Abstract
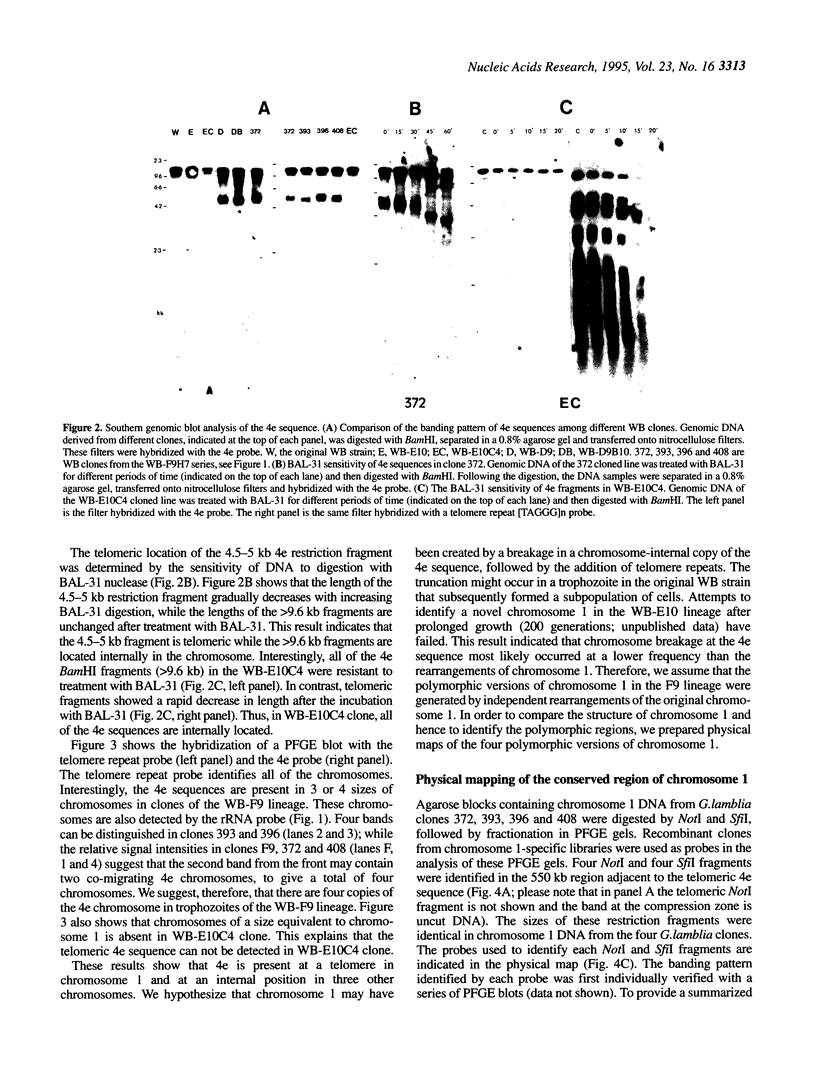
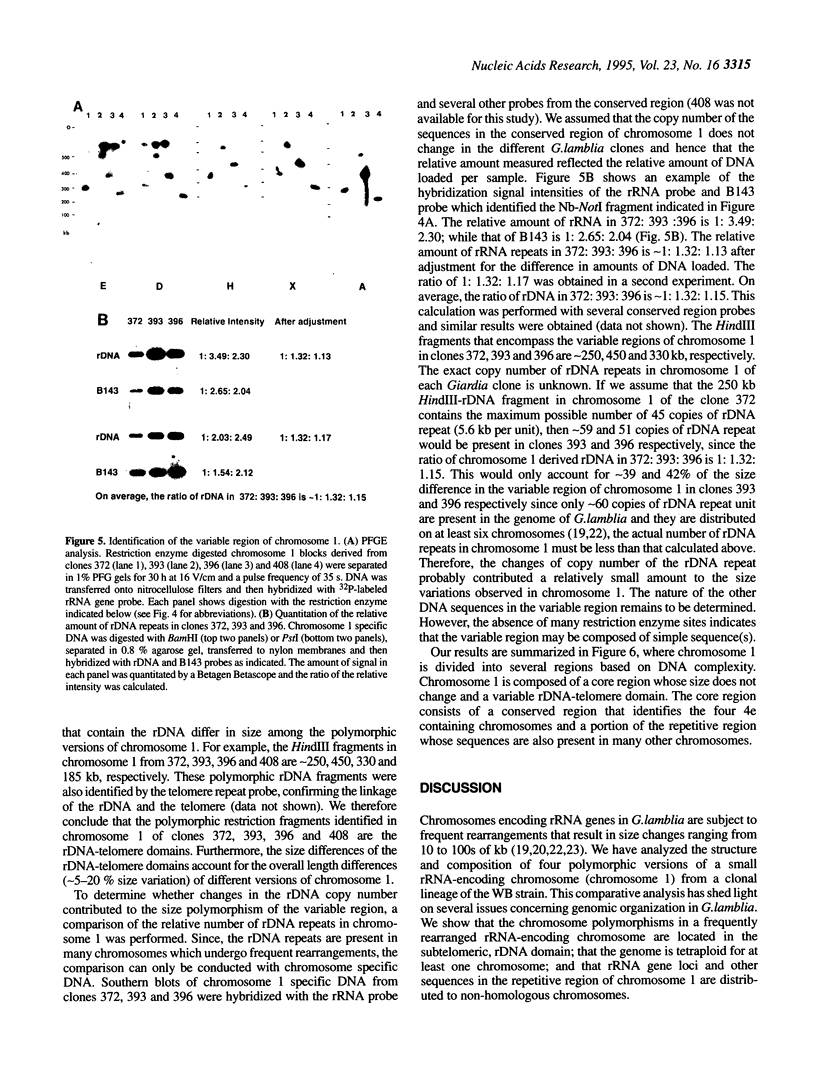
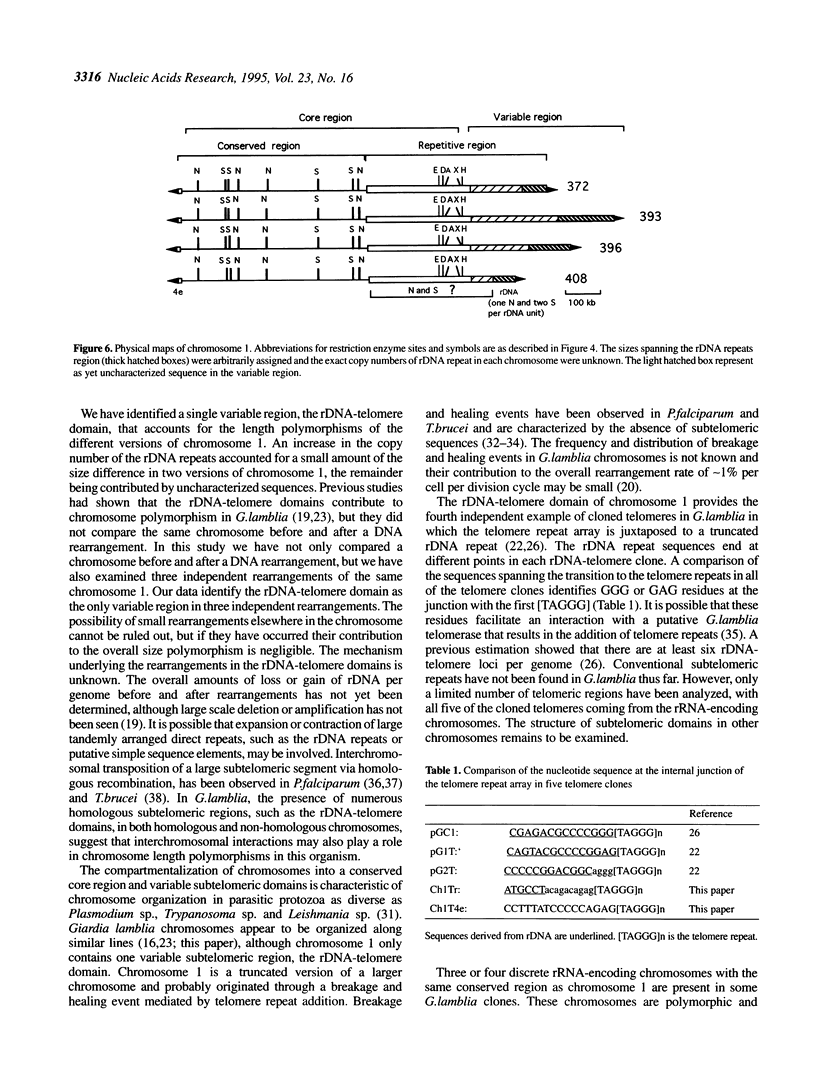
It has been shown previously that the rRNA encoding chromosomes in Giardia lamblia undergo frequent rearrangements with an estimated rate of approximately 1% per cell per division (Le Blancq et al., 1992, Nucleic Acids Res., 17, 4539-4545). Following these observations, we searched for highly recombinogenic regions in one of the frequently rearranged rRNA encoding chromosomes, that is chromosome 1, a small, 1.1 Mb chromosome. Chromosome 1 undergoes frequent rearrangements that result in size variation of 5-20%. We analyzed the structure of chromosome 1 in clonal lineages from the WB strain. The two ends of chromosome 1 comprise telomere repeat [TAGGG] arrays joined to a truncated rRNA gene and a sequence referred to as '4e', respectively. Comparison of the structure of four polymorphic versions of chromosome 1, resulting from independent rearrangement events in four cloned lines, located a single polymorphic region to the variable rDNA-telomere domain. Chromosome 1 is organized into two domains: a core region spanning approximately 850 kb that does not exhibit size heterogeneity among different chromosome 1 and a variable region that spans 185-450 kb and includes the telomeric rRNA genes, referred to as the variable rDNA-telomere domain. The core region contains a conserved region, spanning approximately 550 kb adjacent to the telomeric 4e sequence, which is only present in the 4e containing chromosomes and a 300 kb region of repetitive sequences that are also components of other chromosomes as well. Changes in the number of rDNA repeats accounted for some, but not all, of the size variation. Since there are four chromosomes that share the core region of chromosome 1, we suggest that the genome is tetraploid for this chromosome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam R. D., Aggarwal A., Lal A. A., de La Cruz V. F., McCutchan T., Nash T. E. Antigenic variation of a cysteine-rich protein in Giardia lamblia. J Exp Med. 1988 Jan 1;167(1):109–118. doi: 10.1084/jem.167.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R. D. Chromosome-size variation in Giardia lamblia: the role of rDNA repeats. Nucleic Acids Res. 1992 Jun 25;20(12):3057–3061. doi: 10.1093/nar/20.12.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R. D., Nash T. E., Wellems T. E. Telomeric location of Giardia rDNA genes. Mol Cell Biol. 1991 Jun;11(6):3326–3330. doi: 10.1128/mcb.11.6.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R. D., Nash T. E., Wellems T. E. The Giardia lamblia trophozoite contains sets of closely related chromosomes. Nucleic Acids Res. 1988 May 25;16(10):4555–4567. doi: 10.1093/nar/16.10.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R. D. The biology of Giardia spp. Microbiol Rev. 1991 Dec;55(4):706–732. doi: 10.1128/mr.55.4.706-732.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres: structure and synthesis. J Biol Chem. 1990 Apr 15;265(11):5919–5921. [PubMed] [Google Scholar]
- Boothroyd J. C., Wang A., Campbell D. A., Wang C. C. An unusually compact ribosomal DNA repeat in the protozoan Giardia lamblia. Nucleic Acids Res. 1987 May 26;15(10):4065–4084. doi: 10.1093/nar/15.10.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Carnaby S., Katelaris P. H., Naeem A., Farthing M. J. Genotypic heterogeneity within Giardia lamblia isolates demonstrated by M13 DNA fingerprinting. Infect Immun. 1994 May;62(5):1875–1880. doi: 10.1128/iai.62.5.1875-1880.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Upcroft J. A., Upcroft P. Physical map of a 2 Mb chromosome of the intestinal protozoan parasite Giardia duodenalis. Chromosome Res. 1994 Jul;2(4):307–313. doi: 10.1007/BF01552724. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Thompson J. K., Walliker D., Kemp D. J. Homologous recombination within subtelomeric repeat sequences generates chromosome size polymorphisms in P. falciparum. Cell. 1988 Jun 3;53(5):807–813. doi: 10.1016/0092-8674(88)90097-9. [DOI] [PubMed] [Google Scholar]
- De Jonckheere J. F., Majewska A. C., Kasprzak W. Giardia isolates from primates and rodents display the same molecular polymorphism as human isolates. Mol Biochem Parasitol. 1990 Feb;39(1):23–29. doi: 10.1016/0166-6851(90)90004-6. [DOI] [PubMed] [Google Scholar]
- Edlind T. D., Chakraborty P. R. Unusual ribosomal RNA of the intestinal parasite Giardia lamblia. Nucleic Acids Res. 1987 Oct 12;15(19):7889–7901. doi: 10.1093/nar/15.19.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. B., Korman S. H., Cantor C. R., Smith C. L. Giardia lamblia: haploid genome size determined by pulsed field gel electrophoresis is less than 12 Mb. Nucleic Acids Res. 1991 Apr 25;19(8):1905–1908. doi: 10.1093/nar/19.8.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farthing M. J. Host-parasite interactions in human giardiasis. Q J Med. 1989 Mar;70(263):191–204. [PubMed] [Google Scholar]
- Gottesdiener K., Garciá-Anoveros J., Lee M. G., Van der Ploeg L. H. Chromosome organization of the protozoan Trypanosoma brucei. Mol Cell Biol. 1990 Nov;10(11):6079–6083. doi: 10.1128/mcb.10.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey A., Mitchell R., Upcroft J. A., Boreham P. F., Upcroft P. Complete nucleotide sequence of the ribosomal RNA tandem repeat unit from Giardia intestinalis. Nucleic Acids Res. 1990 Jul 11;18(13):4006–4006. doi: 10.1093/nar/18.13.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth M. F. Immunology of Giardia and Cryptosporidium infections. J Infect Dis. 1992 Sep;166(3):465–472. doi: 10.1093/infdis/166.3.465. [DOI] [PubMed] [Google Scholar]
- Hinterberg K., Mattei D., Wellems T. E., Scherf A. Interchromosomal exchange of a large subtelomeric segment in a Plasmodium falciparum cross. EMBO J. 1994 Sep 1;13(17):4174–4180. doi: 10.1002/j.1460-2075.1994.tb06735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz M., Korman S. H., Shapiro M., Har-Even U., Tamir I., Strauss N., Deckelbaum R. J. Asymptomatic giardiasis in children. Pediatr Infect Dis J. 1989 Nov;8(11):773–779. doi: 10.1097/00006454-198911000-00009. [DOI] [PubMed] [Google Scholar]
- Kabnick K. S., Peattie D. A. In situ analyses reveal that the two nuclei of Giardia lamblia are equivalent. J Cell Sci. 1990 Mar;95(Pt 3):353–360. doi: 10.1242/jcs.95.3.353. [DOI] [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Corcoran L. M., Coppel R. L., Stahl H. D., Bianco A. E., Brown G. V., Anders R. F. Size variation in chromosomes from independent cultured isolates of Plasmodium falciparum. Nature. 1985 May 23;315(6017):347–350. doi: 10.1038/315347a0. [DOI] [PubMed] [Google Scholar]
- Korman S. H., Le Blancq S. M., Deckelbaum R. J., Van der Ploeg L. H. Investigation of human giardiasis by karyotype analysis. J Clin Invest. 1992 Jun;89(6):1725–1733. doi: 10.1172/JCI115774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzer M., Fischer K., Le Blancq S. M. Parasitism and chromosome dynamics in protozoan parasites: is there a connection? Mol Biochem Parasitol. 1995 Mar;70(1-2):1–8. doi: 10.1016/0166-6851(95)00021-r. [DOI] [PubMed] [Google Scholar]
- Le Blancq S. M., Kase R. S., Van der Ploeg L. H. Analysis of a Giardia lamblia rRNA encoding telomere with [TAGGG]n as the telomere repeat. Nucleic Acids Res. 1991 Oct 25;19(20):5790–5790. doi: 10.1093/nar/19.20.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blancq S. M., Korman S. H., Van der Ploeg L. H. Frequent rearrangements of rRNA-encoding chromosomes in Giardia lamblia. Nucleic Acids Res. 1991 Aug 25;19(16):4405–4412. doi: 10.1093/nar/19.16.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blancq S. M., Korman S. H., Van der Ploeg L. H. Spontaneous chromosome rearrangements in the protozoan Giardia lamblia: estimation of mutation rates. Nucleic Acids Res. 1992 Sep 11;20(17):4539–4545. doi: 10.1093/nar/20.17.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. A. The epidemiology of Giardiasis. Parasitol Today. 1985 Oct;1(4):101–105. doi: 10.1016/0169-4758(85)90004-3. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Aggarwal A., Adam R. D., Conrad J. T., Merritt J. W., Jr Antigenic variation in Giardia lamblia. J Immunol. 1988 Jul 15;141(2):636–641. [PubMed] [Google Scholar]
- Nash T. E., Conrad J. T., Merritt J. W., Jr Variant specific epitopes of Giardia lamblia. Mol Biochem Parasitol. 1990 Aug;42(1):125–132. doi: 10.1016/0166-6851(90)90120-b. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Herrington D. A., Losonsky G. A., Levine M. M. Experimental human infections with Giardia lamblia. J Infect Dis. 1987 Dec;156(6):974–984. doi: 10.1093/infdis/156.6.974. [DOI] [PubMed] [Google Scholar]
- Nash T. E., McCutchan T., Keister D., Dame J. B., Conrad J. D., Gillin F. D. Restriction-endonuclease analysis of DNA from 15 Giardia isolates obtained from humans and animals. J Infect Dis. 1985 Jul;152(1):64–73. doi: 10.1093/infdis/152.1.64. [DOI] [PubMed] [Google Scholar]
- Nash T. Surface antigen variability and variation in Giardia lamblia. Parasitol Today. 1992 Jul;8(7):229–234. doi: 10.1016/0169-4758(92)90119-m. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. Large deletions result from breakage and healing of P. falciparum chromosomes. Cell. 1988 Dec 2;55(5):869–874. doi: 10.1016/0092-8674(88)90142-0. [DOI] [PubMed] [Google Scholar]
- Sarafis K., Isaac-Renton J. Pulsed-field gel electrophoresis as a method of biotyping of Giardia duodenalis. Am J Trop Med Hyg. 1993 Jan;48(1):134–144. doi: 10.4269/ajtmh.1993.48.134. [DOI] [PubMed] [Google Scholar]
- Scherf A., Carter R., Petersen C., Alano P., Nelson R., Aikawa M., Mattei D., Pereira da Silva L., Leech J. Gene inactivation of Pf11-1 of Plasmodium falciparum by chromosome breakage and healing: identification of a gametocyte-specific protein with a potential role in gametogenesis. EMBO J. 1992 Jun;11(6):2293–2301. doi: 10.1002/j.1460-2075.1992.tb05288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Upcroft J. A., Boreham P. F., Upcroft P. Geographic variation in Giardia karyotypes. Int J Parasitol. 1989 Aug;19(5):519–527. doi: 10.1016/0020-7519(89)90082-9. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Bernards A., Rijsewijk F. A., Borst P. Characterization of the DNA duplication-transposition that controls the expression of two genes for variant surface glycoproteins in Trypanosoma brucei. Nucleic Acids Res. 1982 Jan 22;10(2):593–609. doi: 10.1093/nar/10.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Smith C. L., Polvere R. I., Gottesdiener K. M. Improved separation of chromosome-sized DNA from Trypanosoma brucei, stock 427-60. Nucleic Acids Res. 1989 Apr 25;17(8):3217–3227. doi: 10.1093/nar/17.8.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Adam R. D. Allele-specific expression of a variant-specific surface protein (VSP) of Giardia lamblia. Nucleic Acids Res. 1994 Jun 11;22(11):2102–2108. doi: 10.1093/nar/22.11.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomerdijk J. C., Kieft R., Borst P. A ribosomal RNA gene promoter at the telomere of a mini-chromosome in Trypanosoma brucei. Nucleic Acids Res. 1992 Jun 11;20(11):2725–2734. doi: 10.1093/nar/20.11.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]