Abstract
Aims/hypothesis
The aim of the study was to determine the transition rate and factors associated with the progression of normo- and low microalbuminuria to diabetic nephropathy (overt proteinuria).
Methods
For 8 years we prospectively observed 1,558 Japanese patients with type 2 diabetes mellitus whose basal urinary albumin:creatinine ratio (UACR) had been measured as <17.0 mg/mmol at entry. The incidence of nephropathy (UACR >33.9 mg/mmol) was determined by measuring UACR twice a year.
Results
Progression to nephropathy occurred in 74 patients. The annual transition rate was 0.67%, and was substantially higher for the low-microalbuminuric group than for the normoalbuminuric group (1.85% and 0.23%, respectively; hazard ratio for the low-microalbuminuric group 8.45, p < 0.01). The hazard ratio for an HbA1c of 7–9% or ≥9% was 2.72 (p < 0.01) or 5.81 (p < 0.01) relative to HbA1c <7.0%, respectively. In comparison with individuals with a systolic blood pressure (SBP) of <120 mmHg, the hazard ratios for patients with an SBP of 120–140 mmHg or ≥140 mmHg were 2.31 (p = 0.06) and 3.54 (p < 0.01), respectively. Smoking also affected progression to proteinuria (hazard ratio 1.99, p < 0.01). In contrast, 30.3% of the low-microalbuminuric group returned to normoalbuminuria (i.e. were in remission).
Conclusions/interpretation
These results suggest that if patients with type 2 diabetes mellitus are receiving treatment from diabetologists for hyperglycaemia and hypertension when they are in the early stages of nephropathy (i.e. normo- or low microalbuminuria), their rate of transition to proteinuria is considerably lowered, and that differentiating patients with low microalbuminuria from those with high microalbuminuria might be clinically useful.
Trial registration
UMIN Clinical Trials Registry C000000222
Funding
The study was funded by the Ministry of Health, Labour and Welfare, Japan.
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-010-2025-0) contains supplementary material, which is available to authorised users.
Keywords: Blood pressure, Diabetic nephropathy, Glycaemic control, Progression, Remission, Smoking
Introduction
Diabetic nephropathy is the most common cause of end-stage renal disease (ESRD) in many countries, including Japan [1–3]. In the UK Prospective Diabetes Study (UKPDS), 24.9% of patients developed microalbuminuria within 10 years of diagnosis of type 2 diabetes, but only 0.8% developed ESRD, as assessed by an elevated plasma creatinine level (>250 μmol/l) or the need for renal replacement therapy [4]. Annual rates of transition between successive stages within the classic paradigm of normoalbuminuria to microalbuminuria to macroalbuminuria to ESRD were 2–3% per year [4].
In Japan, the number of patients requiring renal replacement therapy has increased threefold in less than 15 years [3]. Among 36,017 patients who started haemodialysis in 2007, the number of diabetic patients has reached 15,663 (43.5%) [3]. In Hong Kong, the overall number of people receiving renal replacement therapy increased by 50% between 1995 and 1999, and in the diabetic group, a 100% increase was observed [5]. Thus, Asians have a predisposition to diabetic nephropathy and ESRD. In fact, the recent Japanese Incipient to Overt: Angiotensin II Receptor Blocker, Telmisartan, Investigation on Type 2 Diabetic Nephropathy (INNOVATION) trial revealed that about 50% of diabetic individuals with high microalbuminuria (urinary albumin/creatinine ratio [UACR] between 11.3 and 33.9 mg/mmol [100–300 mg/g]) progressed to proteinuria within 2 years [6], indicating that progression is very rapid once high microalbuminuria develops. On the other hand, intervention using angiotensin receptor blockers (ARBs) such as losartan or telmisartan seems to be very effective in Asians in comparison with Europeans [6, 7]. The Japan Diabetes Complications Study (JDCS) is a nationwide randomised controlled study of type 2 diabetic patients focusing on lifestyle modification [8, 9]. Although the status of control of most classic cardiovascular risk factors, including body weight, glycaemia, serum lipids and blood pressure, did not differ between the two groups during the study period, the incidence of stroke in the intensive lifestyle intervention group (0.55/100 patient-years) was significantly lower than in the control group (0.95/100 patient-years) by Kaplan–Meier analysis, while the incidence of nephropathy did not differ significantly between the groups [9]. Here, we report the rate of transition and factors associated with the development and/or progression of normo- and low microalbuminuria to diabetic nephropathy (overt proteinuria) in this JDCS cohort.
Methods
In 1996, 2,205 patients aged 40–70 years with previously diagnosed type 2 diabetes and HbA1c levels of >6.5% were recruited and registered from 59 hospitals specialising in diabetes care. The protocol for the study, which was in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical/Epidemiological Studies of the Japanese Ministry of Health, Labour and Welfare, received ethics approval from the institutional review boards of all the participating institutions. Written informed consent was obtained from all the patients enrolled. The inclusion criteria for participating patients have been described previously by Sone et al. [8]. A final total of 2,033 patients aged 58.5 ± 6.9 years (mean ± SD) were included in the study, and their diabetes duration was 10.9 ± 7.2 years.
The recruited patients were randomly allocated to either an intensive lifestyle intervention group or a conventional treatment group. Details of the intervention have been described previously by Sone et al. [8, 9]. We selected a cohort of 1,558 patients in whom the mean value of the two-spot UACR was <17.0 mg/mmol (150 mg/g) without microscopic haematuria or other clinical findings indicating other renal diseases. We followed this cohort for 8 years, and measured their body weight, waist/hip circumference and blood pressure at least twice a year. Fasting plasma glucose, HbA1c, serum lipids and serum creatinine levels were also determined twice a year. Spot UACR was also determined at least twice a year using the turbidimetric immunoassay to measure the urinary albumin concentration. We defined normoalbuminuria as a UACR of <3.4 mg/mmol (30 mg/g), and low microalbuminuria as a UACR of 3.4 to 17.0 mg/mmol (30 to 150 mg/g). Estimated glomerular filtration rate (eGFR) was calculated using serum creatinine levels according to the modification of diet in renal disease (MDRD) formula modified for Japanese populations [10].
Statistical analyses
The primary endpoint for the nephropathy analysis was transition from normo- or low microalbuminuria to proteinuria (>33.9 mg/mmol [300 mg/g]) in two consecutive urine samples. Transition to proteinuria was summarised by the annual rate of transition to proteinuria and the remission proportion was defined as those patients whose mean UACR at the final two visits was <3.4 mg/mmol. Risk factors for proteinuria were explored by the following survival analysis methods. Univariate analyses were performed by the Kaplan–Meier method, logrank test, and univariate Cox regression with a 95% CI. Multivariate Cox regression was also used. The SAS software package (version 9.2, SAS Institute, Cary, NC, USA) was used for all analyses, with the level of significance set at p < 0.05.
Results
Tables 1 and 2 give the baseline characteristics and glycaemic and blood pressure control at baseline, and at 4 and 8 years after the start of observation. As shown in Table 2, the proportion of patients who were receiving insulin injections increased from 20.7% to 41.9% over 8 years. The use of antihypertensive agents also increased over this period from 28.2% to 42.0%. In particular, usage of renin–angiotensin system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and/or ARBs increased from 12.3% to 28.4% over 8 years. The use of statins also increased from 20.5% to 31.1%. Over a median follow-up period of 7.98 years, 74 patients developed proteinuria. The annual transition rate was 0.67 per 100 person-years (95% CI 0.53–0.84). For the low-microalbuminuric group, the annual transition rate per 100 person-years was substantially higher than for the normoalbuminuric patients (1.85 [95% CI 1.43–2.41] and 0.23 [95% CI 0.14–0.36]), respectively. On the other hand, remission (i.e. normalisation) occurred in 137 (30.3%) of the 452 individuals with low microalbuminuria (Table 3).
Table 1.
Baseline characteristics of 1,558 patients included in the nephropathy analysis
| Variable | Mean ± SDa |
|---|---|
| n (men/women) | 1,558 (813/745) |
| Age (years) | 58.5 ± 6.9 |
| BMI (kg/m2) | 23.0 ± 2.9 |
| Waist (cm) | 79.4 ± 9.2 |
| SBP (mmHg) | 132.4 ± 15.8 |
| DBP (mmHg) | 76.6 ± 9.5 |
| Fasting plasma glucose (mmol/l) | 8.9 ± 2.4 |
| HbA1c (%) | 7.8 ± 1.3 |
| Duration of diabetes (years) | 10.7 ± 7.1 |
| Serum total cholesterol (mmol/l) | 5.19 ± 0.89 |
| Serum triacylglycerols (mmol/l)b | 1.15 ± 0.82 |
| Serum HDL-cholesterol (mmol/l) | 1.41 ± 0.43 |
| UACR (mg/mmol)b | 1.8 ± 3.0 |
| eGFR (ml min−1 1.73 m−2)b | 81.3 ± 32.1 |
| Current/past/never smoker (%) | 27/24/49 |
| Ethanol intake: 0/1–38/≧38 g/day (%) | 62/31/7 |
DBP, diastolic blood pressure
aUnless otherwise stated
bMedian±interquartile range
Table 2.
Measures of glycaemic and blood pressure control at the baseline and at 4 and 8 years after the start of intervention
| Variable | Baseline | 4 years after start of intervention | 8 years after start of intervention |
|---|---|---|---|
| BMI (kg/m2) | 23.0 ± 2.9 | 23.0 ± 3.0 | 23.0 ± 3.1 |
| SBP (mmHg) | 132.4 ± 15.8 | 132.5 ± 15.4 | 132.5 ± 15.9 |
| DBP (mmHg) | 76.6 ± 9.5 | 75.9 ± 9.1 | 74.0 ± 10.0 |
| Fasting plasma glucose (mmol/l) | 8.9 ± 2.4 | 8.9 ± 2.6 | 8.6 ± 2.5 |
| HbA1c (%) | 7.8 ± 1.3 | 7.7 ± 1.2 | 7.7 ± 2.0 |
| Hypoglycaemic agent (%) | |||
| Any use | 84.4 | 89.3 | 86.6 |
| Insulin | 20.7 | 30.1 | 41.9 |
| Sulfonylurea | 62.3 | 63.3 | 59.7 |
| Alpha-glucosidase inhibitor | 25.9 | 29.9 | 28.8 |
| Biguanide | 7.5 | 16.1 | 32.8 |
| Insulin sensitiser | 1.2 | 8.0 | 9.1 |
| Antihypertensive agent (%) | |||
| Any use | 28.2 | 33.3 | 42.0 |
| ACE inhibitor/ARB | 12.3 | 16.6 | 28.4 |
| Calcium-channel blocker | 20.7 | 24.4 | 27.2 |
| Diuretic | 1.2 | 1.1 | 2.9 |
| Other | 6.0 | 7.1 | 8.6 |
| Statin (%) | 20.5 | 23.7 | 31.1 |
Each value is expressed as mean ± SD or percentage
DBP, diastolic blood pressure
Table 3.
Mean UACR measured at the final two visits stratified by the basal value
| Basal UACR (mg/mmol) | Final UACR (mg/mmol) | |||
|---|---|---|---|---|
| <3.4 | 3.4–17.0 | 17.0–33.9 | ≥33.9 | |
| <3.4 | 817 (73.9) | 244 (22.1) | 27 (2.4) | 18 (1.6) |
| 3.4–17.0 | 137 (30.3) | 203 (44.9) | 56 (12.4) | 56 (12.4) |
| Total | 954 (61.2) | 447 (28.7) | 83 (5.3) | 74 (4.8) |
Data shown are n (%)
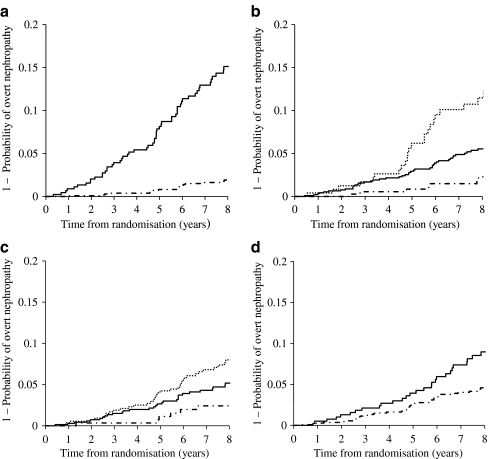
Figure 1 shows the Kaplan–Meier curves for progression to overt nephropathy on the basis of UACR (Fig. 1a), HbA1c level (Fig. 1b), systolic blood pressure (SBP, Fig. 1c) and smoking status (Fig. 1d). As can be seen, patients with higher UACR, higher HbA1c, higher SBP or current smokers had a higher risk for progression to proteinuria. The hazard ratio for the low-microalbuminuric group was 8.45 (p < 0.01) relative to the normoalbuminuric group. Stratification of eGFR to >90, 60–90 and <60 ml min–1 1.73 m–2 did not predict progression to proteinuria. The hazard ratio of HbA1c for a range of 7–9% or for ≥9% was 2.72 (p < 0.01) or 5.81 (p < 0.01) relative to an HbA1c of <7%, respectively. In comparison with individuals with an SBP of <120 mmHg, the hazard ratio for patients with an SBP of 120–140 mmHg or ≥140 mmHg was 2.31 (p = 0.06) and 3.54 (p < 0.01), respectively. Smoking also affected progression to proteinuria, with a hazard ratio of 1.99 (p < 0.01).
Fig. 1.
Kaplan–Meier curves for progression to overt nephropathy according to: UACR (a), HbA1c levels (b), SBP (c) and smoking status (d). a The hazard ratio for the low-microalbuminuric group (solid line) was 8.45 (95% CI 4.97–14.38, p < 0.01) relative to the normoalbuminuric group (dashed–dotted line). b The hazard ratio of HbA1c for a range of 7–9% (solid line) and for ≥9% (dotted line) was 2.72 (95% CI 1.22–6.03, p < 0.01) and 5.81 (95% CI 2.49–13.55, p < 0.01), respectively, relative to an HbA1c of <7% (dashed–dotted line). c The hazard ratio for an SBP of 120–140 mmHg (solid line) or ≥140 mmHg (dotted line) was 2.31 (95% CI 0.96–5.54, p < 0.06) and 3.54 (95% CI 1.50–8.40, p < 0.01), respectively, relative to an SBP of <120 mmHg (dashed–dotted line). d The hazard ratio for current smoking (solid line) was 1.99 (95% CI 1.24–3.18, p < 0.01) relative to past smoking or never smoked (dashed–dotted line)
Table 4 shows risk factors for the development of proteinuria based on multivariate Cox regression analysis. All the factors shown to be significant by univariate analysis—UACR, HbA1c level, SBP level and smoking status—were significantly associated with the development of proteinuria after adjustment for other clinical factors. Multivariate Cox regression analysis showed that the hazard ratio for use of ACE inhibitors and/or ARBs was 1.49 (95% CI 0.83–2.69, p = 0.19) and that the hazard ratio for use of statins was 0.73 (95% CI 0.38–1.41, p = 0.35) in relation to the progression to proteinuria.
Table 4.
Risk factors for progression to proteinuria demonstrated by multivariate Cox regression analysis
| Risk factor | Hazard ratio | 95% CI | p value |
|---|---|---|---|
| Conventional/intervention | 1.01 | 0.63–1.61 | 0.98 |
| Age, +10 years | 1.03 | 0.71–1.49 | 0.87 |
| Sex, woman/man | 0.74 | 0.41–1.34 | 0.32 |
| Duration, +10 years | 1.16 | 0.80–1.68 | 0.44 |
| BMI, +1 kg/m2 | 1.01 | 0.93–1.10 | 0.73 |
| SBP, 120–140/<120 mmHg | 1.90 | 0.73–4.95 | 0.19 |
| SBP, ≥140/<120 mmHg | 2.55 | 0.98–6.63 | 0.05 |
| HbA1c, 7–9/<7% | 2.22 | 1.00–4.96 | 0.05 |
| HbA1c, ≥9/<7% | 4.16 | 1.73–10.04 | <0.01 |
| LDL-cholesterol, ≥4.0/<4.0 mmol/l | 0.85 | 0.48–1.49 | 0.57 |
| Triacylglycerol, ≥2.3/<2.3 mmol/l | 1.60 | 0.88–2.89 | 0.12 |
| HDL-cholesterol, ≥1.0/<1.0 mmol/l | 1.43 | 0.79–2.61 | 0.24 |
| UACR, ≥3.4/<3.4 mg/mmol | 6.98 | 4.02–12.10 | <0.01 |
| Current smoker/past or never smoker | 1.87 | 1.07–3.25 | 0.03 |
| Ethanol intake, ≥38 g/<38 g/day | 0.99 | 0.98–1.01 | 0.38 |
Missing values meant 126 patients were excluded
Discussion
Based on the main result of the JDCS study, which was reported previously by Sone et al., the incidence of stroke in the intensive lifestyle intervention group was significantly lower, by 38%, than in the control group, while the incidence of nephropathy did not differ significantly between the groups [9]. Lifestyle intervention resulted in a small but significant temporary improvement of glycaemic control and only minimal changes in other known risk factors for diabetic complications, including blood pressure, indicating the difficulty of changing the lifestyle of patients with long-term diabetes. In this sense, patients who participated in this study could be considered as representative of the general population of patients with type 2 diabetes. This might explain why there was no difference in the incidence of diabetic nephropathy. The main finding of interest in this study was that the annual incidence of proteinuria was as low as 0.67% (0.67/100 person-years), in marked contrast to previous reports. In the UKPDS, the annual rates of transition from normoalbuminuria to microalbuminuria and from microalbuminuria to macroalbuminuria in newly diagnosed patients with type 2 diabetes were 2% and 2.8% per year, respectively [4]. Ravid et al. [11] reported higher progression rates in type 2 diabetic patients in Israel, i.e. 35% from normoalbuminuria to microalbuminuria and 16% from normoalbuminuria to macroalbuminuria during 7.8 years. In Pima Indians with normotensive type 2 diabetes, Nelson et al. [12] also reported that the rates of progression from normoalbuminuria to microalbuminuria and to macroalbuminuria during 4.7 years was 37.8% and 4.3%, respectively. In Japan, a clinic-based observational 6.8 year longitudinal study of 426 patients who developed diabetes before the age of 30 years revealed that the incidence of proteinuria developing from normoalbuminuria or microalbuminuria was 1.41/100 person-years [13]. In another Japanese clinic-based observational longitudinal study conducted for 6 years, 28% of 216 patients enrolled from 1996 to 1998 showed progression from microalbuminuria to proteinuria [14]. It is difficult to compare the annual incidence of proteinuria with that found in other studies because the stages of nephropathy differ from one study to another. However, the rate of transition to proteinuria in the JDCS seems to be very low. Of course, one of the reasons for this low incidence might be that two-thirds of the enrolled patients had normoalbuminuria and one-third had low microalbuminuria. In contrast, the placebo group in the INNOVATION trial showed a considerably higher transition rate, amounting to 50%, from high microalbuminuria to proteinuria within 2 years, with a UACR between 11.3 and 33.9 mg/mmol [6], although the UACR was determined using the first-voided morning urine. Taken together with these studies, the data suggest that the current treatment by diabetologists along with administration of the usual hypoglycaemic and hypotensive drugs from the stage of normoalbuminuria or low microalbuminuria reduced the annual incidence of proteinuria to a level as low as 0.67/100 person-years. Ideally, however, the inclusion of a control group receiving placebo and matched to the drug-treated diabetic patients would be desirable in order to allow a firm conclusion to be drawn, although admittedly this would be ethically problematic. As the baseline UACR profoundly affected the cumulative incidence of proteinuria, it might be clinically useful to divide patients with microalbuminuria into low- and high-risk groups, i.e. those with low and high microalbuminuria, although the cut-off value remains to be determined.
In the present study, progression to proteinuria was independently associated with higher baseline HbA1c and SBP levels in addition to an elevated baseline UACR. Furthermore, smoking was also a significant predictor of proteinuria. These results are consistent with previous studies [11, 15]. In the UKPDS, the risk factors most highly associated with proteinuria were reported to be urinary albumin, plasma creatinine, waist circumference, SBP, glycaemic control, LDL-cholesterol, and plasma triacylglycerol [15]. Indian-Asian ethnicity was also an independent risk factor for microalbuminuria and/or proteinuria [12, 15]. Smoking and male sex were reported to be independent predictors of proteinuria in addition to plasma cholesterol, mean blood pressure and HbA1c [11]. Based on these epidemiological studies, tight glycaemic control has been reported to be effective for preventing the onset and/or progression of nephropathy in clinical trials such as the Diabetes Control and Complications Trial (DCCT), the Kumamoto study and the UKPDS [16–18]. Strict blood pressure control, especially with ACE inhibitors or ARBs, has also been demonstrated to be effective for delaying the progression of diabetic nephropathy [6, 7, 19–21]. However, in the present study, the initial usage of an ACE inhibitor and/or ARB, or statin was not significantly associated with the prevention of proteinuria. As this study was designed to clarify the effects of lifestyle intervention on subsequent occurrence of diabetic complications, it might have been difficult to recognise the effects of such drugs on the progression of diabetic nephropathy. In some studies, normalisation of microalbuminuria, i.e. remission/regression, has also been reported [6, 12]. In fact, in our study, 30.3% of 452 individuals with low microalbuminuria demonstrated normalisation.
However, following the advent of modern therapeutics, especially hypoglycaemic and antihypertensive agents, diabetic nephropathy is the most common cause of ESRD, and the number of patients being started on haemodialysis is still increasing dramatically in many countries, particularly in Asia. Our data have major clinical relevance because we have demonstrated that the initiation of hypoglycaemic and antihypertensive treatment from the early stage of nephropathy might lower the rate of transition to proteinuria even in the Japanese, who are highly susceptible to diabetic nephropathy. To reduce the number of patients who require haemodialysis, it is very important to measure UACR, make a diagnosis of diabetic nephropathy, define the stage of nephropathy and initiate strict glycaemic and blood pressure control as early as at the normo- or low-microalbuminuria stage.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 44.4 kb)
Acknowledgements
We thank the many diabetologists (the members of the JDCS Group are listed in the Electronic supplementary material [ESM]) and patients at the 59 participating institutions throughout Japan. Part of this study has been reported previously in abstract form ([2008] Diabetes 57 (Suppl 1):A210). This study was financially supported by the Ministry of Health, Labour and Welfare, Japan. The sponsor had no role in the design or conduct of the study.
Duality of interest
The authors declare that there is no conflict of interest associated with the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- ACE
Angiotensin-converting enzyme
- ARB
Angiotensin receptor blocker
- eGFR
Estimated glomerular filtration rate
- ESRD
End-stage renal disease
- INNOVATION
Incipient to Overt: Angiotensin II Receptor Blocker, Telmisartan, Investigation on Type 2 Diabetic Nephropathy
- JDCS
Japan Diabetes Complications Study
- SBP
Systolic blood pressure
- UACR
Urinary albumin/creatinine ratio
- UKPDS
UK Prospective Diabetes Study
References
- 1.Ritz E, Orth S. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999;341:1127–1133. doi: 10.1056/NEJM199910073411506. [DOI] [PubMed] [Google Scholar]
- 2.Caramori MI, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes. 2000;49:1399–1408. doi: 10.2337/diabetes.49.9.1399. [DOI] [PubMed] [Google Scholar]
- 3.Statistic Committee of Japan Hemodialysis Society An overview of dialysis treatment in Japan (as of December 31, 2007) J Jpn Hemodialysis Soc. 2009;42:1–45. doi: 10.4009/jsdt.42.1. [DOI] [Google Scholar]
- 4.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 5.Lui SF, Ho YW, Chu KF, Leung CB, Choy BY. Hong Kong registry 1995–1999. Hong Kong J Nephrol. 1999;1:53–60. doi: 10.1016/S1561-5413(09)60020-X. [DOI] [Google Scholar]
- 6.Makino H, Haneda M, Babazono T, et al. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care. 2007;30:1577–1578. doi: 10.2337/dc06-1998. [DOI] [PubMed] [Google Scholar]
- 7.Chan JCN, Wat NMS, So W-Y, et al. Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes. An Asian perspective from the RENAAL Study. Diabetes Care. 2004;27:874–879. doi: 10.2337/diacare.27.4.874. [DOI] [PubMed] [Google Scholar]
- 8.Sone H, Katagiri A, Ishibashi S, et al. Effects of lifestyle modifications on patients with type 2 diabetes: the Japan Diabetes Complications Study (JDCS) study design, baseline analysis and three-year interim report. Horm Metab Res. 2002;34:509–515. doi: 10.1055/s-2002-34791. [DOI] [PubMed] [Google Scholar]
- 9.Sone H, Tanaka S, Iimuro S, et al. Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicenter randomized controlled trial (the Japan Diabetes Complications Study) Diabetologia. 2010;53:419–428. doi: 10.1007/s00125-009-1622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med. 1998;159:998–1004. doi: 10.1001/archinte.158.9.998. [DOI] [PubMed] [Google Scholar]
- 12.Nelson RG, Knowler WC, Pettitt DJ, Hanson RL, Benett PH. Incidence and determinants of elevated urinary albumin excretion in Pima Indians with NIDDM. Diabetes Care. 1995;18:182–187. doi: 10.2337/diacare.18.2.182. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama H, Okudaira M, Otani T, et al. High incidence of diabetic nephropathy in early-onset Japanese NIDDM patients. Diabetes Care. 1998;21:1080–1085. doi: 10.2337/diacare.21.7.1080. [DOI] [PubMed] [Google Scholar]
- 14.Araki S, Haneda M, Sugimoto T, et al. Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes. 2005;54:2983–2987. doi: 10.2337/diabetes.54.10.2983. [DOI] [PubMed] [Google Scholar]
- 15.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes. U.K. Prospective Diabetes Study 74. Diabetes. 2006;55:1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 16.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 17.Okubo Y, Shichiri M, Kishikawa H, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complication in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diab Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-K. [DOI] [PubMed] [Google Scholar]
- 18.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 19.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 20.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 21.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 44.4 kb)