Abstract
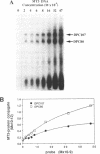
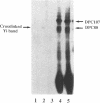
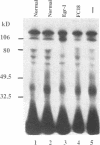
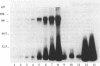
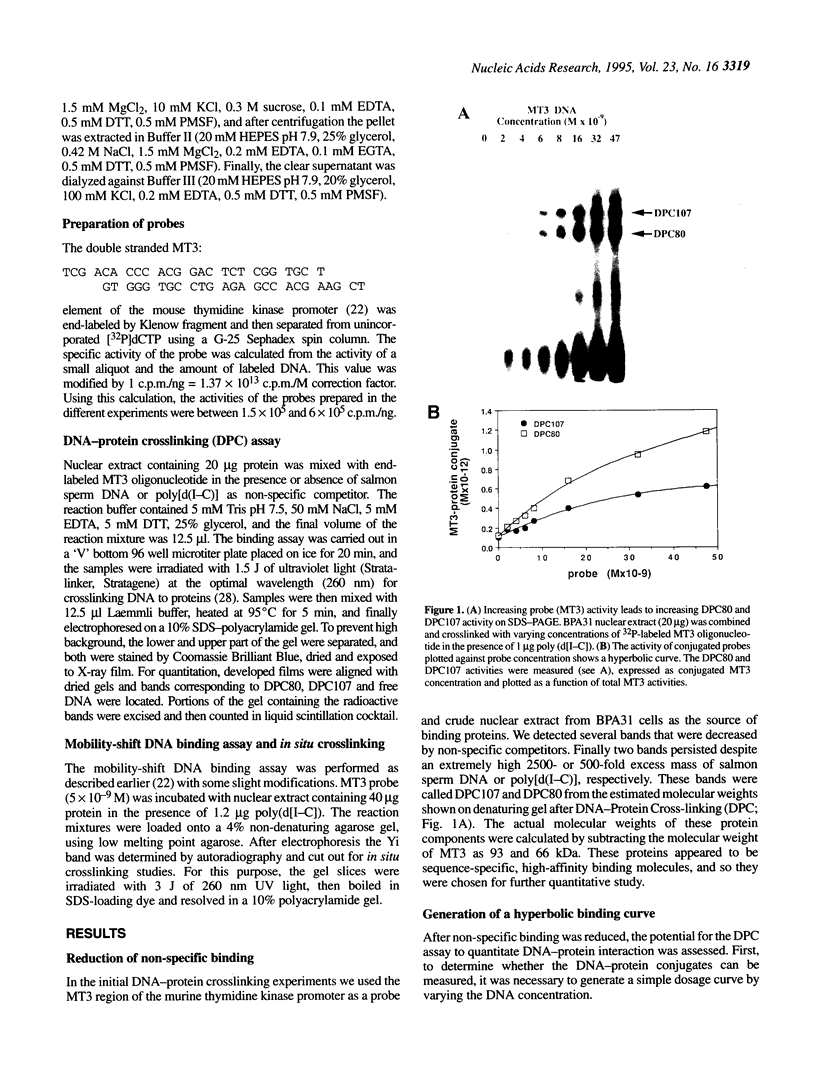
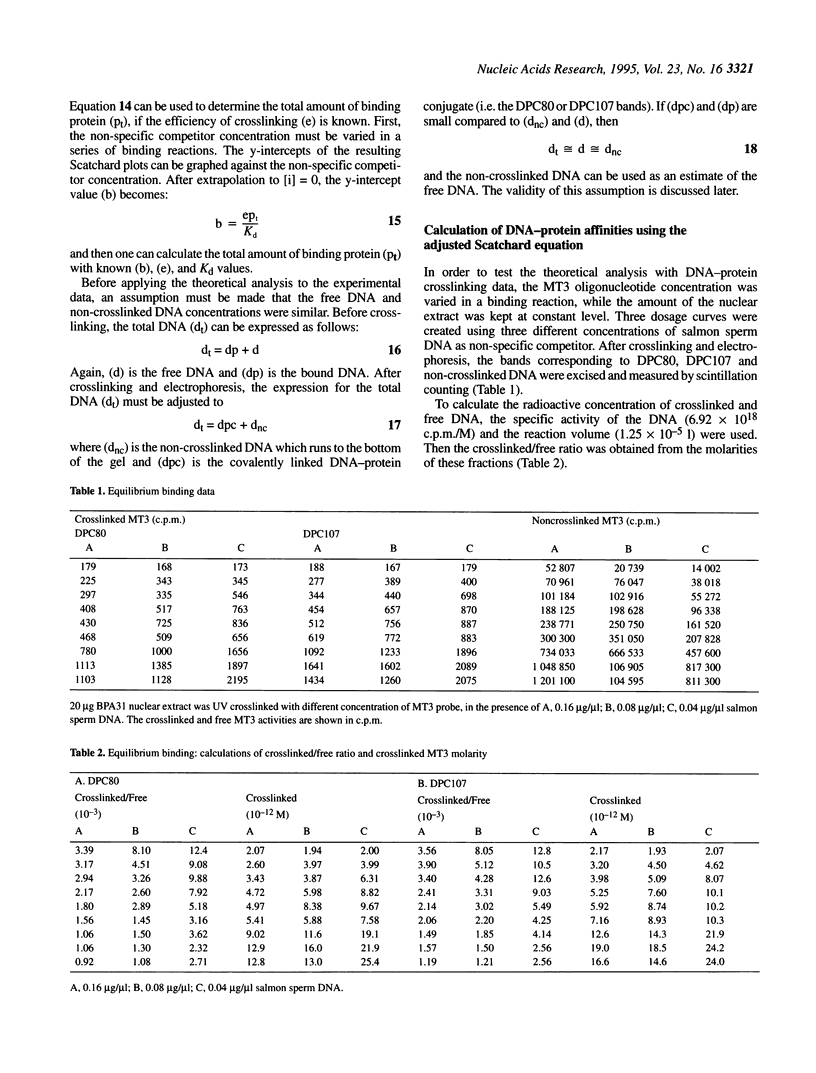
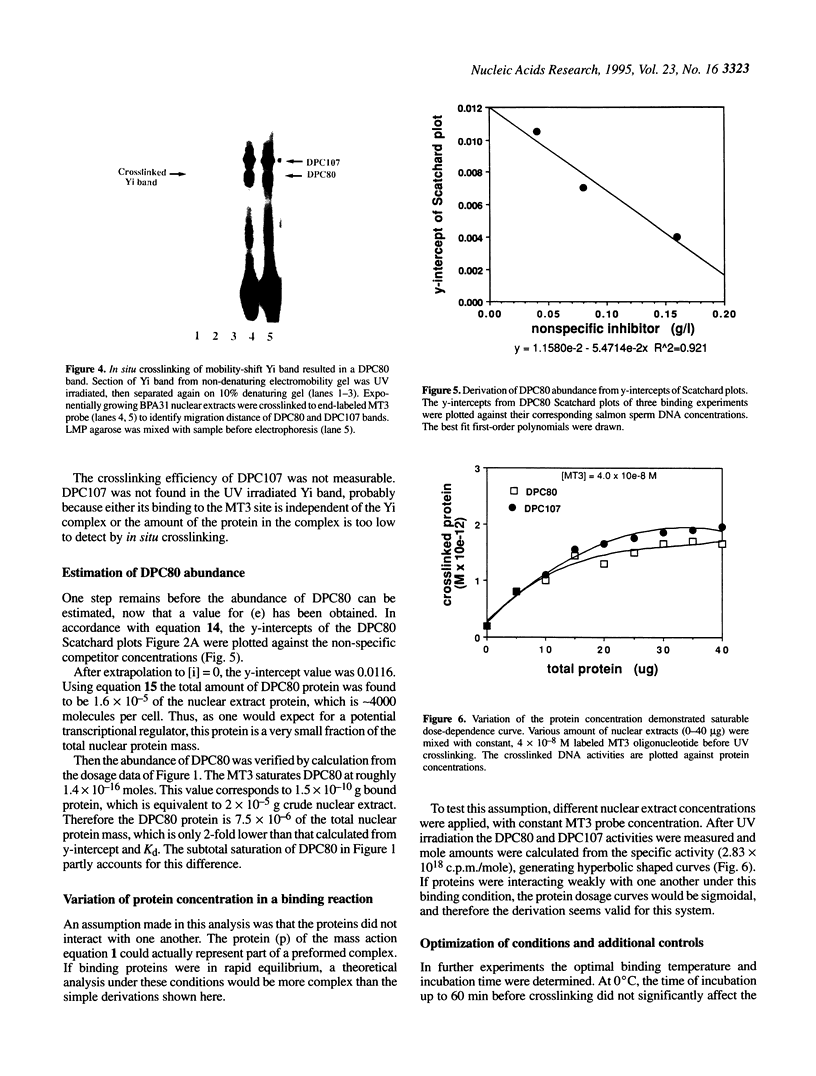
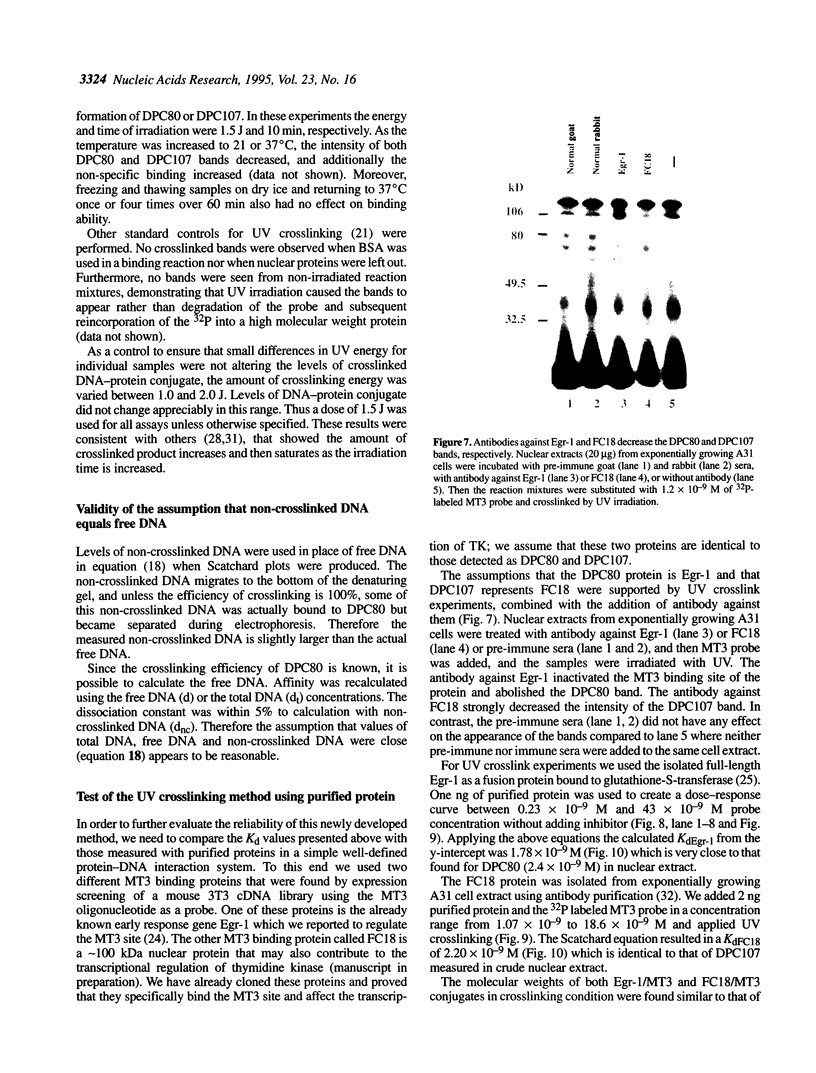
Measurement of the affinity of a protein for a promoter sequence is critical when assessing its potential to regulate transcription. Here we report that the DNA protein crosslinking (DPC) assay can be used to measure affinity, amount and molecular weight of DNA binding proteins to specific and non-specific DNA sequences. By applying a theoretical analysis to evaluate the binding data, it was shown that the affinity constants of two proteins (named DPC80 and DPC107) to the MT3 region of the mouse thymidine kinase promoter were 2 x 10(-9) M, which is 10(4) times higher than to non-specific DNA. Similar affinity constants were found when the purified proteins corresponding to DPC80 and DPC107 instead of nuclear extracts were used to assess the reliability of the DPC assay. A value for crosslinking efficiency was determined as 0.07, however, it is not needed for computation of the DNA-protein affinity, but with it the abundance of a binding protein can be estimated. In summary, the DPC assay is useful for quantifying DNA binding proteins and thereby judging their influence on transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. D., Wick K. L., Matthews K. S. Identification of amino acids in lac repressor protein cross-linked to operator DNA specifically substituted with bromodeoxyuridine. J Biol Chem. 1991 Apr 5;266(10):6113–6119. [PubMed] [Google Scholar]
- Bradley D. W., Dou Q. P., Fridovich-Keil J. L., Pardee A. B. Transformed and nontransformed cells differ in stability and cell cycle regulation of a binding activity to the murine thymidine kinase promoter. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9310–9314. doi: 10.1073/pnas.87.23.9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Repetitive and non-repetitive DNA sequences and a speculation on the origins of evolutionary novelty. Q Rev Biol. 1971 Jun;46(2):111–138. doi: 10.1086/406830. [DOI] [PubMed] [Google Scholar]
- Callan H. G. The organization of genetic units in chromosomes. J Cell Sci. 1967 Mar;2(1):1–7. doi: 10.1242/jcs.2.1.1. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Vayda M. E., Flint S. J. Identification of proteins and protein domains that contact DNA within adenovirus nucleoprotein cores by ultraviolet light crosslinking of oligonucleotides 32P-labelled in vivo. J Mol Biol. 1986 Mar 5;188(1):23–37. doi: 10.1016/0022-2836(86)90477-8. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Yang U. C., Flint S. J. Comparison of the interactions of the adenovirus type 2 major core protein and its precursor with DNA. Nucleic Acids Res. 1986 Mar 25;14(6):2721–2735. doi: 10.1093/nar/14.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Carthew R. W., Sharp P. A. A single polypeptide possesses the binding and transcription activities of the adenovirus major late transcription factor. Mol Cell Biol. 1986 Dec;6(12):4723–4733. doi: 10.1128/mcb.6.12.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. General model for the chromosomes of higher organisms. Nature. 1971 Nov 5;234(5323):25–27. doi: 10.1038/234025a0. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Organization, transcription, and regulation in the animal genome. Q Rev Biol. 1973 Dec;48(4):565–613. doi: 10.1086/407817. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Dou Q. P., Fridovich-Keil J. L., Pardee A. B. Inducible proteins binding to the murine thymidine kinase promoter in late G1/S phase. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1157–1161. doi: 10.1073/pnas.88.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franza B. R., Jr, Josephs S. F., Gilman M. Z., Ryan W., Clarkson B. Characterization of cellular proteins recognizing the HIV enhancer using a microscale DNA-affinity precipitation assay. 1987 Nov 26-Dec 2Nature. 330(6146):391–395. doi: 10.1038/330391a0. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Rauscher F. J., 3rd, Josephs S. F., Curran T. The Fos complex and Fos-related antigens recognize sequence elements that contain AP-1 binding sites. Science. 1988 Mar 4;239(4844):1150–1153. doi: 10.1126/science.2964084. [DOI] [PubMed] [Google Scholar]
- Fried M. G., Crothers D. M. Kinetics and mechanism in the reaction of gene regulatory proteins with DNA. J Mol Biol. 1984 Jan 25;172(3):263–282. doi: 10.1016/s0022-2836(84)80026-1. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman T. M., Lefever E., Shimkus M. Affinity chromatography of DNA labeled with chemically cleavable biotinylated nucleotide analogs. Anal Biochem. 1986 Jul;156(1):48–55. doi: 10.1016/0003-2697(86)90152-1. [DOI] [PubMed] [Google Scholar]
- Hilton M. D., Whiteley H. R. UV cross-linking of the Bacillus subtilis RNA polymerase to DNA in promoter and non-promoter complexes. J Biol Chem. 1985 Jul 5;260(13):8121–8127. [PubMed] [Google Scholar]
- Hockensmith J. W., Kubasek W. L., Vorachek W. R., von Hippel P. H. Laser cross-linking of nucleic acids to proteins. Methodology and first applications to the phage T4 DNA replication system. J Biol Chem. 1986 Mar 15;261(8):3512–3518. [PubMed] [Google Scholar]
- Holley R. W., Baldwin J. H., Kiernan J. A., Messmer T. O. Control of growth of benzo(a)pyrene-transformed 3T3 cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3229–3232. doi: 10.1073/pnas.73.9.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasher M. S., Pintel D., Ward D. C. Rapid enrichment of HeLa transcription factors IIIB and IIIC by using affinity chromatography based on avidin-biotin interactions. Mol Cell Biol. 1986 Sep;6(9):3117–3127. doi: 10.1128/mcb.6.9.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S., Sheng N., Dennis D. Yeast RNA polymerase I. Derivatization of the 190 and 135 subunits by 4-thiouridine monophosphate positioned uniquely at the 3' terminus of an enzyme-bound 32P-containing transcript initiated by a triribonucleotide primer on synthetic single-stranded DNA. J Biol Chem. 1990 May 15;265(14):7787–7792. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marciniak R. A., Garcia-Blanco M. A., Sharp P. A. Identification and characterization of a HeLa nuclear protein that specifically binds to the trans-activation-response (TAR) element of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 May;87(9):3624–3628. doi: 10.1073/pnas.87.9.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill B. M., Williams K. R., Chase J. W., Konigsberg W. H. Photochemical cross-linking of the Escherichia coli single-stranded DNA-binding protein to oligodeoxynucleotides. Identification of phenylalanine 60 as the site of cross-linking. J Biol Chem. 1984 Sep 10;259(17):10850–10856. [PubMed] [Google Scholar]
- Miller W. T., Kaiser E. T. Probing the peptide binding site of the cAMP-dependent protein kinase by using a peptide-based photoaffinity label. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5429–5433. doi: 10.1073/pnas.85.15.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskimins W. K., Roberts M. P., McClelland A., Ruddle F. H. Use of a protein-blotting procedure and a specific DNA probe to identify nuclear proteins that recognize the promoter region of the transferrin receptor gene. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6741–6744. doi: 10.1073/pnas.82.20.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar G., Crozat A., Pardee A. B. The immediate-early gene Egr-1 regulates the activity of the thymidine kinase promoter at the G0-to-G1 transition of the cell cycle. Mol Cell Biol. 1994 Aug;14(8):5242–5248. doi: 10.1128/mcb.14.8.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro Y., DiPaolo J. A. Changes in the uptake of 2-deoxy-D-glucose in BALB-3T3 cells chemically transformed in culture. J Cell Physiol. 1974 Apr;83(2):193–201. doi: 10.1002/jcp.1040830205. [DOI] [PubMed] [Google Scholar]
- Pauli U., Chiu J. F., Ditullio P., Kroeger P., Shalhoub V., Rowe T., Stein G., Stein J. Specific interactions of histone H1 and a 45 kilodalton nuclear protein with a putative matrix attachment site in the distal promoter region of a cell cycle-regulated human histone gene. J Cell Physiol. 1989 May;139(2):320–328. doi: 10.1002/jcp.1041390214. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Perrouault L., Le Doan T., Chassignol M., Thuong N., Hélène C. Sequence-specific binding and photocrosslinking of alpha and beta oligodeoxynucleotides to the major groove of DNA via triple-helix formation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1349–1353. doi: 10.1073/pnas.85.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkus M., Levy J., Herman T. A chemically cleavable biotinylated nucleotide: usefulness in the recovery of protein-DNA complexes from avidin affinity columns. Proc Natl Acad Sci U S A. 1985 May;82(9):2593–2597. doi: 10.1073/pnas.82.9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer J. C., Rudd C. E. A 32-kD GTP-binding protein associated with the CD4-p56lck and CD8-p56lck T cell receptor complexes. Science. 1991 Oct 18;254(5030):439–441. doi: 10.1126/science.1925604. [DOI] [PubMed] [Google Scholar]
- Wick K. L., Matthews K. S. Interactions between lac repressor protein and site-specific bromodeoxyuridine-substituted operator DNA. Ultraviolet footprinting and protein-DNA cross-link formation. J Biol Chem. 1991 Apr 5;266(10):6106–6112. [PubMed] [Google Scholar]