Abstract
The NFKB pathway performs pivotal roles in diverse physiological processes, such as immunity, inflammation, proliferation, and apoptosis. NFKB is kept inactive in the cytoplasm through association with inhibitors (IKB), and translocates to the nucleus to activate its target genes after the IKBs are phosphorylated and degraded. Here we demonstrate that loss of function of SHARPIN leads to activation of NFKB signaling in skin resulting in the development of an idiopathic hypereosinophilic syndrome (IHES) with eosinophilic dermatitis in C57BL/KaLawRij-Sharpincpdm/RijSunJ mice with clonal expansion of B-1 B cells and CD3+CD4−CD8− T cells. Transcription profiling in skin revealed constitutive activation of classical NFKB pathways predominantly by overexpressed members of IL1 family. Compound null mutants for both the IL1 receptor accessory protein (Il1raptm1Roml) and SHARPIN (Sharpincpdm) resulted in mice having decreased skin disease severity. Inhibition of IKBA degradation by the proteasome inhibitor, bortezomib, alleviated the dermatitis in Sharpincpdm mice. These results indicate that absence of SHARPIN causes IHES with eosinopholic dermatitis by NFKB activation and bortezomib may be an effective treatment for skin problem of IHES.
Keywords: Sharpin, bortezomib, NFKB, HES, mice
INTRODUCTION
Hypereosinophilic syndromes (HES) are a rare, heterogeneous group of hematological and systemic diseases characterized by unexplained blood and tissue eosinophilia either mediated by extrinsic cytokines or as a consequence of intrinsic mutations in hematopoietic stem cells leading to predominantly eosinophil differentiation (Fletcher and Bain, 2007; Kahn et al., 2008; Leiferman, 1995; Roufosse et al., 2007; Sheikh and Weller, 2009). Eosinophilic infiltration into multiple tissues and eosinophilia are the prominent features of chronic proliferative dermatitis mouse (CPDM) due to a spontaneous null mutation in the Sharpin gene (Gijbels et al., 1995; HogenEsch et al., 1993; Seymour et al., 2007). These mutant mice were initially recognized by the phenotype of erythematous, scaly areas of alopecia due to marked epidermal hyperplasia and dermal granulocytic infiltration. The molecular mechanism that caused the severe phenotype seen in Sharpincpdm/Sharpincpdm mice was unknown due to the limited understanding of the biological function(s) of Sharpin (Daigo et al., 2003; Lim et al., 2001).
The NFKB signaling pathway serves a crucial role in regulating the transcriptional responses of multifunctional physiological processes that include cell division, cell survival, differentiation, immunity, and inflammation (Gerondakis et al., 2006; Gilmore, 2006). It can induce different kinetic or phenotypic properties in various cell types or even in the same clonal cell population in other cellular environments (Mercurio and Manning, 1999; Pasparakis et al., 2006).
Dysregulation of the NFKB pathway is involved in many diseases, particularly ones associated with chronic inflammation, immunodeficiency, or cancer (Aradhya and Nelson, 2001; Baldwin, 2001; Courtois, 2005; Courtois and Gilmore, 2006). NFKB is expressed in the cytoplasm of virtually all cell types, where its activity is controlled by inhibitors of NFKB (IKBs) (Hayden and Ghosh, 2004). In the classical NFKB pathway, binding of ligands (e.g, TNF, IL1) to their receptors (TNFR, IL1R) triggers polyubiquitination of factors such as TNF receptor-associated factors (e.g, TRAF 2, TRAF6), leading to phosphorylation, ubiquitination, and degradation of IKBs. In the alternative pathway, NFKB2/p100 is phosphorylated by the conserved helix-loop-helix ubiquitous kinase (CHUK) and then polyubiquitinated. Proteolysis of the carboxyl-terminal of p100 follows and the RELB/p52 transcription factor is released. In both classical and alterative pathways, translocation of NFKB also induces transcription of IKBs which in turn bind to nuclear NFKB and export it back into the cytosol as a negative feedback circuit (Kim et al., 2009a). The yeast two-hybrid (Y2H) screen with TRAF2 as the bait (UCSD Nature Signaling Gateway) identified SHARPIN (alternative symbol: RBCKL1) as one of its prey in B cell lines, indicating that SHARPIN may interact with TRAF2 to regulate activation of NFKB signaling.
A series of functional studies were conducted to dissect the molecular mechanism of eosinophilic dermatitis seen in Sharpincpdm/Sharpincpdm mutant mice and shed light on similar human diseases.
RESULTS
Phenotype of idiopathic hypereosinophilic syndrome in Sharpincpdm/Sharpincpdm mice
The Sharpincpdm/Sharpincpdm mutant mouse was first reported as having chronic, severe, mixed granulocytic inflammation with epidermal hyperproliferation and puritic, scaly, and ulcerated cutaneous lesions (HogenEsch et al, 2003; Gijbels et al, 1996). Many visceral organs are affected and characterized by mixed granulocytic, primarily eosinophilic infiltration, which makes chronic proliferative dermatitis in mice an eosinophilic disorder (Simon and Simon, 2007). In order to further address which eosinophilic disorder Sharpincpdm/Sharpincpdm mimics, specific parameters for several known human eosinophilic diseases were tested. Hematologic counting identified eosinophilia of 2685±56, 1770±65, 1040±34, 1230±44 eosinophils/ul in mutant (Sharpincpdm/Sharpincpdm) mice compared to 250±35, 150±55, 200±70, 160±42 eosinophils/ul in +/+ mice respectively at the age of 4, 6, 8, and 10 weeks. The eosinophil counts in +/+ mouse peripheral blood are in the range of humans (0–350/ul) (Rothenberg, 1998). Persistent and marked eosinophilia, lack of other associations with eosinophilia (such as infectious diseases and allergic diseases), and systemic eosinophilic inflammation suggests that Sharpincpdm/Sharpincpdm mutant mice represent a mouse model for one or more of the human idiopathic hypereosinophilic syndromes (Leiferman, 1995).
To further determine if the Sharpincpdm/Sharpincpdm mice mimics the lymphocytic variant of hypereosinophilic syndromes, FACS analysis was performed using splenocytes. The wet weight of Sharpincpdm/Sharpincpdm spleens (0.2±0.01 g) was approximately three times of that of +/+ mice (0.07±0.01 g). The percentage of B cells, T cells, and dendritic cells were decreased in the mutant mouse spleens. However, normalization by spleen wet weight significantly altered these numbers (Supplemental Table 1). The B-1 B cells (CD45R+CD11b+) had the greatest increase in Sharpincpdm/Sharpincpdm mutant mice either before or after normalization. The percentage of inflammatory cells (eosinophils, macrophages, monocytes, and polymorphonuclear leukocytes) was also significantly increased. CD3+CD4+ helper T cells and CD3+CD8+ cytotoxic T cells were not significantly different from those of controls after normalization but both percentage and total counts of CD3+CD4−CD8− T cells were significantly increased in Sharpincpdm/Sharpincpdm mutant mice (Supplemental Table 2). CD3−CD4+ T cells were also detected in Sharpincpdm/Sharpincpdm mutants but this number was not as significant as that in human HES patients (Supplemental Figure 1) (Simon et al., 1999).
Activated NFKB signaling revealed by gene expression arrays and confirmed by Immunohistochemistry
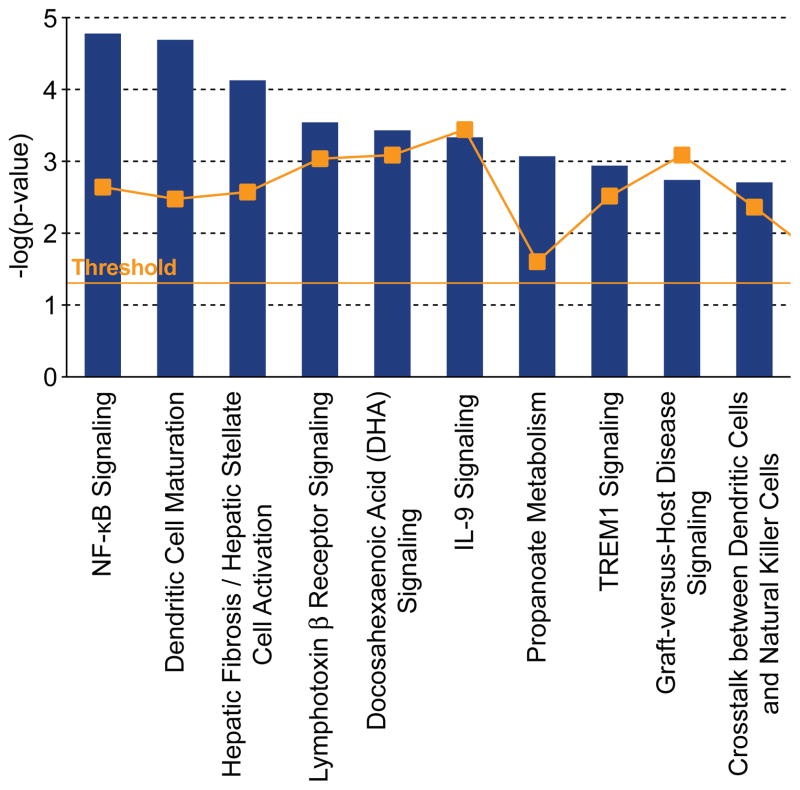
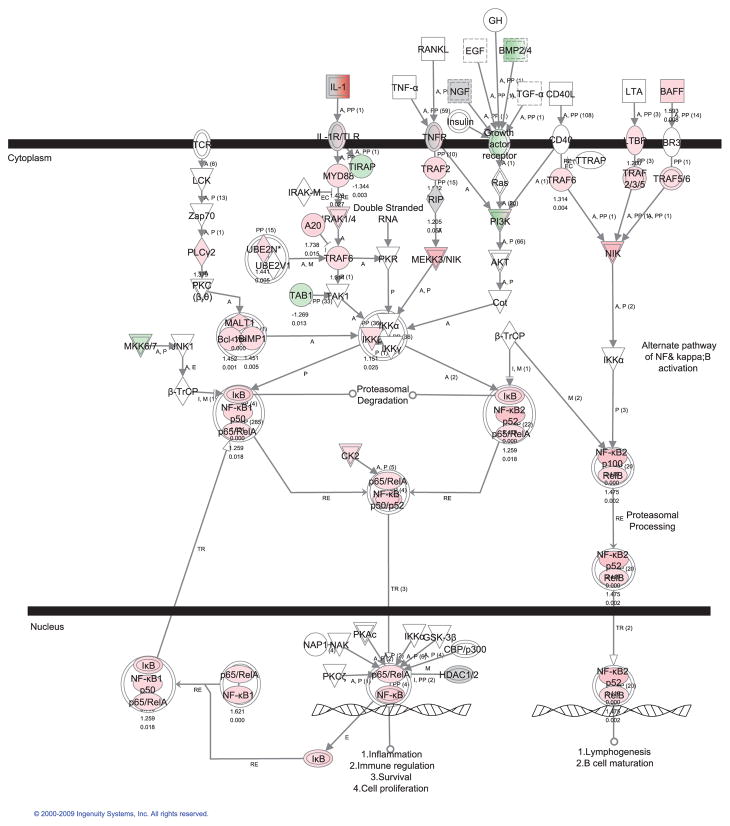
A longitudinal transcriptome analysis of dorsal skin from Sharpincpdm/Sharpincpdm mutant mice compared to age and gender matched +/+ mice at 2-, 4-, 6-, 8-, and 10-weeks of age was done using Ingenuity Pathway Analysis® (IPA) software. Analysis of the 2-week group identified downregulation of Sharpin and upregulation of interleukin 1 family, member 6 (Il1f6), stefin A3 (Stfa3), complement component 3 (C3), and serine peptidase inhibitor clade A member 3N (Serpina3n) with a q value below 0.05, which represented the earliest molecular changes in the skin of these mutant mice. This concurs with essentially no histologic abnormalities in the skin of mutant mice at this age. Pathway analysis revealed that NFKB signaling was the most significantly upregulated pathway along with disease development (Figure 1). Most of genes in NFKB pathway (Nishikori, 2005) are progressively upregulated in the skin of Sharpincpdm/Sharpincpdm mutant mice (Figure 2, Supplemental Figure 2) and IL1 pathway members, especially Il1f6, Il1f8, and Il1f9, are most dramatically upregulated (p<0.0001) suggesting the contributing role of Il1 stimuli for NFKB activation. NFKB activation was validated by immunohistochemical localization of phosphorylation of NFKB p65 in skin of Sharpincpdm/Sharpincpdm mice (Figure 3A) compared to that in control mice (Figure 3B). Interaction between TRAF2 and SHARPIN suggested by yeast two-hybrid screen implicates where SHARPIN may act in the NFKB pathway (UCSD Nature Signaling Gateway).
Figure 1. Top 10 canonical pathways.
NFKB signaling was highly associated pathways in the skin of Sharpincpdm/Sharpincpdm mutant mice based on Ingenuity Pathway Analysis® software.
Figure 2. Activation of NFKB signaling in the skin of Sharpincpdm/Sharpincpdm mutant mice.
Pathway analysis of transcriptome data in skin using Ingenuity Pathway Analysis® software suggested activation of both classical and alterative NFKB pathways. Most of molecules in NFKB pathways were upregulated, such as p65/RelA and NFKB p52/RelB in the mutant group from the pooled expression data of 2-, 4-, 6-, 8-, and 10-week old mice.  upregulation;
upregulation;  downregulation; □ no significant change
downregulation; □ no significant change
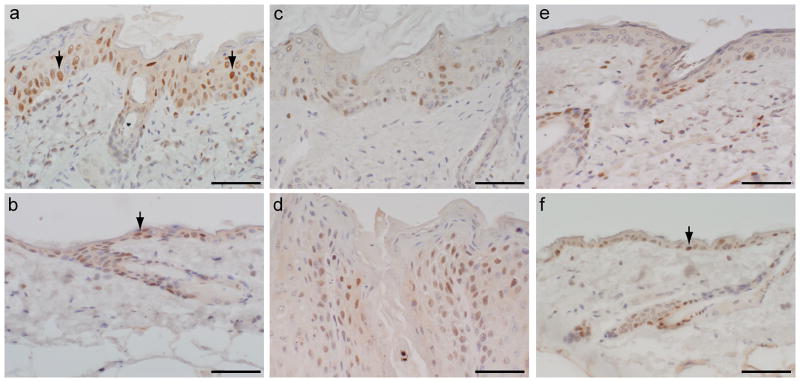
Figure 3. NFKB p65 phosphorylation.
NFKB p65 was over phosphorylated in the skin of Sharpincpdm/Sharpincpdm mutant mice (arrows indicated positive, brown, nuclei). NFKB p65 phosphorylation was decreased by inactivation of Il1rap and especially by bortezomib inhibition in Sharpincpdm/Sharpincpdm mutant mice. Bortezomib did not affect NFKB p65 phosphorylation in skin of control mice. a: Sharpincpdm/Sharpincpdm mutant mice; b: +/+ control; c: l1raptm1Roml/Il1raptm1Roml; d: l1rap+/l1rap+, Sharpincpdm/Sharpincpdm mutant mice; e: Sharpincpdm/Sharpincpdm mutant mice with bortezomib treatment; f: +/+ control with bortezomib treatment. Bar = 5 um.
Improved Sharpincpdm/Sharpincpdm mutant skin lesions in Il1raptm1Roml/Il1raptm1Roml, Sharpincpdm/Sharpincpdm compound mutants
Among eleven IL1 members, IL1F6, IL1F8, and IL1F9, which activate NFKB in an IL1RAP-dependent manner (Barksby et al., 2007), are most significantly upregulated. Therefore, loss of the IL1RAP protein should abrogate IL1 signaling. To test this hypothesis C57BL/KaLawRijSunJ-Sharpincpdm/Sharpincpdm male mice were crossed with B6;129S1-Il1raptm1Roml/Il1raptm1Roml /J female mice. Eight Il1raptm1Roml/Il1raptm1Roml, Sharpincpdm/Sharpincpdm; 13 +/ Il1raptm1Roml, Sharpincpdm/Sharpincpdm; and 7 +/+, Sharpincpdm/Sharpincpdm F2 female progeny were evaluated at 10 weeks of age (Figure 4A, B). The skin lesions were significantly reduced in Il1raptm1Roml/Il1raptm1Roml, Sharpincpdm/Sharpincpdm compound mutants that had mild alopecia and scaling. Histology revealed decreased severity of the skin lesions but not total resolution of diseases (Figure 4C, D) as demonstrated by epidermal thickness (Figure 4E, p<0.0001). +/Il1raptm1Rom, Sharpincpdm/Sharpincpdm and +/+, Sharpincpdm/Sharpincpdm mutant mice were similarly affected. Numerous phosphorylated NFKB p65 positive cells were still present in skin of the compound mutants (Figure 3C) but decreased compared to that of +/+, Sharpincpdm/Sharpincpdm mutant mice (Figure 3D), indicating that other stimuli, beyond IL1RAP modulation, contribute to NFKB activation and in turn the skin phenotype.
Figure 4. Lack of Il1rap improved the skin disease in Sharpincpdm/Sharpincpdm mutant mice.
Loss of Il1rap in Sharpincpdm/Sharpincpdm mutant mice resulted in mice appearing clinically normal (A, C) compared to Il1rap+/+, Sharpincpdm/Sharpincpdm mutant mice (B, D). Histology revealed decreased severity of the skin lesions but not total resolution of diseases as shown by epidermal thickness (E;1 represents +/+, Sharpincpdm/Sharpincpdm; 2 represents l1raptm1Roml/Il1raptm1Roml, Sharpincpdm/Sharpincpdm; 3 represents l1raptm1Roml/Il1raptm1Roml, +/+; and 4 represents +/+, +/+). Note: size of PCR products: Il1rap band (168bp), Neo (280bp). Bar = 5 um.
Bortezomib alleviates inflammation in Sharpincpdm/Sharpincpdm mutant mice by blocking NFKB activation
Bortezomib inhibits IKB degradation, thereby preventing translocation of NFKB into the nucleus (Ma and Adjei, 2009). To test whether inhibition of this pathway would improve the disease in Sharpincpdm/Sharpincpdm mutant mice, 0.5 mg/kg of bortezomib was injected intraperitoneally twice a week for 6 weeks into mutant and +/+ control mice with equal volumes of physiological saline used as a treatment control. Macroscopically, Sharpincpdm/Sharpincpdm mice treated with bortezomib looked like +/+ control mice except for small body size. As shown in Figure 5, while mildly hyperplastic compared to the wildtype control, skin from bortezamib treated mice was significantly (P<0.0001) reduced in thickness compared to the vehicle treated Sharpincpdm mutant mice. NFKB p65 phosphorylation was inhibited by bortezomib in the skin of both Sharpincpdm/Sharpincpdm mice (Figure 3E) and close to that of the +/+ controls (Figure 3F), implicating the drug effect on blockage of NFKB activation and in turn reducing the severity of the skin phenotype. NFKB p65 phosphorylation in the +/+ controls was not obviously affected by administration of bortezomib which might be due to a feedback mechanism for NFKB’s role in the physiological function of skin (Gilmore, 2006; Kaufman and Fuchs, 2000; Kim et al., 2009b). Hypereosinophilia and the damage in other organs were still present in Sharpincpdm/Sharpincpdm mutant mice (data not shown).
Figure 5. NFKB inhibition by bortezomib treatment reduced the skin problem in Sharpincpdm/Sharpincpdm mutant mice.
a, e: Bortezomib-treated Sharpincpdm/Sharpincpdm mutant mice. Alopecia on the dorsal skin with partial histologic resolution. Histologically, skin lesions were improved but still present with moderate acanthosis and mild orthokeratotic hyperkeratosis. b, f: Bortezomib-treated +/+ controls. No skin lesions. Normal skin histology. c, g: Saline-treated Sharpincpdm/Sharpincpdm mutant mice. Severe scaling, alopecia, and ulcers on the dorsal skin. Marked acanthosis, orthokeratotic hyperkeratosis, apoptosis, and granulocytic infiltration in the dermis. d, h: Saline-treated +/+ controls. No skin lesions. Normal skin histology. i: Measurement of epidermal thickness. Bar = 5 um.
DISCUSSION
We show here that SHARPIN negatively regulates pro-inflammatory NFKB signaling primarily through the IL1 pathway in the skin of Sharpincpdm/Sharpincpdm mutant mice. Bortezomib treatment reduces the severity of the skin disease and improves survival in a mouse model mimicking idiopathic hypereosinophilic syndrome caused by loss of function of SHARPIN.
Human hypereosinophilic syndrome is a relatively rare syndrome with a heterogeneous set of symptoms presumably due to multiple etiologies (Kahn et al., 2008; Roufosse et al., 2007). Unmasking the molecular basis for such diseases has led to the development of semi-molecular classification schemes for categorizing patients based on recurrent genetic alterations (Gotlib, 2008). Except for a transgenic mouse model expressing the fusion gene FIP1-like 1/platelet-derived growth factor receptor alpha (FIP1L1/PDGFRA, F/P), no other animal models have been reported to date for HES (Yamada et al., 2008). The vast majority of HES cases are neither associated with mutations in hematopoiesis nor lymphoid clones. Analytical parameters cannot be simply converted from humans to mice or vice versa (Sundberg and King, 1996). However, the peripheral blood eosinophil counts in wildtype C57BL/KaLawRijSunJ mice were 0.17×109 cells/L while that of healthy human is approximately 0.35×109 cells/L, which suggests that the diagnostic threshold of HES (1.5×109 eosinophils/L) can be used as one criteria for identification of an HES model in mice (Kahn et al., 2008). Based on the diagnostic criteria for HES in humans, Sharpincpdm/Sharpincpdm mutant mice could be a spontaneous mouse model for one type of HES considering eosinophil associated with multiorgan damage and persistent eosinophilia (2.02×109/L). Simon et al. proposed a new classification of eosinophilic disorders based on the primary cause as mutations in stem cells or cytokines secreted by T cells or tumor cells (Simon and Simon, 2007). Alternatively, there could be mutations in stem cells leading to the clonal expansion of T cells which can secrete cytokines inducing eosinophilia. There are some human HES cases showing aberrant T cell phenotypes such as elevated CD3−CD4+ T cells and CD3+CD4−CD8− T cells (Cogan et al., 1994; Roufosse et al., 2004; Simon et al., 1999). These T cells have been shown to produce Th2 cytokines: IL5, responsible for eosinophil differentiation, maturation, activation, and prolonged survival in the periphery and IL4, which induces B cell class-switching leading to IgE production, a common feature of the lymphocytic variant of HES. Although insignificant numbers of CD3−CD4+ were detected in Sharpincpdm/Sharpincpdm mutant mice, FACS data present here indicated that at least two lymphocyte subpopulations, CD3+CD4−CD8− T cells and B-1B cells, are abnormally expanded in Sharpincpdm/Sharpincpdm mutant mice. In contrast to the predominant population of B-2 B cells produced in the bone marrow, B-1 B cells are a minor population of B lymphocytes that are found in multiple tissues in the response to bacteria and self-antigens and participate in T-cell-mediated alloimmune response (Montecino-Rodriguez and Dorshkind, 2006; Nogueira-Martins and Mariano, 2009). Significant increases of B-1 B cells in Sharpincpdm/Sharpincpdm mutant mice may be partially related to the downregulation of sialic acid binding Ig-like lectin G (Siglecg) (transcriptome profiling data using spleen, not shown here) which limits the expansion of B-1 B cells by down regulating NFKB activation (Ding et al., 2007). Significant increases of B-1 B cells in mutant mice provide a tool to study its biological function.
As described above, Sharpincpdm/Sharpincpdm mutant mice present a very complicated disorder including chronic inflammation, proliferation, apoptosis, immune response, and organ development abnormities (HogenEsch et al., 1993; HogenEsch et al., 1999; Seymour et al., 2006), all of which implicate that Sharpin plays diverse roles in crucial transduction signaling or pathways. Pathway analysis by gene expression profiling in skin revealed the constitutive activation of NFKB signaling in the skin of Sharpincpdm/Sharpincpdm mutant mice as disease developed predominantly through IL1 cascades. IL1 family members are known to alter the host response to an inflammatory, infectious, or immunological challenges (Dinarello, 2009). The IL1 family comprises 11 ligands (IL1F1 to IL1F11) and 3 receptors (IL1RI, Il1RAP, and IL1RII) (Barksby et al., 2007; Boraschi and Tagliabue, 2006). Although other IL1 family members also showed upregulation at the mRNA level in skin of Sharpincpdm/Sharpincpdm mutant mice, IL1F6, IL1F8, and IL1F9 were the most significantly elevated, suggesting the importance of this subfamily in skin inflammation. IL1F6, IL1F8, and IL1F9 activate pathways leading to NFKB and MAPKs signaling in an IL1RAP and IL1Rrp2 dependent manner (Towne et al., 2004). IL1F6 transgenic mice exhibit psoriasiform skin abnormalities that are dependent on IL1RRP2 and IL1RAP (Blumberg et al., 2007). Furthermore, expression of IL1F2 was also increased in psoriasiform skin lesions in flaky skin (Ttc7fsn/Ttc7fsn) mice (Schon et al., 2001). The skin disease improved in the compound mutant Il1raptm1Roml/Il1raptm1Roml, Sharpincpdm/Sharpincpdm mice suggesting that IL1RAP dependent signals induced by IL1F6, IL1F8, and IL1F9 play major roles in the development of skin damage in these mice.
NFKB transduction pathways can be activated by many diverse stimuli such as TNF and LPS in addition to IL1 family members. Protesome inhibition, blockading a common link of the complicated NFKB circuit using bortezomib, reduced skin lesion severity in Sharpincpdm/Sharpincpdm mutant mice. Through reversible inhibition of this major proteolytic system, bortezomib potentially interacts with multiple signaling pathways critical for inflammation, tumor cell growth, survival, and apoptosis (Adams et al., 1999; Dicato et al., 2006). Bortezomib blocks proliferation and induces apoptosis and was approved by United States Food and Drug Administration for initial treatment of patients with multiple myeloma (Curran and McKeage, 2009; Ma and Adjei, 2009). This agent also inhibits the proteasomal degradation of NFKBIA, downregulating inducible NFKB signaling. Macroscopic and microscopic observations indicated that bortezomib administration resulted in a moderate improvement in the skin phenotype in Sharpincpdm/Sharpincpdm mutant mice, again suggesting SHARPIN plays a critical role in regulating proinflammatory NFKB signaling in the skin. Regarding the therapeutic effect on dermatitis, steroids, the primary treatment of the FIP1L1/PDGFRA negative form of HES, were also shown to have moderate effects on dermatitis in Sharpincpdm/Sharpincpdm mutant mice (Gijbels et al., 2000). Moreover, treatment with recombinant IL12 resulted in almost complete resolution of the skin disease in Sharpincpdm/Sharpincpdm mutant mice (HogenEsch et al., 2001) which implied that some Th2 cytokines can cause dermatitis in an NFKB independent manner. The dissociation between eosinophilia and the clinical effect of bortezomib is consistent with a previous report that anti-IL5 decreases the absolute eosinophil count in peripheral blood but not the severity of dermatitis in Sharpincpdm/Sharpincpdm mice (Renninger et al., 2009), indicating that infiltration of eosinophils into skin may be a secondary event to NFKB activation and has both pro-inflammatory and anti-inflammatory roles in the development of dermatitis of these mice. Although eosinophilia is the hallmark of human hypereosinophilic diseases, the relationship between the absolute eosinophil count and end-organ damage in this syndrome is inconsistent (Boucher et al., 2008; Plotz et al., 2003). Clinical trials in humans demonstrated that anti-IL5 therapy results in a rapid decrease in peripheral blood eosinophil numbers. Moreover, improvement of symptoms in human patients with lymphocytic variants of hypereosinophilic syndromes, in eosinophilic esophagitis and chronic rhinitis with nasal polyposis, were observed with anti-IL5 therapy. In contrast, in HES patients with bronchial asthma or atopic eczema, anti-IL5 therapy showed only moderate or no clinical effects (Simon et al., 2007). Future studies will have to identify those eosinophilic diseases in which anti-IL5 antibodies are effective.
In conclusion, this study identified a mouse model mimicking IHES with eosinophilic dermatitis caused by loss of function of SHARPIN which negatively regulates proinflammatory NFKB signaling primarily in the IL1 pathway in the skin. Bortezomib has therapeutic benefits in these mutant mice suggesting potential value for IHES patients.
MATERIALS AND METHODS
Mice
C57BL/KaLawRij-Sharpincpdm/RijSunJ (JR# 007599), C57BL/KaLawRijSunJ +/+, and B6;129S1-Il1raptm1Roml/J (JR# 003284) mice were maintained in a humidity, temperature, and light cycle (12:12) controlled vivarium under specific pathogen-free conditions (http://jaxmice.jax.org). Sharpincpdm/Sharpincpdm mutant mice of both sexes are equally affected. As female mutant mice do not breed, they were used for all experiments unless indicated otherwise. Mice were housed in double-pen polycarbonate cages at a maximum capacity of four mice per pen. Mice were allowed free access to autoclaved food (NIH 31, 6% fat; LabDiet 5K52, Purina Mills, St. Louis, MO) and acidified water (pH 2.8–3.2). All work was done with Institutional Animal Care and Use Committee approval.
Antibodies
The fluorochrome-conjugated monoclonal antibodies (mAb) to mouse antigens used for flow cytometry were purchased from BD Bioscience (San Diego, CA): anti-CD2 FITC (RM2-S), anti-CD3e PC7 (KT31.1), anti-CD4 APC (RM4-5), anti-CD8 biotin (YTS 169.4), anti-CD25 PE (PC61), anti-CD95 PE (JO2), anti-FOXP3 APC (259D/C7), CD45R/B220 biotin (RA3-6B2), CD11b/MAC-1 PE (M1/70), CD11c FITC (N418), and GR-1/LY6G AC7 (RB6-8C5). The phosphorylated NFKB p65 antibody was obtained from Cell Signaling (Danvers, MA).
Complete blood counts
Whole blood (275 μl) was drawn from the retro-orbital sinus through EDTA-coated microhematocrit tubes directly into Eppendorf tubes containing 30 μl 20% EDTA in PBS. The additional anticoagulant is critical in preventing microclot formation. Complete blood counts were determined immediately after obtaining the samples using an Advia 120 Multispecies whole blood analyzer (Bayer Corporation, Tarrytown, NY). Three Sharpincpdm mutant and three +/+ control mice were used for each time point.
Flow cytometric analysis
Splenocytes were harvested from mice according to protocol A (http://openwetware.org/wiki/Isolation_of_murine_splenocytes). Cells were then incubated with various combinations of mAb for 25 min at 4°C, washed twice with FACS buffer, and fixed with 1% paraformaldehyde. Flow cytometric analysis was performed by using conventional multiparameter procedures. Analysis was carried out on a FACScan (Becton Dickinson, Franklin Lakes, NJ) with CELLQuest software for acquisition and FlowJo software (Tree Star, Ashland, OR) for analysis. Viable cells were gated by propidium iodide exclusion.
Transcriptional profiling and pathway analysis
RNA isolation, cDNA preparation, and microarray hybridization
Dorsal interscapular thoracic skin and spleen were collected from 3 Sharpincpdm/Sharpincpdm and 3 +/+ female mice at 2, 4, 6, 8, and 10 weeks of age to provide a cross sectional transcription profile to determine the molecular pathogenesis of the disease. Total RNA was isolated by TRIzolPlus ™ kit according to the manufacturer’s protocols. cDNA was then hybridized onto GeneChip® Mouse Gene 1.0 ST Arrays (Affymetrix). Raw intensity values were normalized to positive control genes and each gene was normalized to the age-matched control. The fold change of the hybridization intensity of samples was used to represent the relative gene expression level. Data were analyzed through the use of Ingenuity Pathways Analysis (Ingenuity® Systems, www.ingenuity.com).
Bortezomib administration
Lyophilized bortezomib was obtained from L.C. laboratories (Velcade, Woburn, MA). Stock bortezomib solution (5 mg/mL) was prepared in physiologic saline (Hospira, Lake Forest, IL) and stored at −20°C. The stocks were diluted in physiologic saline immediately before use.
Groups of 4 week old, female Sharpincpdm/Sharpincpdm or +/+ mice (N = 10 mice per group) were injected intraperitoneally with 0.5 mg/kg body weight bortezomib or an equivalent volume of physiologic saline twice weekly for 6 weeks. Body weights and gross photos were obtained before every injection and at the time of necropsy. Complete blood cell counts were evaluated 3 days after the last injection. A complete necropsy was performed on each mouse (Seymour et al., 2004). Spleen, liver, brain, and kidneys were weighed at the time of necropsy. Tissues were processed routinely, paraffin embedded, sectioned at 6 um, and stained with hematoxylin and eosin (H&E). Histology was evaluated by an experienced veterinary pathologist (JPS).
Creation of Il1rap-Sharpin compound mutant mice
Il1raptm1Roml targeted mutant mice with a selectable Neo cassette were obtained from The Jackson Laboratory (B6;129S1-Il1raptm1Roml/J). Homozygous B6;129S1-Il1raptm1Roml/J females were crossed with homozygous C57BL/KaLawRij-Sharpincpdm/RijSun males. Phenotypes of 10 week old, female F2 progeny were compared between groups of each genotype (Il1raptm1Roml/Il1raptm1Roml, Sharpincpdm/Sharpincpdm; +/ Il1raptm1Roml, Sharpincpdm/Sharpincpdm; +/+, Sharpincpdm/Sharpincpdm). Sharpin genotypes were identified by direct sequencing for PCR products amplified with primers as below: TGTGTTTGTGTGCATTGGTG (forward) and CCCACGGACGGTGTAACAAA (reverse). Primer pairs for both Neo and Il1rap were included in the same PCR reaction to determine Il1rap deletion. Primers for Il1rap fragment were: 5′-ACTACAGCACTGCCCATTCC-3′ (forward), and 5′-TGTAATTGCCCGTGTCATTG-3′ (reverse). Primers for Neo fragment were: 5′-CTTGGGTGGAGAGGCTATTC-3′ (forward), and 5′-AGGTGAGATGACAGGAGATC-3′ (reverse). Il1rap+/+, +/Il1raptm1Roml, and Il1raptm1Roml/Il1raptm1Roml was identified by the presence of only the Il1rap band (168bp), both Neo (280bp) and Il1rap (168bp) bands, and only the Neo band (280bp), respectively.
Immunohistochemistry
Dorsal skin was collected and fixed by immersion in Fekete’s acid alcohol formalin solution and then transferred after 12 hr into 70% ethanol until processed (Mikaelian et al., 2004). Fixed tissues were embedded routinely in paraffin and serially sectioned at 6 um. Paraffin sections were stained at 1:50 for phosphorylated NFKB p65 (Ser276, Cell Signaling, Danvers, MA).
Measurement of epidermal thickness
Epidermal thickness of 10 fields for each sample, one sample per mouse, and 3 mice per group was measured. Every field included at least one full length hair follicle as an internal standard for orientation. The epidermal thickness was measured from the basement membrane to the top of the stratum granulosum.
Statistical analysis
Data were expressed as mean ± SE. Statistical comparisons between two groups were performed by the Student’s t-test. Statistical significance was determined as P<0.05.
Supplementary Material
Acknowledgments
This work was supported by NIH grants (AR049288 to JPS and AI060707 to RS).
Abbreviation
- CPDM
chronic proliferative dermatitis mutation
- IHES
idiopathic hyper-eosinophilic syndrome
- FACS
fluorescence activated cell sorting
- Il1rap
IL1 receptor accessory protein
- IPA
Ingenuity Pathway Analysis®
- Sharpin
gene
- SHARPIN
protein, SHANK-associated RH domain interacting protein
- RBCKL1
protein kinase C-interacting protein RBCC like 1
Footnotes
CONFLICT OF INTEREST: Dr. Sundberg had a research with the Procter & Gamble Company at the time this work was performed. However, that project was unrelated to this study.
References
- Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–22. [PubMed] [Google Scholar]
- Aradhya S, Nelson DL. NF-kappaB signaling and human disease. Curr Opin Genet Dev. 2001;11:300–6. doi: 10.1016/s0959-437x(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barksby HE, Lea SR, Preshaw PM, Taylor JJ. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007;149:217–25. doi: 10.1111/j.1365-2249.2007.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H, Dinh H, Trueblood ES, Pretorius J, Kugler D, Weng N, et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J Exp Med. 2007;204:2603–14. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraschi D, Tagliabue A. The interleukin-1 receptor family. Vitam Horm. 2006;74:229–54. doi: 10.1016/S0083-6729(06)74009-2. [DOI] [PubMed] [Google Scholar]
- Boucher RM, Gilbert-McClain L, Chowdhury B. Hypereosinophilic syndrome and mepolizumab. N Engl J Med. 2008;358:2838–9. doi: 10.1056/NEJMc080856. author reply 9–40. [DOI] [PubMed] [Google Scholar]
- Cogan E, Schandene L, Crusiaux A, Cochaux P, Velu T, Goldman M. Brief report: clonal proliferation of type 2 helper T cells in a man with the hypereosinophilic syndrome. N Engl J Med. 1994;330:535–8. doi: 10.1056/NEJM199402243300804. [DOI] [PubMed] [Google Scholar]
- Courtois G. The NF-kappaB signaling pathway in human genetic diseases. Cell Mol Life Sci. 2005;62:1682–91. doi: 10.1007/s00018-005-5031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene. 2006;25:6831–43. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- Curran MP, McKeage K. Bortezomib: a review of its use in patients with multiple myeloma. Drugs. 2009;69:859–88. doi: 10.2165/00003495-200969070-00006. [DOI] [PubMed] [Google Scholar]
- Daigo Y, Takayama I, Ward SM, Sanders KM, Fujino MA. Novel human and mouse genes encoding a shank-interacting protein and its upregulation in gastric fundus of W/WV mouse. J Gastroenterol Hepatol. 2003;18:712–8. doi: 10.1046/j.1440-1746.2003.03046.x. [DOI] [PubMed] [Google Scholar]
- Dicato M, Boccadoro M, Cavenagh J, Harousseau JL, Ludwig H, San Miguel J, et al. Management of multiple myeloma with bortezomib: experts review the data and debate the issues. Oncology. 2006;70:474–82. doi: 10.1159/000099284. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Ding C, Liu Y, Wang Y, Park BK, Wang CY, Zheng P. Siglecg limits the size of B1a B cell lineage by down-regulating NFkappaB activation. PLoS One. 2007;2:e997. doi: 10.1371/journal.pone.0000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher S, Bain B. Diagnosis and treatment of hypereosinophilic syndromes. Curr Opin Hematol. 2007;14:37–42. doi: 10.1097/00062752-200701000-00008. [DOI] [PubMed] [Google Scholar]
- Gerondakis S, Grumont R, Gugasyan R, Wong L, Isomura I, Ho W, et al. Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene. 2006;25:6781–99. doi: 10.1038/sj.onc.1209944. [DOI] [PubMed] [Google Scholar]
- Gijbels MJ, Elliott GR, HogenEsch H, Zurcher C, van den Hoven A, Bruijnzeel PL. Therapeutic interventions in mice with chronic proliferative dermatitis (cpdm/cpdm) Exp Dermatol. 2000;9:351–8. doi: 10.1034/j.1600-0625.2000.009005351.x. [DOI] [PubMed] [Google Scholar]
- Gijbels MJ, Zurcher C, Kraal G, Elliott GR, HogenEsch H, Schijff G, Savelkoul HF, Bruijnzeel PL. Pathogenesis of skin lesions in mice with chronic proliferative dermatitis (cpdm/cpdm) Am J Pathol. 148:941–50. [PMC free article] [PubMed] [Google Scholar]
- Gijbels MJ, HogenEsch H, Blauw B, Roholl P, Zurcher C. Ultrastructure of epidermis of mice with chronic proliferative dermatitis. Ultrastruct Pathol. 1995;19:107–11. doi: 10.3109/01913129509014610. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–4. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Gotlib J. Chronic eosinophilic leukemia/hypereosinophilic syndrome. Cancer Treat Res. 2008;142:69–106. [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- HogenEsch H, Gijbels MJ, Offerman E, van Hooft J, van Bekkum DW, Zurcher C. A spontaneous mutation characterized by chronic proliferative dermatitis in C57BL mice. Am J Pathol. 1993;143:972–82. [PMC free article] [PubMed] [Google Scholar]
- HogenEsch H, Janke S, Boggess D, Sundberg JP. Absence of Peyer’s patches and abnormal lymphoid architecture in chronic proliferative dermatitis (cpdm/cpdm) mice. J Immunol. 1999;162:3890–6. [PubMed] [Google Scholar]
- HogenEsch H, Torregrosa SE, Boggess D, Sundberg BA, Carroll J, Sundberg JP. Increased expression of type 2 cytokines in chronic proliferative dermatitis (cpdm) mutant mice and resolution of inflammation following treatment with IL-12. Eur J Immunol. 2001;31:734–42. doi: 10.1002/1521-4141(200103)31:3<734::aid-immu734>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Kahn JE, Bletry O, Guillevin L. Hypereosinophilic syndromes. Best Pract Res Clin Rheumatol. 2008;22:863–82. doi: 10.1016/j.berh.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Kaufman CK, Fuchs E. It’s got you covered. NF-kappaB in the epidermis. J Cell Biol. 2000;149:999–1004. doi: 10.1083/jcb.149.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kolch W, Cho KH. Multiple roles of the NF-{kappa}B signaling pathway regulated by coupled negative feedback circuits. FASEB J. 2009a doi: 10.1096/fj.09-130369. [DOI] [PubMed] [Google Scholar]
- Kim D, Kolch W, Cho KH. Multiple roles of the NF-kappaB signaling pathway regulated by coupled negative feedback circuits. FASEB J. 2009b;23:2796–802. doi: 10.1096/fj.09-130369. [DOI] [PubMed] [Google Scholar]
- Leiferman KM. Hypereosinophilic syndrome. Semin Dermatol. 1995;14:122–8. doi: 10.1016/s1085-5629(05)80007-0. [DOI] [PubMed] [Google Scholar]
- Lim S, Sala C, Yoon J, Park S, Kuroda S, Sheng M, et al. Sharpin, a novel postsynaptic density protein that directly interacts with the shank family of proteins. Mol Cell Neurosci. 2001;17:385–97. doi: 10.1006/mcne.2000.0940. [DOI] [PubMed] [Google Scholar]
- Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–37. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Manning AM. Multiple signals converging on NF-kappaB. Curr Opin Cell Biol. 1999;11:226–32. doi: 10.1016/s0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- Mikaelian I, Nanney LB, Parman KS, Kusewitt DF, Ward JM, Naf D, et al. Antibodies that label paraffin-embedded mouse tissues: a collaborative endeavor. Toxicol Pathol. 2004;32:181–91. doi: 10.1080/01926230490274335. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Dorshkind K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006;27:428–33. doi: 10.1016/j.it.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nishikori M. Classical and alternative NF-kB activation pathways and their roles in lymphoid malignancies. J Clin Exp Hematopathol. 2005;45:15–24. [Google Scholar]
- Nogueira-Martins MF, Mariano M. B-1 cell participation in T-cell-mediated alloimmune response. Immunobiology. 2009 doi: 10.1016/j.imbio.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Luedde T, Schmidt-Supprian M. Dissection of the NF-kappaB signalling cascade in transgenic and knockout mice. Cell Death Differ. 2006;13:861–72. doi: 10.1038/sj.cdd.4401870. [DOI] [PubMed] [Google Scholar]
- Plotz SG, Simon HU, Darsow U, Simon D, Vassina E, Yousefi S, et al. Use of an anti-interleukin-5 antibody in the hypereosinophilic syndrome with eosinophilic dermatitis. N Engl J Med. 2003;349:2334–9. doi: 10.1056/NEJMoa031261. [DOI] [PubMed] [Google Scholar]
- Renninger ML, Seymour RE, Whiteley LO, Sundberg JP, Hogenesch H. Anti-IL5 decreases the number of eosinophils but not the severity of dermatitis in Sharpin-deficient mice. Exp Dermatol. 2009 doi: 10.1111/j.1600-0625.2009.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338:1592–600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- Roufosse F, Cogan E, Goldman M. Recent advances in pathogenesis and management of hypereosinophilic syndromes. Allergy. 2004;59:673–89. doi: 10.1111/j.1398-9995.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi: 10.1186/1750-1172-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon M, Behmenburg C, Denzer D, Schon MP. Pathogenic function of IL-1 beta in psoriasiform skin lesions of flaky skin (fsn/fsn) mice. Clin Exp Immunol. 2001;123:505–10. doi: 10.1046/j.1365-2249.2001.01421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour R, Ichiki T, Mikaelian I, Boggess D, Silva KA, Sundberg JP. Necropsy methods. In: Hedrich H, editor. Laboratory mouse. Elsevier Science; London: 2004. pp. 495–516. [Google Scholar]
- Seymour R, Sundberg JP, Hogenesch H. Abnormal lymphoid organ development in immunodeficient mutant mice. Vet Pathol. 2006;43:401–23. doi: 10.1354/vp.43-4-401. [DOI] [PubMed] [Google Scholar]
- Seymour RE, Hasham MG, Cox GA, Shultz LD, Hogenesch H, Roopenian DC, et al. Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes Immun. 2007;8:416–21. doi: 10.1038/sj.gene.6364403. [DOI] [PubMed] [Google Scholar]
- Sheikh J, Weller PF. Advances in diagnosis and treatment of eosinophilia. Curr Opin Hematol. 2009;16:3–8. doi: 10.1097/MOH.0b013e32831c841f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D, Braathen LR, Simon HU. [Anti-interleukin-5 therapy for eosinophilic diseases] Hautarzt. 2007;58(122):4–7. doi: 10.1007/s00105-006-1273-x. [DOI] [PubMed] [Google Scholar]
- Simon D, Simon HU. Eosinophilic disorders. J Allergy Clin Immunol. 2007;119:1291–300. doi: 10.1016/j.jaci.2007.02.010. quiz 301–2. [DOI] [PubMed] [Google Scholar]
- Simon HU, Plotz SG, Dummer R, Blaser K. Abnormal clones of T cells producing interleukin-5 in idiopathic eosinophilia. N Engl J Med. 1999;341:1112–20. doi: 10.1056/NEJM199910073411503. [DOI] [PubMed] [Google Scholar]
- Sundberg JP, King LE., Jr Mouse mutations as animal models and biomedical tools for dermatological research. J Inves Dermatol. 1996;106:368–76. doi: 10.1111/1523-1747.ep12343152. [DOI] [PubMed] [Google Scholar]
- Towne JE, Garka KE, Renshaw BR, Virca GD, Sims JE. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J Biol Chem. 2004;279:13677–88. doi: 10.1074/jbc.M400117200. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Sanchez-Aguilera A, Brandt EB, McBride M, Al-Moamen NJ, Finkelman FD, et al. FIP1L1/PDGFRalpha synergizes with SCF to induce systemic mastocytosis in a murine model of chronic eosinophilic leukemia/hypereosinophilic syndrome. Blood. 2008;112:2500–7. doi: 10.1182/blood-2007-11-126268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.