Abstract
Background
Euro 1.1 billion were spent in 2009 for disease management programs (DMPs) in Germany, among them the DMP for type 2 diabetes mellitus (T2DM). Earlier studies of DMPs failed to take sufficient account of patient- and physician-related selection effects. We used innovative methods to study the medical benefit of the DMP for T2DM among insurees of the Techniker Krankenkasse, a German health insurance provider.
Methods
For this study, we analyzed claims data of the Techniker Krankenkasse from 2006 to 2008. We developed and implemented a sophisticated control group design based on propensity score interval matching. We considered a large number of variables in the baseline assessment, including socio-economic parameters, comorbidities, levels of nursing care, drug expenses, and hospital expenses.
Results
The DMP participants did not differ from the control group with respect to the incidence of relevant comorbidities. They underwent emergency hospitalization somewhat less frequently than the control group and also generated lower costs for inpatient treatment. In every three-month period studied, the DMP participants received more prescriptions, had more contacts with physicians in private practice, and submitted higher claims for health insurance benefits than the control group.
Conclusion
The current DMP for T2DM in Germany is not adequately effective. This study does not reveal any clear medical benefit from DMP participation. Selection effects were largely eliminated by means of a sophisticated control group design. Analyses of other DMPs with this method are currently being planned.
The first of six disease management programs (DMPs) in Germany, the diabetes mellitus type 2 (T2DM) DMP, was introduced in 2003. Since that date, around 5.5 million persons with statutory health insurance have taken part in DMPs. In 2009 the costs amounted to over 1.1 billion euros. However, the use of the DMPs, which are very administration-heavy compared to those in some other countries, has not yet been satisfactorily evaluated.
The quality control report in 2008 (1) on the DMPs in North Rhine and the ELSID study (2) show an apparent success of the DMPs, but they do not fulfill the requirements of a scientifically based study because their control group design is inadequate (3). Recently, the FAZ (Frankfurter Allgemeine Zeitung) reported on a study by Berthold et al. (4) based on clinical treatment data that raised doubts about the effectiveness of the DMPs in their present form. Regret was expressed at the lack of engagement of the associations of statutory health insurance physicians (i.e., those physicians who work within the public health system) and the major health insurers, whose data pools would enable extensive analyses.
The evaluations presented here are based on routine data from the statutory health insurers that cover all sectors: outpatient and inpatient treatments, medical prescriptions, socioeconomic variables, levels of care, etc. A conscious decision was made not to analyze the DMP documentation, the completeness and content of which are debatable. Instead, we employed more robust indicators on the basis of the routine data.
To exclude selection effects relating to both patients and treating physicians as far as possible, an innovative control group design was used, employing propensity score interval matching (5). The T2DM DMP was the first to be analyzed, as an example, since this DMP accounts for more than 50% of all DMP registrations.
The aim of the study was to answer the following questions:
Does the DMP improve the medical outcome in terms of a reduced incidence of disease-related co-morbidities or a reduction in emergency inpatient admissions?
Can a subgroup of patients be identified, on the basis of sociodemographic characteristics, co-morbidities, or the use of medical care, in whom the DMP shows a statistically significant effect?
Methods
Underlying data
The population on which the analysis was based was made up of all persons insured with the Techniker Krankenkasse (TK) (a statutory health insurer) between 1 January 2006 and 31 December 2008. The data for 2006 served as baseline data (data at the start point), which were required for the design of the control group. The data for 2007 and 2008 were those used for the retrospective analysis in the search for an effect of the DMP. The following groups were defined:
DMP participants (n = 84 410). This group included insured persons who were taking part in the T2DM DMP on 31 December 2008. The patient was offered the opportunity of taking part in a DMP either by the insurer or by the treating physician who organized his or her registration on the DMP with the insurer. Insured persons who were registered in other DMPs were excluded.
Candidates for a DMP who did not register (non-DMP-participants; n = 144 910). This group included insured persons with at least one documented primary or secondary diagnosis of T2DM (ICD E11) at the time of discharge as an inpatient and/or at least two confirmed outpatient diagnoses, who at no time had decided to take part in any DMP.
Matching control group (n = 23 180). This was a subgroup of the non-DMP-participants who in the year before the earliest possible DMP registration (i.e., mainly in 2006) were comparable to the DMP participants in respect of sociodemographic characteristics, co-morbidities, and use of health care services.
Aims of the analysis
In order to represent in comparative form the medical outcomes in the described groups across quarter-years in relation to possible DMP registration, for all insured persons the first quarter in which they could be (or could have been) registered for a DMP was designated as quarter 0. The earliest this could be was the first quarter (January to March) of 2007. With a maximum 2-year observation period (2007–2008), up to seven more quarters could be evaluated.
We compared new occurrences of typical co-morbidities. The data on which this was based was the first-time documentation of primary and secondary diagnoses as stated at the time of hospital discharge or the outpatient diagnoses marked “G” for confirmed (“gesichert”) in the period 2007–2008. The co-morbidities regarded as typical were:
Stroke (ICD I63)
Blindness (ICD H54.0, H54.1, H54.4)
Peripheral arterial occlusive disease (PAOD) (ICD I70.2, I73.9)
Diabetic polyneuropathy (ICD G63.2)
Ischemic heart disease (ICD I20–I25)
End-stage renal failure requiring renal replacement therapy (ICD N18.0)
Myocardial infarction (ICD I21).
The OPS code (“Operationsschlüssel,” operation code) 5–865 (foot amputation) was also evaluated.
In addition to these “hard” end points, newly occurring metabolic disorders (ICD E78) and hypertension (ICD I10) during the observation period were analyzed, in order to be able to evaluate treatment success also in terms of maintenance of normal values.
With regard to the use of health care services over time, the costs for hospital stays and medical prescriptions were compared between the groups. The following variables were also studied:
Use of pharmacotherapy, measured in defined daily dose (DDD)
Frequency of emergency inpatient admissions
Number of outpatient physician contacts
Use of medical services provided by general practitioners and physicians (EBM points) (EBM: “Einheitlicher Bewertungsmaßstab,” uniform evaluation standard).
Matching procedure
To minimize selection effects in the groups, a control group was formed on the basis of the non-DMP-participants, by which insured persons who were not registered for a DMP were matched in respect of the following criteria:
Age
Sex
Level of care
Socioeconomic characteristics (education, professional status)
Level of drug costs and hospital costs
Extent of drug prescriptions (in DDD)
Relevant co-morbidities (ICD I21, I63, I70.2, I73.9, H54.0, H54.1, H54.4, N18.0, G632, OPS 5–865).
Hence, the group is comparable with the DMP participants in relation to the year before the earliest possible DMP registration.
Using propensity score interval matching (5), it was ensured that the baseline situations of the DMP participants and the control group were comparable in terms of the broad surrogates mentioned. Otherwise, no meaningful statements could be made on the basis of a comparison, since the non-DMP participants were on average healthier, but at the same time a higher percentage of them required nursing care.
Statistical tests
Statistical tests were carried out using the statistical software PASW Statistics 18 and the SAS Enterprise Guide V. 4.1. The chi-square test was used for binary variables and the Mann–Whitney U test for continuous variables (6). Both tests are valid for samples that are independent of each other. Since, strictly speaking, this independence no longer exists after matching, the resulting p-values can only serve to evaluate differences provisionally. For subgroup analysis, a decision tree was constructed using the chi-square automatic interaction detectors (CHAID) algorithm, which was then intended, using the 2006 data, to dichotomize whether a co-morbidity was documented for the first time within the observation period. Subgroups with more than 20% probability for the new occurrence of a co-morbidity were investigated for differences between DMP participants and the control group, since any effect of the program might be expected to show especially where complication risks are high.
Results
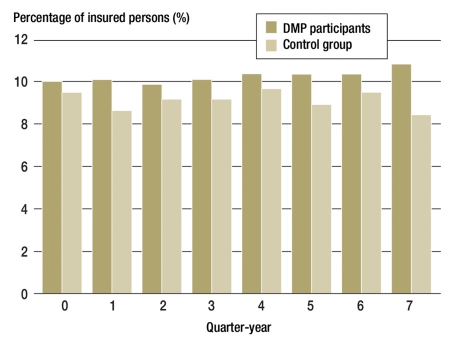
A total of 5 428 979 persons were continuously insured with the TK from 1 January 2006 to 31 December 2008, and T2DM was diagnosed in 242 541 of these. Figure 1 shows the insured numbers in the groups over time.
Figure 1.
Group sizes in the eight quarter-years of the observation period:
Quarter 0 is the first quarter of the observation period in which registration in a DMP was possible. A large proportion of the insured persons was analyzed over the entire 2-year observation period (righthand columns). Since newly diagnosed persons were added into the groups over the course of the 2-year observation period, the groups for short observation periods are the largest (lefthand columns).
There were statistically significant differences between the DMP participants and non-DMP-participants in almost all respects, but these differences mostly disappeared after matching (Table).
Table. Statistical differences (p-values) between the DMP participants and the insured persons in the control group.
| Total | DMP participants | Control group | p-value | ||
| N = 84 410 | N = 23 180 | ||||
| Binary variable | n | % | n | % | Chi-square test |
| Sex (male) | 54 595 | 64.68 | 14 919 | 64.36 | 0.3716 |
| Education*1: | |||||
| Secondary school completed | 17 628 | 20.88 | 4849 | 20.92 | 0.9073 |
| University entrance requirement completed | 1587 | 1.88 | 431 | 1.86 | 0.8366 |
| University degree completed | 6777 | 8.03 | 1834 | 7.91 | 0.562 |
| Occupation*1: | |||||
| White collar/blue collar worker | 14 317 | 16.96 | 3878 | 16.73 | 0.4053 |
| Self-employed | 3759 | 4.45 | 1006 | 4.34 | 0.4576 |
| Unemployed | 8081 | 9.57 | 2184 | 9.42 | 0.4865 |
| Receiving social benefits | 451 | 0.53 | 123 | 0.53 | 0.9459 |
| Student | 155 | 0.18 | 41 | 0.18 | 0.8309 |
| Pensioner | 57 197 | 67.76 | 15 822 | 68.26 | 0.1519 |
| Level of care*2: | |||||
| Level 1 | 1438 | 1.7 | 393 | 1.7 | 0.9322 |
| Level 2 | 713 | 0.84 | 190 | 0.82 | 0.7116 |
| Level 3 | 97 | 0.11 | 38 | 0.16 | 0.0619 |
| Co-morbidities/events: | |||||
| Myocardial infarction | 837 | 0.99 | 231 | 1 | 0.9462 |
| Stroke | 1432 | 1.7 | 394 | 1.7 | 0.9729 |
| PAOD | 5700 | 6.75 | 1544 | 6.66 | 0.6211 |
| Blindness | 434 | 0.51 | 114 | 0.49 | 0.672 |
| Renal failure | 283 | 0.34 | 77 | 0.33 | 0.9426 |
| Polyneuropathy | 5741 | 6.8 | 1591 | 6.86 | 0.7386 |
| Foot amputation | 164 | 0.19 | 41 | 0.18 | 0.5902 |
| Continuous variables | Mean | Standard deviation | Mean | Standard deviation | Mann-Whitney U test |
| Age | 63.8 | 10.22 | 63.56 | 12.61 | 0.001 |
| Hospital costs (euros) | 1158.16 | 4119.93 | 1277.80 | 5699.04 | 0.2947 |
| Drug prescription costs (euros) | 1164.89 | 3969.69 | 1309.52 | 3055.26 | <0.0001 |
| Drug prescriptions (DDD) | 1438.24 | 1333.39 | 1440.76 | 1365.08 | 0.0856 |
*1Due to incomplete entries in the database, percentages do not add up to 100%;
*2levels of care: level 1 = custodial care; level 2 = intermediate care; level 3 = skilled care (highest level of care); DMP, disease management program; PAOD, peripheral arterial occlusive disease; DDD, defined daily dose
Co-morbidities
No clear differences were seen between the DMP participants and the control group in relation to the incidence of co-morbidities or foot amputations. Only newly occurring polyneuropathies were more often documented among the DMP participants in every quarter of the observation period (incidence in the 2-year observation period: 5.9% vs. 3.1%).
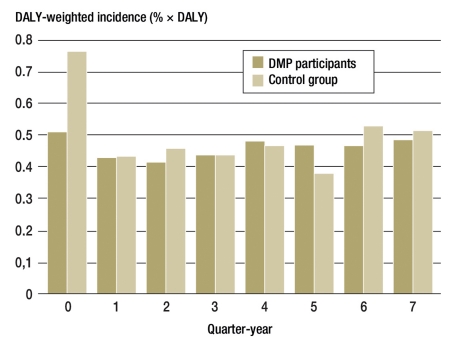
Since the co-morbidities are not comparable in respect of their disease burden, the percentages of the individual newly occurring co-morbidities were weighted by means of disability-adjusted life years (DALYs) (7) and summed. This showed no significant differences between the groups (Figure 2; p = 0.949).
Figure 2.
Overall proportion of newly occurring co-morbidities, weighted for disability-adjusted life years (DALY)
Product sums from the incidence of newly occurring disease-related co-morbidities weighted for their respective disease burdens (by DALY). In the control group of insured persons, co-morbidities were more frequent in the quarter in which T2DM was diagnosed
Drug use
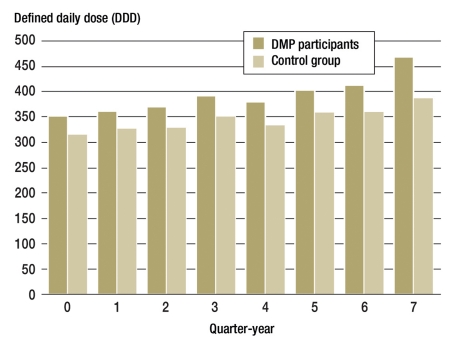
In terms of both costs and prescriptions as measured in DDD, the use of drugs was higher in the DMP participant group than in the control group for all quarters (Figure 3).
Figure 3.
Average use of drugs per insured person measured in terms of median defined daily doses (DDD) of all prescriptions filled
It cannot be discerned that the DMP in association with the risk structure equalization regulations (Risikostrukturausgleichsverordnung, RSAV) has led to less prescription of insulin analogues (Figure 4), although in the framework of the DMP these are supposed to be used only as second-line treatment (8).
Figure 4.
Percentages of insured persons treated with insulin analogues (ATC A10AD)
who received at least one insulin analogue (ATC A10AD) in the form of insulin lispro (ATC A10AD04) or insulin aspartate (ATC A10AD05). (ATC A10AD is the code for insulins and analogues for injection, intermediate-acting combined with fast-acting)
Use of healthcare services
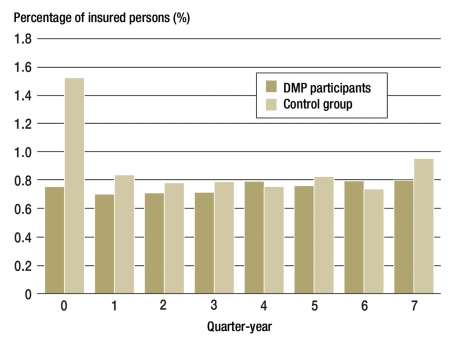
In relation to hospital costs and emergency admissions (Figure 5), the results for the DMP participants were slightly better. It was not possible to analyze the range of reasons for emergency admissions—e.g. failure to maintain blood sugar levels correctly—because the number of cases was too low.
Figure 5.
Percentages of insured persons admitted to hospital as an emergency
T2DM was more frequently recognized and diagnosed in connection with emergency treatment in the control group
As to the number of doctor appointments and outpatient services used, as measured in EBM points, the DMP participants made use of health services more in all quarters of the observation period.
Subgroup analysis
Among the 86 subgroups of the CHAIS tree, statistically significant differences were shown between the DMP participants and the control group, provided the insured persons in 2006 had more than 3165 DDD prescribed (chi-square test, p = 0.006). Complications were recorded in 1813/8441 (21.5%, DMP participants) and 586/2432 insured persons, respectively (24.1%, control group).
Discussion
Discussion of methods
The evaluation related to the period 2006–2008; 2006 was needed for the formation of a representative (matching) control group. Limiting the observation period to effectively 2 years had the advantage that from 2006 the data for outpatient diagnoses were more reliable, since the health insurers have been able to give the additional marker “G” for a confirmed diagnosis (“gesicherte Diagnose”) only since 1 January 2006. For foot amputation, T2DM is not the only reason for this operation (it can also be done for “smoker’s leg” or following trauma), but this consideration is not a major one, since it is the same for all groups investigated. In the context of the present evaluation, no epidemiological statements can be made about the prevalence or incidence of co-morbidities or operative interventions.
The reason for the differences in relation to age and drug costs that persisted despite matching is the large size of the sample, which makes even slight differences in distribution statistically significant. Thus, there is a significant difference at the p = 0.001 level between the age structure of the DMP participants and the control group, although the means are 63.80 and 63.56 years respectively. Neither does looking at the corresponding histograms give any indication of relevant differences that would have had to be taken into account during matching.
The self-selection effect could, for lack of social psychometric data, possibly not be controlled for by the data on sex, age, education and occupation: one might imagine, for example, that insured persons who were more health-conscious or more active overall would more often decide to take part in a DMP, or that they would more often be classed as suited to a DMP (9). In this way, observed effects may be due less to the program and more to characteristics of the insured persons.
Limitations of the present study are the lack of knowledge about how long diabetes had already existed in those with a documented diagnosis at the start of 2006, and the limited duration of the study. Although the evaluations (Figure 1) indicate that the DMP participants and the control group had a comparable previous history, this assumption can not be verified from the routine data on which the study relied. Much more important, however, is the comparative shortness of the observation period (maximum 2 years), in the course of which intervention effects may not have become sufficiently clear. This leads to the demand for a study that not only covers the whole country, but also evaluates possible program effects over a longer term. The methodology presented here enables important findings in addition to the already available quality reports and evaluation of clinical parameters (4).
Discussion of results
By means of iterative propensity score matching, we were able to put together a control group that was comparable to the DMP participants in respect of many influential variables. As Figure 1 shows, T2DM was diagnosed in almost all the persons in the study groups before the observation period began, so some late complications of diabetes were to be expected. Differences in disease severity were largely ruled out by the matching process. However, it could be argued that the DMP participants might have been regarded by their physicians as more at risk for co-morbidities and been registered for the DMP for that reason. This hypothesis cannot be tested on the basis of the routine data from statutory health insurers.
Interestingly, in the first quarter of (possible) DMP registration (quarter 0), the control group experienced co-morbidities much more often than the DMP participants. This is particularly evident in respect of singular major events such as myocardial infarction or stroke. Here it may be assumed that the significance of the new diagnosis T2DM was originally subordinate and DMP registration did not take place.
In the sum of all co-morbidities, the control group had an advantage over the DMP participants, although differences in documentation behavior cannot be ruled out, e.g., in the documentation of polyneuropathy. On this point, it might be supposed that the DMP physicians would take more care over diagnosis and/or documentation. These kinds of consideration underline how complex the interpretation is. After DALY weighting (Figure 2), statistically significant differences between the groups ceased to be visible.
In relation to the more frequent use of outpatient services and drug prescriptions, it should be remembered that more intensive healthcare may be interpreted not just as a cost driver, but actually as a desired effect, if it achieves better prevention of cardiovascular complications.
Summary and future perspective
For the first time, a DMP has been evaluated with a sophisticated control group approach. Routine hard data from a statutory health insurer were examined in respect of:
Medical usefulness
Spectrum of drugs prescribed
Frequency of physician contacts in the outpatient sector
EBM services used
Spectrum of reasons for emergency inpatient admission
Overall, the picture of program effects was uneven, but it did not show, what has been shown elsewhere, that the T2DM DMP was useful (1, 2, 10). Thus, our findings confirm the recent study results of Schäfer et al. (11) and of Heiner Berthold and colleagues at the Charité Berlin (personal communication). Because of matching, the DMP participants studied were comparable to the non-DMP-participants in the control group in respect of relevant co-morbidities and disease duration (Table). Only the assumption that the patients who register for a DMP are those who are at particular risk of complications would help to explain the lack of program effects in relation to the outcome variables. The only signs of a desired program effect are the somewhat lower hospital costs and the slightly smaller number of emergency inpatient admissions—possibly a result of more effective patient education.
Against the background of the more than 1.1 billion euros expended on DMPs in 2009, the conclusion that must be drawn for more effective allocation of resources is that DMPs, in the form in which they are currently practiced in Germany, should be closed down. One or two individual elements appear to be worth retaining. For example, expert interviews underlined that “reminders” sent from health insurers to DMP participants were helpful. The subgroup analysis results support case management of those whose diabetes is most serious. Shortly after DMPs were introduced, the Institute for Health and Social Research (Institut für Gesundheits- und Sozialforschung, IGES) recommended in a report (12) that DMPs should be individualized with behavior-modifying elements for selected patients. Other helpful elements appear to be additional offers of support to promote competence in the person providing treatment, e.g. offers to professionalize the patient interviews, which to date is not compulsory in medical students’ training.
To be able to evaluate the DMP in an ideal way requires prospective randomized studies by institutes whose conflicts of interest in the field of study are minimized. In the discussion of the evaluation of the DMPs, the possibility of a controlled study was supposed to be tested (9). However, this was not implemented in a binding way. To generate as much evidence as possible on the effectiveness of the DMPs, the study situation needs to be improved. In addition, it is also possible to reach further findings using solid scientific methods of data analysis. In particular, evaluating hard end points over the course of several years could contribute to this. In regard to the present study, a longer observation period would certainly be desirable, even though the six-figure numbers of insured persons mean that even minor effects would be revealed. Nevertheless, particularly for DMPs with low registration numbers, a DMP evaluation that includes all statutory health insurers should be demanded. If the ideal case of a prospective randomized study continues to prove impossible to realize, the present study offers a proven methodical way of approach based on routine data from the statutory health insurers.
Key Messages.
By using a suitable methodical approach, it is possible to put together a control group that is comparable to the participants in disease management programs retrospectively, and thus largely to exclude selection effects.
With regard to the incidence of relevant co-morbidities, no clear differences are visible between the DMP participants and the control group.
The number of emergency admissions and the costs of hospital stays are slightly lower among DMP participants.
In every quarter-year investigated, the DMP participants received more prescriptions for medication, contacted non-hospital doctors more frequently, and received more health care services (EBM points).
Overall, it is not possible to see a clear medical benefit from taking part in a DMP.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
The mission of the Scientific Institute of the TK for Usefulness and Efficiency in Public Health (Wissenschaftliches Institut der TK für Nutzen und Effizienz im Gesundheitswesen, WINEG) is to investigate the value of innovations and new programmatic approaches within the statutory health insurance framework. The authors declare that because they belong to the Techniker Krankenkasse, a potential conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Qualitätssicherungsbericht. www.kvno.de/downloads/qualbe_dmp08.pdf. 2008. Disease-Management-Programme in Nordrhein. (letzter Zugriff am 27.07.2010) [Google Scholar]
- 2.Miksch A, Laux G, Ose D, et al. Is there a survival benefit within a German primary care-based disease management program? Am J Manag Care. 2010;16:49–54. [PubMed] [Google Scholar]
- 3.Birnbaum DS, Braun S. Evaluation von Disease Management Programmen - Bewertung der Methodik und der ersten Ergebnisse aus gesundheitsökonomischer Sicht. Z Evid Fortbild Qual Gesundheitswesen. 2009 doi: 10.1016/j.zefq.2009.07.002. doi:10.1016/j.zefq.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 4.von Lutterotti N. Disease-Management - Patienten im Datenverlies. www.faz.net/, Abschnitt „Diabetiker.Programme“. (letzter Zugriff am 18.01.2011) [Google Scholar]
- 5.Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–50. [Google Scholar]
- 6.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. JASA. 1952;47:583–621. [Google Scholar]
- 7.World-Health-Organization Global Burden of Disease 2004 Update. www.who.int/healthinfo/global_burden_disease/daly_disability_weight/en/index.html. Disability Weights for Diseases and Conditions. (letzter Zugriff am 27.07.2010) [Google Scholar]
- 8.Verordnung über das Verfahren zum Risikostrukturausgleich in der gesetzlichen Krankenversicherung (Risikostruktur-Ausgleichsverordnung - RSAV Anlage 1 (zu §§ 28b bis 28g) www.gesetze-im-internet.de/rsav/anlage_1_66.html. Anforderungen an strukturierte Behandlungsprogramme für Diabetes mellitus Typ 2. (last accessed on 27.01.2010) [Google Scholar]
- 9.Ullrich W, Marschall U, Graf C. Vesorgungsmerkmale des Diabetes mellitus in Disease-Management-Programmen. Ein Vergleich von in die DMP eingeschriebenen und nichteingeschriebenen Versicherten mit Diabetes. Diabetes, Stoffwechsel und Herz. 2007;16:407–441. [Google Scholar]
- 10.Elkeles T, Kirschner W, Graf C, Kellermann-Mühlhoff P. Versorgungsunterschiede zwischen DMP und Nicht-DMP aus der Sicht der Versicherten. Ergebnisse einer vergleichenden Versichertenbefragung von Typ-2-Diabetikern der Barmer. Gesundheit & Sozialpolitik. 2008;1:10–18. [Google Scholar]
- 11.Schäfer I, Küver C, Gedrose B, et al. The disease management program for type 2 diabetes in Germany enhances process quality of diabetes care - a follow-up survey of patient’s experiences. BMC health services research. 2010;10 doi: 10.1186/1472-6963-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Häussler B, Berger U. Bedingungen für effektive Disease-Management-Programme. Baden-Baden: Nomos. 2004 [Google Scholar]