Abstract
Vagal afferent neurons (VAN) express the cholecystokinin (CCK) type 1 receptor (CCK1R) and, as predicted by the role of CCK in inducing satiation, CCK1R−/− mice ingest larger and longer meals. However, after a short fast, CCK1R−/− mice ingesting high fat (HF) diets initiate feeding earlier than wild-type mice. We hypothesized that the increased drive to eat in CCK1R−/− mice eating HF diet is mediated by ghrelin, a gut peptide that stimulates food intake. The decrease in time to first meal, and the increase in meal size and duration in CCK1R−/− compared to wild-type mice ingesting high fat (HF) diet was reversed by administration of GHSR1a antagonist D-(Lys3)-GHRP-6 (p<0.05). Administration of the GHSR1a antagonist significantly increased expression of the neuropeptide cocaine and amphetamine-regulated transcript (CART) in VAN of HF-fed CCK1R−/− but not wild-type mice. Administration of the GHSR1a antagonist decreased neuronal activity measured by immunoreactivity for fos protein in the nucleus of the solitary tract (NTS) and the arcuate nucleus of both HF-fed wild-type and CCK1R−/− mice. The data show that hyperphagia in CCK1R−/− mice ingesting HF diet is reversed by blockade of the ghrelin receptor, suggesting that in the absence of the CCK1R, there is an increased ghrelin-dependent drive to feed. The site of action of ghrelin receptors is unclear, but may involve an increase in expression of CART peptide in VAN in HF-fed CCK1R−/− mice.
Keywords: ghrelin, CCK, nodose ganglia, c-fos, neuronal activation
1.0 Introduction
Ghrelin is produced primarily by enteroendocrine cells in the gastric epithelium and is the endogenous ligand for the growth hormone secretagogue receptor (GHS-R1a). Plasma levels of ghrelin are high during fasting; in particular, there is a preprandial peak consistent with a role for ghrelin in meal initiation (1) and acute peripheral administration of exogenous ghrelin increases food intake in humans and experimental animals (2). However, the site of action of ghrelin endogenously released from gastric endocrine cells or peripherally administered ghrelin is not clear. GHSR1a are located on several populations of neurons shown to be involved in the regulation of food intake, including the arcuate nucleus of the hypothalamus, the brainstem and vagal afferent neurons (VAN) (3–7). Functional ablation of VAN via perineural capsaicin treatment, total subdiaphragmatic or selective gastric vagotomy inhibits the ability of ghrelin to increase food intake in mice and rats (5, 8–9) and truncal vagotomy associated with gastric surgery inhibited the stimulatory effect of ghrelin in humans (10), although this has been not been verified in one study in rats (11). In addition, ghrelin has been shown to influence VAN function; ghrelin inhibits the neuronal discharge of gastric mechanoreceptor fibers and increases the discharge of subdiaphragmatic vagal afferents innervating the intestine to distention stimulus (12–13).
There are data to suggest that cholecystokinin (CCK), a gut peptide that inhibits food intake, and ghrelin interact in the control of food intake. Prior administration of ghrelin inhibits the effects of CCK to reduce feeding, and administration of CCK prior to ghrelin inhibits the ability of ghrelin to induce feeding (8, 14) The site of this interaction between these two peptides is unknown. A similar interaction between CCK and ghrelin was reported on vagal afferent fiber discharge (5). Moreover, ghrelin inhibits CCK- or feeding-induced alteration of peptide expression by vagal afferent neurons (4); CCK induces expression of cocaine- and amphetamine-regulated transcript (CART) peptide in VAN, an effect inhibited by administration of ghrelin. Thus CCK and ghrelin interact at the level of the vagus nerve, yet whether there are functional consequences of this interaction on feeding behavior remain unknown.
We and others have previously shown that CCK1R−/− mice lack short term satiety, resulting in the ingestion of longer and larger meals (15–16). Moreover, we extended these observations to show that the hyperphagia was more pronounced in animals ingesting HF diet. An unexpected finding was a marked decrease in the time to the first meal after a short (6 hour) fast in CCK1R−/− mice, particularly when the mice were ingesting a diet high in fat and calories (16). These data suggests that lack of the CCK1R can disrupt orexigenic signaling, but the possible mechanism and pathway by which this occurs are unknown. Given the possible role of ghrelin in meal initiation, and previous studies showing interaction of CCK and ghrelin in the regulation of feeding and VAN function, we hypothesized that the increase in time to first meal in CCK1R−/− mice may be mediated by ghrelin and that this interaction may occur at the level of VAN.
To test this hypothesis, we measured meal patterns in wildtype and CCK1R−/− mice and determined the role of GHSR1a using the specific receptor antagonist, D-(Lys3)-GHRP-6 (17). To determine whether there were any changes in the vagal afferent pathway, we measured expression of CART peptide in VAN using immunocytochemistry. To determine the possible site of action of blockade of the ghrelin receptor on meal patterns, we also measured neuronal activation using immunohistochemistry for c-fos protein in CCK1R−/− and WT mice in the nucleus of the solitary tract (NTS), the region where vagal afferent terminate in the brainstem, and in the arcuate nucleus of the hypothalamus, a site of expression of GHSR1a and a possible site of action of ghrelin to increase food intake.
2.0 Materials and Methods
2.1 Animals
Age matched (25–30g body weight, 8 weeks) adult male CCK1R−/− mice (Mouse Biology Program, UC Davis) and their wildtype controls 129Sv (Taconic, Oxnard, CA) mice were used in all experiments (18). All mice were individually housed on wire bottom cages and maintained on a 12-h light, 12-h dark cycle (lights on at 3:00 am for feeding behavior; otherwise lights on at 6:00 am) in a temperature-controlled room (23°C). For experiments where neural tissues were collected, animals had ad libitum access to water and either standard laboratory chow (Lab Diet 5001), or 38% high fat (HF) diet (Cat. No. D12451Research Diets). Animals used for meal pattern studies were fed either a 10% low fat (LF) or 45% high fat (HF) diet (Bioserv Custom Dustless Precision Pellets, Frenchtown, NJ) for 2 weeks. Prior to treatment or experiment day, all mice were placed in wire-bottom cages during short term or overnight fasts. All animal procedures were approved by the Institutional Animal Care and Use Committee at University of California, Davis.
2.2 Drugs/Peptides
D-(Lys3)-GHRP-6 (Cat. # H-3108) was purchased from Bachem (King of Prussia, PA) and reconstituted in 0.9% saline and administered intraperitoneally (ip) following either a short term (6 hour) or overnight fast on wire-bottom cages.
2.3 Food intake analysis
Meal pattern analysis was performed as previously described (16). Briefly, mice were fed either LF or HF isocaloric (3.4kcal/g), isonitrogenous (21% of energy) 20mg pelleted diets. Mice (n=3–6 per treatment group) were fasted daily in wire bottom cages for 6 hours during the light cycle (9:00 am to 3:00 pm). Each experiment lasted 15 days. Mice were acclimatized to the diets and the feeding paradigm for 5 days. Following the acclimation period, meal pattern data were recorded for 10 consecutive days with treatment injections. Body weight was measured at 3:00 pm prior to placement in the meal pattern analysis cages. Feeding patterns (meal duration, meal size) were measured continuously from 3:00 pm to 9:00 am using food intake monitoring cages (The Habitest® System, Coulbourn Instruments, Allentown, PA). In this system, infrared pellet-sensing photo beams control the pellet dispensers and pellets are delivered in response to removal of the previous pellet. Data were recorded from EZ count software and analyzed using Spike2 (version 5.07, Cambridge Electronic Design 1988–2004), SigmaStat (version3.11, Systat Software Inc. 2004) and Graph Prism® (version 3.02, GraphPad Software Inc. 1994–2000). The parameters for defining a meal (acquisition of at least 4 pellets within 10 min, preceded or followed by 10 min of no feeding) were based on two previously published studies using mice and our previous study (15–16, 19).
2.4 Immunohistochemistry of CART in nodose ganglia neurons
Mice were fed HF diets for two weeks (n=4–6 per group) with free access to water. Nodose ganglia collected for CART immunostaining were taken from LF saline – treated mice, and from HF fed mice of both genotypes treated with saline or D-(Lys3)-GHRP6 (2.8 µg/kg). Following an overnight fast, mice were given an intraperitoneal injection of saline or drug treatment and after 90 minutes, were deeply anesthetized (sodium pentobarbital, Beuthanasia 0.05ml/rat) and transcardially perfused with 0.1% heparinized 0.9% NaCl at 4°C, followed by 1mL/gm body weight of 4% paraformaldehyde dissolved in PBS (PFS-PBS) at 4°C. Nodose ganglia were collected and post-fixed for 2 hours in 4% PFA, then transferred and stored at 4°C in 25% sucrose-PBS until further processing. Frozen nodose ganglia were sectioned at 10um on a cryostat and placed onto Fisher Superfrost/Plus slides. Slides were blocked with 20% goat serum-PBS for 30 minutes, followed by primary antibody incubation of rabbit-anti-rat-CART (1:200, Cat. No.H00360, Phoenix Pharmaceuticals, Inc., Burlingame, CA) at 37°C. Following three serial washes in PBS at room temperature, donkey or goat anti-rat AlexaFluor 488 secondary antibody was applied and incubated at 37°C for 30 minutes. Slides were washed overnight and coverslips mounted using Fluoro-Gel mounting media (Cat. No. 17985-10, Electron Microscopy Sciences, Hatfield, PA), dried overnight at room temperature, and stored at −20°C until ready for imaging. Confocal images were made using an Olympus FV1000 Laser Scanning Confocal Microscope (Olympus America Inc., Melville, NY) at 20× oil objective and analyzed for neuronal labeling using Scion Image (Beta 4.0.2, Scion Corporation, 2000). 5–8 photomicrographs were analyzed per mouse; the level of CART expression was quantified by determination of the number of positively labeled pixels was normalized to total number of pixels and expressed as percent positive labeled pixels.
2.5 c-fos Immunohistochemistry in the hindbrain and forebrain
Mice were fed a 45% high fat diet, (129sv and CCK1R−/−, n=4–6 for all groups) for two weeks ad libitum. Animals were fasted overnight on wire-bottom cages with ad libitum water. Animals were then given an intraperitoneal injection of drug treatment of 0.1µL total volume of either saline or D-(Lys3)-GHRP-6 (2.8 µg/kg). After 90 minutes, animals were deeply anesthetized with sodium pentobarbital (Beuthanasia 0.05mL/animal) and transcardially perfused with 4% paraformaldehyde. Whole brains were removed and stored in 4% paraformaldehyde for post-fixation, then transferred to PBS and stored at 4°C until vibratome sectioning.
Regions of the hindbrain and forebrain were cut rostrocaudally and categorized into areas of the fourth ventricle, area postrema, and post-area postrema for the hindbrain (18, 20) and the third ventricle was used as a landmark for the arcuate nucleus in the forebrain. 100µm sections were cut using a Series 1000 Vibratome in cold PBS and blocked with goat serum-PBS (2% goat serum, 0.2% Triton X-100, 0.1% bovine serum albumin in PBS) for 2 hours at 37°C. Next, sections were incubated with c-Fos primary antibody (sc-52, Santa Cruz Biotechnology, Santa Cruz, CA) for 3 hours at 37°C, serially washed three times in PBS and treated with biotinylated goat anti-rabbit IgG secondary antibody (Vector Labs, Burlingame, CA) for 1 hour at 37°C. Following two serial washes of PBS, sections were incubated with ABC solution from the Standard Elite Vectastain ABC Kit (Vector Labs) for 1.5 hours at 37°C. Ni-3’3’-diaminobenzidine (Cat.No. D-8000-5G, Sigma Aldrich Chemicals) was dissolved in PBS (30mg/100mL) and added to sections for 5 minutes followed by the addition of 30% H2O2 to each section with the reaction stopped after 5 minutes with three serial washes using cold PBS. All reagents contained penicillin streptomycin (Cat.No. 15140-122, Gibco, Carlsbad, CA) antibiotic treatment to prevent bacterial growth on sections. Sections were then mounted onto Fisher Superfrost/Plus slides and dehydrated in six serial Whatman jars of distilled water/ethanol/xylene solutions. Coverslips were mounted using permanent mounting media (Cat. No. 6419, Tissue-Tek-Glas, Torrance, CA) and allowed to dry overnight. c-Fos images were taken in rostrocaudal order (rostral (bregma −8.00 to −7.92 mm), mid-NTS (−7.76 to −7.32 mm) and caudal to the area postrema (−7.08 to −6.48 mm) using an Olympus Provis AX70 light microscope (Olympus Optical Co., Tokyo, Japan) at 20× oil objective and analyzed by Scion Image (Beta 4.0.2, Scion Corporation, 2000).
For each mouse brain, 4–8 sections were imaged to include bilateral dorsal vagal complex (2 images per section, one left side and one right side, for a total of 8–12 analyzed values). Using the Scion Image analysis software, the margins of the NTS were defined using a dashed outline and the number of fos positive neurons in that area counted. The total number of pixels in that same area was quantified and used to normalize the number of neurons to the area of the NTS. Data are therefore expressed as fos density (ie the number of fos (+) nuclei divided by total number of pixels in the ipsilateral NTS). Thus, 8–12 values per mouse brain were obtained and an average of these values was calculated. This average was then used to provide the mean and standard error of the mean per group.
2.6 Statistics and data analysis
Data are presented as means ± SE. Meal pattern data were recorded using EZ count software and analyzed using Spike2 (version 5.07, Cambridge Electronic Design 1988–2004). Statistical analyses were performed using GraphPad Prism version 3.02 (GraphPad Software, San Diego, CA) and SigmaStat (version3.11, Systat Software Inc. 2004). Protein expression data in nodose neurons was quantified by Scion Image version 4.02 (Scion, Frederick, MD) using set fluorescence threshold values (average of 8–10 analyzed images used as the mean value per animal) and compared by Student’s t-test. CART peptide expression in nodose neurons, c-fos-IR in hindbrain and arcuate nucleus were compared by two-way ANOVA followed by post hoc analysis with Holm-Sidak’s multiple comparison test for the effects of genotype, diet or treatment. Differences in values were considered significant at P<0.05.
3.0 Results
3.1 Effect of GHSR1a receptor antagonist on meal patterns
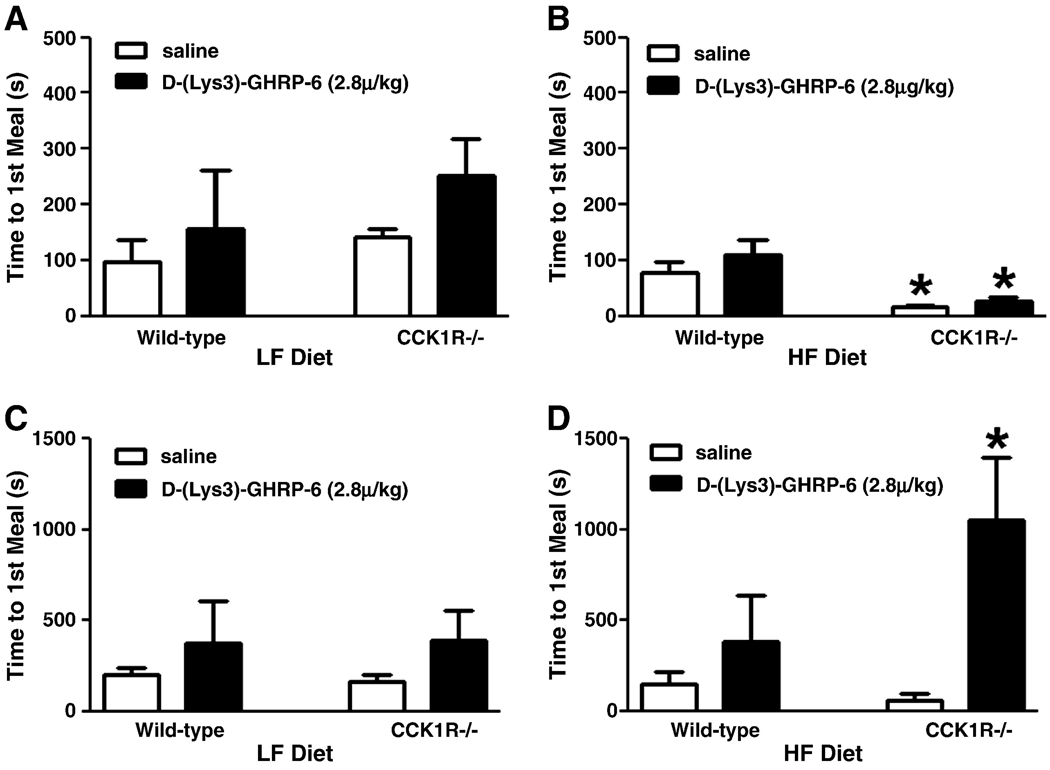
As previously described (16), there was no difference in time to first meal between wild-type and CCK1R−/− mice ingesting LF diet (Fig. 1A); when ingesting HF diets, there was a decrease in the time to the first meal in CCK1R−/− mice compared to wild-type controls (Fig 1B, p<0.05). Administration of the ghrelin receptor antagonist D-(Lys3)-GHRP-6 (2.8µg/kg, IP15 min, Fig. 1B) had no significant effect on time to first meal in HF fed wild-type or CCK1R mice. However, administration of D-(Lys3)-GHRP-6 (8µg/kg, Fig. 1D) significantly delayed the time to first meal in CCK1R null mice ingesting HF diets (p<0.05).
Figure 1. Effect of administration of the GHSR1a antagonist on meal patterns in wild-type and CCK1R−/− mice.
Time to first meal following a 6-hr fast in wild-type and CCK1R−/− mice (n=3–6) treated with saline or D-(Lys3)-GHRP-6 (2.8µg/kg, 8µg/kg). Mice were maintained on either low-fat (LF) (A, C) or high-fat (HF) (B, D) diets for two weeks. * P<0.05 indicating effect of treatment (saline or D-(Lys3)-GHRP-6) or genotype (wild-type or CCK1R−/−), respectively, by two-way ANOVA (treatment×genotype), followed by Bonferroni’s post-hoc test. Values represent mean ± SEM.
CCK1R−/− mice ingesting either LF or HF diets ate larger, longer first meals compared to wild-type mice (Table 1, p<0.05)(16). Administration of the GHSR1a antagonist had no effect on meal patterns in wild-type mice on either diet but significantly decreased the size and duration of the first meal in CCK1R null mice ingesting HF, but not LF, diet (Table 1).
Table 1.
Meal pattern analysis for first meal size and duration for wild-type and CCK1R−/− mice fed LF or HF diets following a 6 hr fast treated with saline or D-(Lys3)-GHRP-6 (2.8µg/kg). Significant differences indicated by different superscript letters.
| Low fat diet | High fat diet | ||||
|---|---|---|---|---|---|
| WT | CCK1R−/− | WT | CCK1R−/− | ||
| 1st meal size | Vehicle | 405 ± 32a | 851 ± 87a | 983 ± 91b | 1496 ± 125b |
| (mg) | Antagonist | 506 ± 27a | 894 ± 146ab | 869 ± 100ab | 506 ± 68a |
| 1st meal duration | Vehicle | 204 ± 42a | 1397 ± 202ab | 1056 ± 242b | 1681 ± 242a |
| (secs) | Antagonist | 322 ± 73ab | 1191 ± 245ab | 931 ± 295ab | 931 ± 295b |
3.2 Effect of GHSR1a antagonist on neurochemical phenotype of vagal afferent neurons
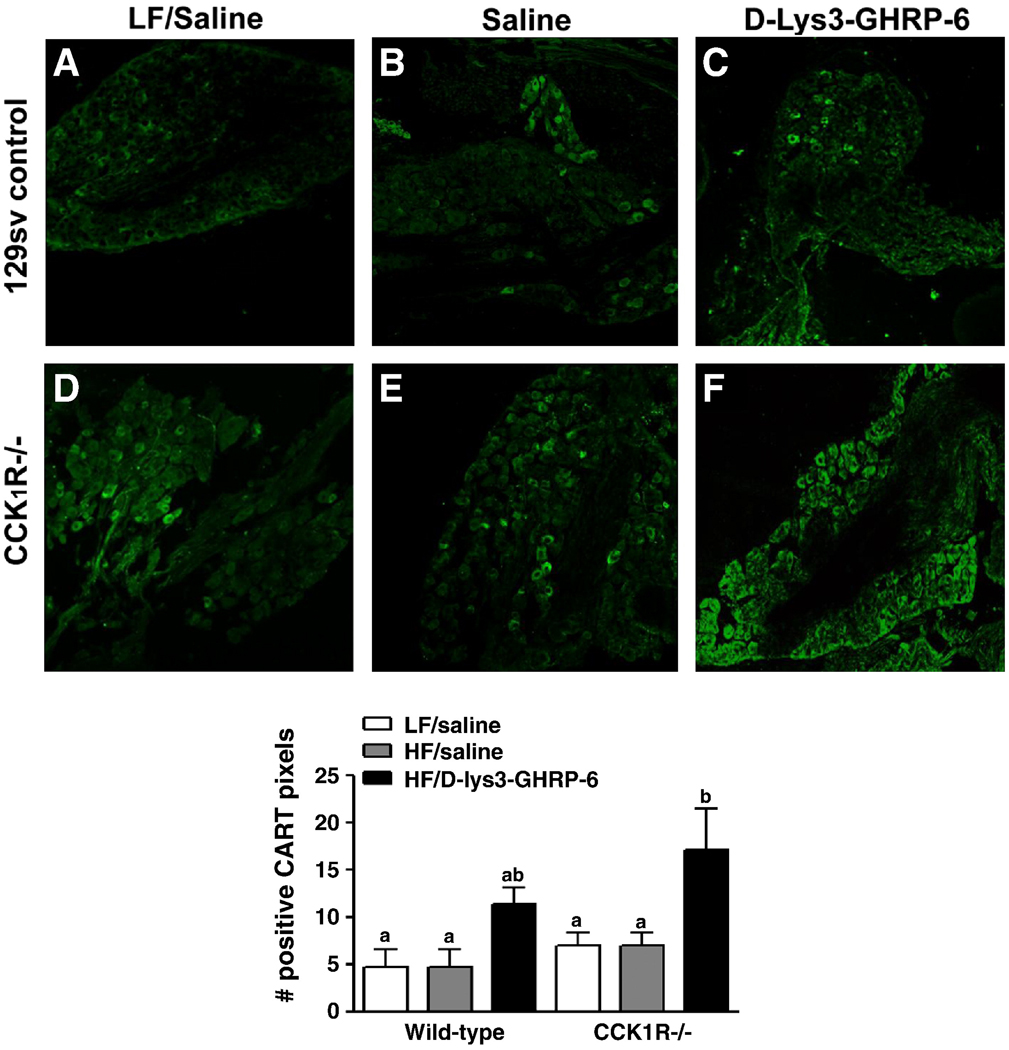
It has previously been shown that CCK or refeeding upregulates CART expression in vagal afferent neurons and that this may be involved in mediating the anorexigenic effect of CCK (21–22). Therefore, CART protein expression was quantified by immunofluorescent labeling in the nodose ganglia of wild-type and CCK1R−/− mice ingesting a HF diet. There was no difference in baseline CART protein levels in mice of either genotype following administration of saline (Fig. 2). Administration of D-(Lys3)-GHRP-6 (2.8µg/kg) in wildtype mice had no effect on levels of CART expression; in contrast in CCK1R−/− mice, there was a two-fold increase of CART protein expression following administration the ghrelin receptor antagonist compared to vehicle-treated mice (Fig. 2, p<0.05). These data suggests that in fasting CCK1R null mice ingesting HF diets ghrelin is producing a constitutive decrease in CART expression in VAN.
Figure 2. Effect of administration of the GHSR1a antagonist on CART peptide expression in nodose neurons of wild-type and CCK1R−/− mice.
Representative immunofluorescent images of immunoreactivity for CART peptide in sections of nodose ganglia from wild-type (A,B,C) and CCK1R−/− (D,E,F) mice treated with saline or D-(Lys3)-GHRP-6 (2.8µg/kg). The number of positively labeled pixels were counted (8–10 sections per animal) and normalized to the total number of pixels and expressed as a percentage (8–10 sections per animal, (n=4–6). a,b indicate p<0.05 by Student’s t-test. Values represent mean ± SEM.
3.3 Effect of GHSR1a antagonist on neuronal activity in the NTS and arcuate nucleus
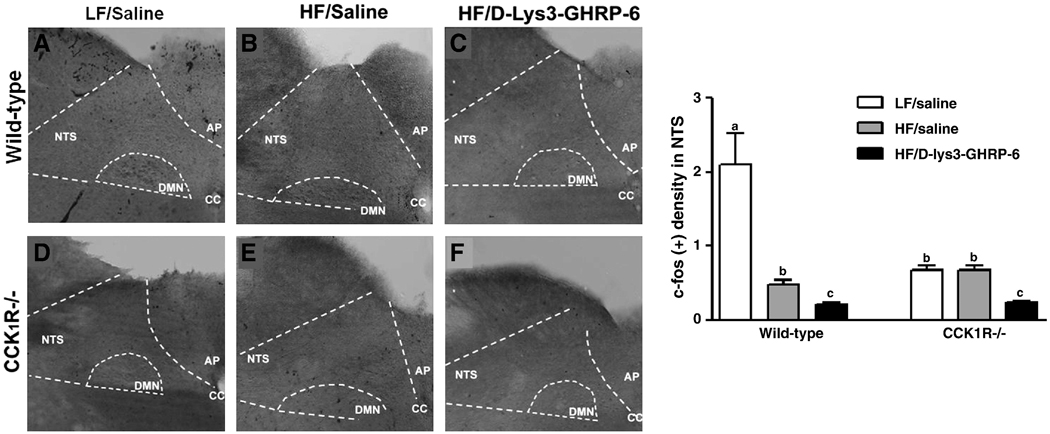
The effect of administration of D-(Lys3)-GHRP-6 on neuronal activity as measured by c-fos-IR in the NTS was determined in CCK1R−/− mice fed a HF diet and compared to neuronal activity in LF fed vehicle-treated mice. Following an overnight fast, basal fos protein-IR was low in mice of both genotypes ingesting whether a LF or HF diet; there was significantly less fos in the NTS of CCK1R−/− mice compared to wildtype mice fed a LF diet (Fig. 3, p<0.05). Administration of the ghrelin receptor antagonist to mice ingesting HF diets had little effect c-fos-IR in both genotypes.
Figure 3. Effect of ghrelin and D-(Lys3)-GHRP-6 on c-fos-IR in hindbrain neurons.
Representative photomicrographs of the NTS of wild-type and CCK1R−/− mice (n=4–6) treated with saline or D-(Lys3)-GHRP-6 (2.8µg/kg). c-fos-immunoreactivity was quantified as labeling density, where the number of c-fos neurons in the NTS region was counted and normalized to total background area. a,b indicate p<0.05 by two-way ANOVA followed by Bonferroni’s post-hoc test. Values represent mean ± SEM.
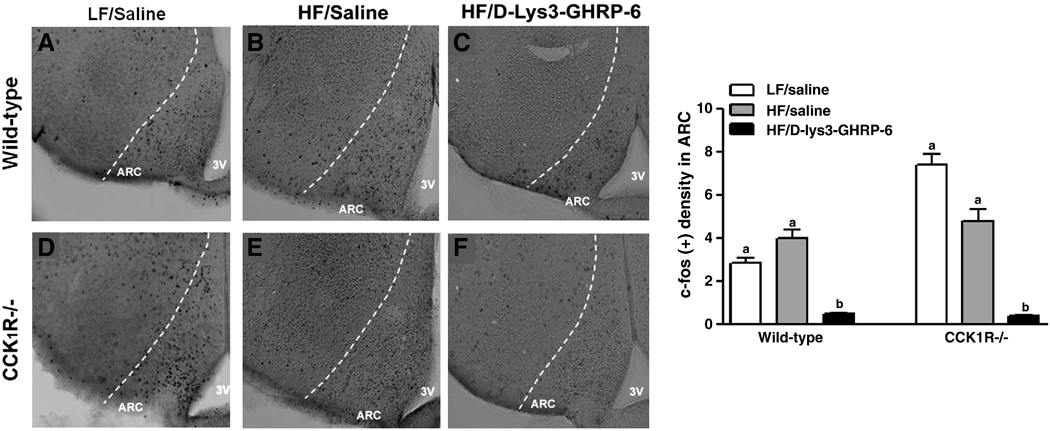
In the ARC, there was a 2.5-fold higher baseline level of c-fos-IR neurons in LF fed CCK1R−/− mice compared to wild-type controls (Fig. 4, p<0.05). There was no difference in baseline c-fos-IR between saline-treated wild-type and CCK1R−/− mice fed HF diet; administration of D-(Lys3)-GHRP-6 significantly reduced neuronal activation in both wild-type and CCK1R−/− mice to near nadir levels (Fig. 4, p<0.05).
Figure 4. Effect of ghrelin and D-(Lys3)-GHRP-6 on c-fos-IR in ARC neurons.
Representative photomicrographs of the arcuate nucleus of wild-type and CCK1R−/− mice (n=4–6) treated with saline or D-(Lys3)-GHRP-6 (2.8µg/kg). c-fos-immunoreactivity is represented as labeling density, where the number of c-fos in the NTS region was counted and normalized to total background area (Aii, Bii). a,b indicate p<0.05 by two-way ANOVA followed by Bonferroni’s post-hoc test. Values represent mean ± SEM.
4.0 Discussion
The main finding of the present study is that blockade of GHSR1a in CCK1R−/− mice ingesting a HF diet delays the onset of the first meal and decreases first meal size and duration. Blockade of ghrelin receptors had no effect on meal parameters in wildtype mice ingesting either LF or HF diet, or in CCK1R−/− mice ingesting LF diet. Thus ghrelin receptor blockade only influenced the hyperphagic meal patterns seen in the CCK1R−/− mice ingesting HF diets. These data suggests that in the absence of CCK1R and during ingestion of HF diets, ghrelin may play a significant role in initiating food intake. Previous work has shown that exogenous administration of ghrelin initiates feeding, but the effects of administration of the ghrelin receptor on meal patterns has not previously been reported in mice fed HF diets. It is interesting to note that in ghrelin or GHSR1a knockout mice, or the combination of both, there is no increase in body weight or hyperphagia (23–24), also suggesting that ghrelin and its receptor may be important in regulation of food intake when normal eating is disrupted as during ingestion of HF diet and compromised anorexigenic signaling, in this case modeled by using CCK1R null mice. Taken together our data supports a role for the ghrelin receptor in determining hyperphagic meal patterns in rodents ingesting high fat diets.
Ghrelin receptors have been localized to several structures in the brain and also to vagal afferent neurons. Ghrelin administered either centrally or peripherally can inhibit food intake; the action of ghrelin on neurons in the arcuate nucleus is well delineated (2). Although early work suggested that peripheral ghrelin can act via a vagal afferent pathway to inhibit food intake, this was not substantiated in another carefully controlled study (11). However, little is known about the role of the vagal afferent pathway and increase in food intake in response to ghrelin in animals ingesting high fat foods, and it is possible that ghrelin receptor on vagal afferent may play a role in regulation of food intake only when meal patterns are altered by high fat diets. CCK and ghrelin interact at the level of the vagus in a number of ways but the site of action of these interactions is unclear and given that peripheral ghrelin can act in the CNS may be at the level of the hypothalamus where peripheral signals are integrated (14). However, it has been shown that CCK and ghrelin interact at the level of vagal afferents. The ability of CCK to increase firing in vagal afferent neurons to elicit reflex changes in GI function and food intake has been well documented (25); however, recent evidence has shown that CCK also controls expression of anorexigenic and orexigenic peptides and receptors in vagal afferent neurons (26). In fasted rats, expression of CART (cocaine-and amphetamine regulated transcript) is low and increased by feeding via a CCK1R mediated pathway (21). This may explain the mechanism by which CART plays a role in mediating the effects of CCK to inhibit food intake. Feeding or exogenous administration of CCK also reduces expression of the orexigenic peptide MCH, and MCH1Rs and CB1Rs, thus enhancing orexigenic signaling and decreasing anorexigenic signaling in the postprandial period. Ghrelin inhibits this CCK-induced neurochemical switch (4, 21); the action of ghrelin may be important in mediating the vagal afferent component of its orexigenic phenotype.
In the current study, we found that administration of the ghrelin receptor antagonist increased expression of CART. These data suggests that in the absence of the CCK1R and when maintained on a HF diet, the ghrelin receptor puts a “brake” on the expression of CART, which may result in a decrease in anorexigenic drive via the vagal afferent pathway and play a role in the hyperphagic meal patterns in CCK1R−/− mice. Thus, loss of functional CCK1R may allow ghrelin receptors in the vagal afferent pathway to further augment the drive to initiate meals by dampening satiety signaling via reduced expression of CART. This may be particularly relevant in HF diet-induced overconsumption, as this has been shown to diminish the sensitivity of the vagal afferent pathway to CCK (27-28).
In order to determine a role for the vagal afferent pathway, we measured fos in the nucleus of the solitary tract, the site of termination of VAN and in the arcuate nucleus, a site of expression of ghrelin receptors. Anorectic peptides and postprandial nutrient stimuli have been shown to induce c-fos-IR in hindbrain neurons and central ARC although fewer studies have studied the effects of peripheral administration of orexigenic peptides on neuronal activity. Ghrelin has also been shown to induce c-fos-IR in the same regions by increasing neuronal activation in the nucleus tractus of the solitarius (NTS) of the DVC and ARC (29), but the models used were not associated with any metabolic compromise. In this study, we extend these findings by using the CCK1R−/− mouse and HF feeding where meal patterns are altered toward a more obesigenic phenotype. Ghrelin receptors are also located on neurons in the dorsal vagal complex and central ARC (7). Thus, blockade of ghrelin receptors by [D-Lys3]-GHRP-6 may be attenuating meal initiating events via a vagal afferent pathway or within the central nervous system. In the NTS, administration of the ghrelin receptor antagonist produced only a small but significant decrease in neuronal activity in the NTS in all groups; this suggests that the NTS may not be the main site of integration for the hyperphagic meal patterns seen with HF feeding or in the absence of the CCK1R. Neuronal activation in the ARC was markedly reduced following GHSR1a blockade in both CCK1R−/− and wild-type mice ingesting HF diets. This attenuation in activity in central neurons of both the NTS and ARC may be representative of the reduction in first meal size, duration, and short-term food intake in both strains of mice.
Ghrelin seems to play a critical role in meal patterns and food intake, particularly when these are disrupted by the absence of normal satiety factors, such as seen during ingestion of HF diets. Indeed, it has recently been demonstrated that ghrelin-induced increase in food intake is attenuated in mice fed a high fat diet (30). Consumption of and adaptation to palatable HF diets has been shown to lead to hyperphagia and adiposity (28, 31). In rodents, this has been shown to be associated with an increase in expression of orexigenic receptors at the level of the nodose ganglion (32) and decreased sensitivity to central anorectic peptide signaling (33). Ingestion of HF diets is characterized by prolonged and larger meals in both rodents and humans (34–35), also seen in the present study. We demonstrate for the first time that in the absence of the CCK1R, ghrelin plays a role in determining time to meal initiation as well as size of the first meal.
Acknowledgements
Work funded by NIH DK41004 (HER) and by funds from T32 NIH NCRR RR07038 (EM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 2.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89(1):71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Mondal MS, Date Y, Yamaguchi H, Toshinai K, Tsuruta T, Kangawa K, et al. Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regul Pept. 2005;126(1−2):55–59. doi: 10.1016/j.regpep.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 4.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1289–G1297. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- 5.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123(4):1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 6.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 7.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494(3):528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, et al. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology. 2005;146(8):3518–3525. doi: 10.1210/en.2004-1240. [DOI] [PubMed] [Google Scholar]
- 9.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120(2):337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 10.le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, Ghatei MA, et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab. 2005;90(8):4521–4524. doi: 10.1210/jc.2004-2537. [DOI] [PubMed] [Google Scholar]
- 11.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26(43):11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page AJ, Slattery JA, Milte C, Laker R, O'Donnell T, Dorian C, et al. Ghrelin selectively reduces mechanosensitivity of upper gastrointestinal vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2007;292(5):G1376–G1384. doi: 10.1152/ajpgi.00536.2006. [DOI] [PubMed] [Google Scholar]
- 13.Murray CD, Booth CE, Bulmer DC, Kamm MA, Emmanuel AV, Winchester WJ. Ghrelin augments afferent response to distension in rat isolated jejunum. Neurogastroenterol Motil. 2006;18(12):1112–1120. doi: 10.1111/j.1365-2982.2006.00848.x. [DOI] [PubMed] [Google Scholar]
- 14.Kobelt P, Tebbe JJ, Tjandra I, Stengel A, Bae HG, Andresen V, et al. CCK inhibits the orexigenic effect of peripheral ghrelin. Am J Physiol Regul Integr Comp Physiol. 2005;288(3):R751–R758. doi: 10.1152/ajpregu.00094.2004. [DOI] [PubMed] [Google Scholar]
- 15.Bi S, Scott KA, Kopin AS, Moran TH. Differential roles for cholecystokinin a receptors in energy balance in rats and mice. Endocrinology. 2004;145(8):3873–3880. doi: 10.1210/en.2004-0284. [DOI] [PubMed] [Google Scholar]
- 16.Donovan MJ, Paulino G, Raybould HE. CCK(1) receptor is essential for normal meal patterning in mice fed high fat diet. Physiol Behav. 2007;92(5):969–974. doi: 10.1016/j.physbeh.2007.07.003. PMCID: 2675541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA. Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J Neuroendocrinol. 2002;14(7):580–586. doi: 10.1046/j.1365-2826.2002.00810.x. [DOI] [PubMed] [Google Scholar]
- 18.Whited KL, Thao D, Lloyd KC, Kopin AS, Raybould HE. Targeted disruption of the murine CCK1 receptor gene reduces intestinal lipid-induced feedback inhibition of gastric function. Am J Physiol Gastrointest Liver Physiol. 2006;291(1):G156–G162. doi: 10.1152/ajpgi.00569.2005. [DOI] [PubMed] [Google Scholar]
- 19.Powley TL, Chi MM, Baronowsky EA, Phillips RJ. Gastrointestinal tract innervation of the mouse: afferent regeneration and meal patterning after vagotomy. Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R563–R574. doi: 10.1152/ajpregu.00167.2005. [DOI] [PubMed] [Google Scholar]
- 20.Donovan MJ, Paulino G, Raybould HE. Activation of hindbrain neurons in response to gastrointestinal lipid is attenuated by high fat, high energy diets in mice prone to diet-induced obesity. Brain Res. 2009;1248:136–140. doi: 10.1016/j.brainres.2008.10.042. PMCID: 2649666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci. 2007;27(11):2876–2882. doi: 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Lartigue G, Dimaline R, Varro A, Raybould H, De la Serre CB, Dockray GJ. Cocaine- and amphetamine-regulated transcript mediates the actions of cholecystokinin on rat vagal afferent neurons. Gastroenterology. 2010;138(4):1479–1490. doi: 10.1053/j.gastro.2009.10.034. PMCID: 2847060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115(12):3564–3572. doi: 10.1172/JCI26002. PMCID: 1297251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, et al. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115(12):3573–3578. doi: 10.1172/JCI26003. PMCID: 1297252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raybould HE. Mechanisms of CCK signaling from gut to brain. Curr Opin Pharmacol. 2007;7(6):570–574. doi: 10.1016/j.coph.2007.09.006. PMCID: 2692370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurones. Neuroscience. 2006;137(4):1405–1415. doi: 10.1016/j.neuroscience.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 27.Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides. 1998;19(8):1407–1415. doi: 10.1016/s0196-9781(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 28.Savastano DM, Covasa M. Adaptation to a high-fat diet leads to hyperphagia and diminished sensitivity to cholecystokinin in rats. J Nutr. 2005;135(8):1953–1959. doi: 10.1093/jn/135.8.1953. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143(1):155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- 30.Gardiner JV, Campbell D, Patterson M, Kent A, Ghatei MA, Bloom SR, et al. The hyperphagic effect of ghrelin is inhibited in mice by a diet high in fat. Gastroenterology. 2010;138(7):2468–2476. doi: 10.1053/j.gastro.2010.02.012. 76 e1. [DOI] [PubMed] [Google Scholar]
- 31.Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1211–1218. doi: 10.1098/rstb.2006.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulino G, Barbier de la Serre C, Knotts TA, Oort PJ, Newman JW, Adams SH, et al. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab. 2009;296(4):E898–E903. doi: 10.1152/ajpendo.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2002;283(4):R941–R948. doi: 10.1152/ajpregu.00245.2002. [DOI] [PubMed] [Google Scholar]
- 34.Green SM, Wales JK, Lawton CL, Blundell JE. Comparison of high-fat and high-carbohydrate foods in a meal or snack on short-term fat and energy intakes in obese women. Br J Nutr. 2000;84(4):521–530. [PubMed] [Google Scholar]
- 35.Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, et al. Acute exposure to a high-fat diet alters meal patterns and body composition. Physiol Behav. 2010;99(1):33–39. doi: 10.1016/j.physbeh.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]