hickson ID. RecQ helicases: multifunctional genome caretakers. Nat Rev Cancer. 2009;9(9):644–654. doi: 10.1038/nrc2682. [DOI] [PubMed] [Google Scholar]
- 6.Agrelo R, Cheng WH, Setien F, et al. Epigenetic inactivation of the premature aging Werner syndrome gene in human cancer. Proc Natl Acad Sci U S A. 2006;103(23):8822–8827. doi: 10.1073/pnas.0600645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blander G, Kipnis J, Leal JF, et al. Physical and functional interaction between p53 and the Werner's syndrome protein. J Biol Chem. 1999;274(41):29463–29469. doi: 10.1074/jbc.274.41.29463. [DOI] [PubMed] [Google Scholar]
- 8.Opresko PL, Calvo JP, von Kobbe C. Role for the Werner syndrome protein in the promotion of tumor cell growth. Mech Ageing Dev. 2007;128(7–8):423–436. doi: 10.1016/j.mad.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Chun SG, Yee NS, Holland JM, et al. Pancreatic adenocarcinoma associated with Werner's Syndrome (adult-onset progeria) Gastrointest Cancer Res. 2010;4:xx–xx. (in press) [PMC free article] [PubMed] [Google Scholar]
- 10.Chun SG, Yee NS. Werner's Syndrome as a hereditary risk factor for exocrine pancreatic cancer: Potential role of WRN in pancreatic tumorigenesis and patient-tailored therapy. Cancer Biol Ther. 2010;10(5):xx–xx. doi: 10.4161/cbt.10.5.12763. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramírez CL, Cadiñanos J, Varela I, et al. Human progeroid syndromes, aging and cancer: new genetic and epigenetic insights into old questions. Cell Mol Life Sci. 2007;64(2):155–170. doi: 10.1007/s00018-006-6349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domínguez-Gerpe L, Araújo-Vilar D. Prematurely aged children: molecular alterations leading to Hutchinson-Gilford progeria and Werner syndromes. Curr Aging Sci. 2008;1(3):202–212. doi: 10.2174/1874609810801030202. [DOI] [PubMed] [Google Scholar]
- 13.Leistritz DF, Hanson N, Martin GM, Oshima J. Werner Syndrome. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. Gene Revie. Seattle (WA): University of Washington, Seattle; 1993–2002. [PubMed] [Google Scholar]
- 14.Usui M, Ishii S, Yamawaki S, Hirayama T. The occurrence of soft tissue sarcomas in three siblings with Werner's syndrome. Cancer. 1984;54(11):2580–2586. doi: 10.1002/1097-0142(19841201)54:11<2580::aid-cncr2820541146>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Goto M, Miller RW, Ishikawa Y, Sugano H. Excess of rare cancers in Werner syndrome (adult progeria) Cancer Epidemiol Biomarkers Prev. 1996;5(4):239–246. [PubMed] [Google Scholar]
- 16.Satoh M, Imai M, Sugimoto M, et al. Prevalence of Werner's syndrome heterozygotes in Japan. Lancet. 1999;353(9166):1766. doi: 10.1016/S0140-6736(98)05869-3. 22. [DOI] [PubMed] [Google Scholar]
- 17.Nordyke EC. The peopling of Hawai‘i. Honolulu: University Press of Hawai‘i; 1977. [Google Scholar]
- 18.Le Marchand L. Do Japanese have an increased susceptibility to colorectal cancer? Hawai‘i Med J. 2006;65(10):296–297. [PubMed] [Google Scholar]
- 19.Lynch HT, Lynch JF, Lynch PM, Attard T. Hereditary colorectal cancer syndromes: molecular genetics, genetic counseling, diagnosis and management. Fam Cancer. 2008;7(1):27–39. doi: 10.1007/s10689-007-9165-5. [DOI] [PubMed] [Google Scholar]
- 20.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361(15):1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 21.O'Donnovan P, Livingston PM. BRCA1 and BRCA2: breast/ovarian cancer susceptibility gene products and participants in DNA double strand break repair. Carcinogenesis. 2010 doi: 10.1093/carcin/bgq069. in press. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez BY, Goodman MT. Ethnic disparities in colorectal cancer incidence and mortality in Hawai‘i. Hawai‘i Med J. 2004;63:54–56. [PubMed] [Google Scholar]
- 23.Massip L, Garand C, Paquet ER, et al. Vitamin C restores healthy aging in a mouse model for Werner syndrome. FASEB J. 2010;24(1):158–172. doi: 10.1096/fj.09-137133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grigorova M, Balajee AS, Natarajan AT. Spontaneous and X-ray-induced chromosomal aberrations in Werner syndrome cells detected by FISH using chromosome-specific painting probes. Mutagenesis. 2000;15(4):303–310. doi: 10.1093/mutage/15.4.303. [DOI] [PubMed] [Google Scholar]
- 25.Dong YP, Seki M, Yoshimura A, et al. WRN functions in a RAD18-dependent damage avoidance pathway. Biol Pharm Bull. 2007;30(6):1080–1083. doi: 10.1248/bpb.30.1080. [DOI] [PubMed] [Google Scholar]
- 26.Ariyoshi K, Suzuki K, Goto M, et al. Increased chromosome instability and accumulation of DNA double-strand breaks in Werner syndrome cells. J Radiat Res (Tokyo) 2007;48(3):219–231. doi: 10.1269/jrr.07017. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya H, Kato A, Kai N, et al. A case of Werner syndrome with three primary lesions of malignant melanoma. J Dermatol. 2005;32(9):737–744. doi: 10.1111/j.1346-8138.2005.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 28.Walton NP, Brammar TJ, Coleman NP. The musculoskeletal manifestations of Werner's syndrome. J Bone Joint Surg Br. 2000;82(6):885–888. doi: 10.1302/0301-620x.82b6.10457. [DOI] [PubMed] [Google Scholar]
- 29.Martin GM, Sprague CA, Epstein CJ. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970;23(1):86–92. [PubMed] [Google Scholar]
- 30.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138(6):2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]