Abstract
Objective
The World Health Organization (WHO) recently changed its first-line antiretroviral treatment guidelines in resource-limited settings. The cost-effectiveness of the new guidelines are unknown.
Design
Comparative effectiveness and cost-effectiveness analysis using a model of HIV disease progression and treatment.
Methods
Using a simulation of HIV disease and treatment in South Africa, we compared the life expectancy, quality-adjusted life expectancy, lifetime costs, and cost-effectiveness of five initial regimens. Four are currently recommended by the WHO: tenofovir/lamivudine/efavirenz; tenofovir/lamivudine/nevirapine; zidovudine/lamivudine/efavirenz; and zidovudine/lamivudine/nevirapine. The fifth is the most common regimen in current use: stavudine/lamivudine/nevirapine. Virologic suppression and toxicities determine regimen effectiveness and cost-effectiveness.
Results
Choice of first-line regimen is associated with a difference of nearly 12 months of quality-adjusted life expectancy, from 135.2 months (tenofovir/lamivudine/efavirenz) to 123.7 months (stavudine/lamivudine/nevirapine). Stavudine/lamivudine/nevirapine is more costly and less effective than zidovudine/lamivudine/nevirapine. Initiating treatment with a regimen containing tenofovir/lamivudine/nevirapine is associated with an incremental cost-effectiveness ratio of $1,045 per quality-adjusted life year compared with zidovudine/lamivudine/nevirapine. Using tenofovir/lamivudine/efavirenz was associated with the highest survival, fewest opportunistic diseases, lowest rate of regimen substitution, and an incremental cost-effectiveness ratio of $5,949 per quality-adjusted life year gained compared with tenofovir/lamivudine/nevirapine. Zidovudine/lamivudine/efavirenz was more costly and less effective than tenofovir/lamivudine/nevirapine. Results were sensitive to the rates of toxicities and the disutility associated with each toxicity.
Conclusion
Among the options recommended by WHO, we estimate only three should be considered under normal circumstances. Choice among those depends on available resources and willingness to pay. Stavudine/lamivudine/nevirapine is associated with the poorest quality-adjusted survival and higher costs than zidovudine/lamivudine/nevirapine.
Keywords: Cost-effectiveness analysis, comparative effectiveness, antiretroviral therapy, treatment guidelines, World Health Organization, South Africa
Introduction
Increasing the quality of and access to antiretroviral therapy (ART) in Africa are important public health priorities. The World Health Organization's (WHO) guidelines for treatment of HIV infection in adults and adolescents were changed at the end of 2009.[1] The new guidelines aim to improve treatment effectiveness and reduce risk of toxicities for millions of infected individuals residing in resource-limited and highly affected regions.
For treatment-naïve individuals, the new guidelines include the following four regimens:
Tenofovir + lamivudine + efavirenz;
Tenofovir + lamivudine + nevirapine;
Zidovudine + lamivudine + efavirenz; and
Zidovudine + lamivudine + nevirapine.
Compared with the previous WHO guidelines from 2006, the new guidelines recommend against the use of stavudine due to its toxicity profile and suggest that “in settings where stavudine regimens are used as the principal option for starting ART, countries should develop a plan to move towards zidovudine- or tenofovir-based first-line regimens.”[1] Reasons for the changes include emerging data reflecting the safety and efficacy of tenofovir in resource-limited settings, long-term metabolic toxicities associated with stavudine, and the availability of generic tenofovir. However, stavudine in combination with lamivudine and nevirapine is the cheapest and most common regimen in current use throughout Africa, and phasing it out will be a challenge.
Regimens containing tenofovir and efavirenz are similar in many respects to those recommended in the US and other developed countries.[2-4] However, the new guidelines are also tailored for a public health approach in regions with limited resources. Because tenofovir and efavirenz are among the most expensive antiretroviral drugs, other regimens that include zidovudine and nevirapine are also recommended. The guidelines attempt to reconcile considerations of effectiveness and budget constraints, but they do not include a cost-effectiveness analysis. Recent literature provides partial insights into the cost-effectiveness of the WHO strategies, but leaves out comparisons of regimens containing efavirenz and nevirapine, and does not examine the implications for the most highly affected country in sub-Saharan Africa.[5] We provide a comparative analysis of the four recommended first-line regimens as well as stavudine/lamivudine/nevirapine, and suggest implications for future guidelines and drug development.
Methods
Overview
We adopt a mathematical simulation model of the clinical course of HIV infected individuals based on demographic and clinical data from South Africa. Details of the model and assumptions are presented in the Methods Appendix. We compare the effectiveness and costs of five alternative ART first-line regimens (four recommended by the WHO and the ART combination in most common use in sub-Saharan Africa):
Tenofovir + lamivudine + efavirenz
Tenofovir + lamivudine + nevirapine
Zidovudine + lamivudine + efavirenz
Zidovudine + lamivudine + nevirapine
Stavudine + lamivudine + nevirapine
We follow each person's health in one-month increments and compare the effectiveness and cost-effectiveness of the regimens listed above.
Treatment Choice
Our population has similar demographic and disease characteristics as that of a published South Africa cohort.[6-8] We assume individuals are followed in a treatment facility with CD4 counts, and initiated treatment once their CD4 counts drop below 350 cells/μl, as suggested in the recent WHO guidelines. Because most individuals in southern Africa present to care late in the course of their illness, the majority of individuals in our model are placed on treatment soon after presentation to care.[9] We follow cohorts of 100,000 patients in each strategy. Effectiveness is most strongly determined by the regimen's ability to suppress viral load. Suppressed viral load is associated with a rise in CD4 counts, which in turn is associated with lower mortality. All the model parameters, including rates of viral suppression for each regimen, rates of opportunistic diseases, and mortality are shown in Table 1.
Table 1.
Model Parameters and Assumptions
| Variable | Base Case | Range | Source | ||
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age structure of infected population presenting to care | Badri[8], Dorrington[28], IDB[29] | ||||
| 15-25 years old | 34% | ||||
| 25-35 years old | 39% | ||||
| 35-50 years old | 27% | ||||
| CD4 count at presentation, cells/μl (mean ± SD) | 144 ± 121 | ART-LINC[9] | |||
| Viral load at presentation, log copies/ml (mean ± SD) | 5.0 ± 0.8 | Badri[6] | |||
| Disease Progression Variables | |||||
| Decline in CD4 (cells/μl/mo), viral load 103-106 | Holmes[21], Mellors[30], Rodriguez[31], PLATO[32] | ||||
| Baseline CD4 >500 cells/μl | 3.9-5.9 | ± 50% | |||
| Baseline CD4 351-500 cells/μl | 2.6-3.8 | ± 50% | |||
| Baseline CD4 201-350 cells/μl | 1.7-2.6 | ± 50% | |||
| Baseline CD4 <200 cells/μl | 1.3-2.0 | ± 50% | |||
| Monthly probability of developing severe opportunistic diseases (%), by CD4 | <50 cells/μl | 51-200 cells/μl | 201-350 cells/μl | >350 cells/μl | Holmes[21] |
| Oral candidiasis | 3.50% | 2.04% | 1.26% | 0.57% | |
| Chronic diarrhea | 2.00% | 0.49% | 0.18% | 0.00% | |
| Esophageal candidiasis | 1.46% | 0.34% | 0.09% | 0.06% | |
| Wasting syndrome | 1.29% | 0.23% | 0.02% | 0.00% | |
| Severe bacterial | 1.15% | 0.04% | 0.03% | 0.00% | |
| Pulmonary TB | 1.15% | 0.71% | 0.47% | 0.11% | |
| Extrapulmonary TB | 0.98% | 0.47% | 0.18% | 0.05% | |
| PCP | 0.67% | 0.05% | 0.02% | 0.00% | |
| CMV | 0.52% | 0.07% | 0.02% | 0.00% | |
| Cryptococcal meningitis | 0.52% | 0.05% | 0.00% | 0.00% | |
| Risk of HIV death by CD4 cell count | Badri[7], ART-LINC[9] | ||||
| CD4 <50 cells/μl | 2.1%/mo | ± 10% | |||
| CD4 51-200 cells/μl | 1.7%/mo | ± 10% | |||
| CD4 201-350 cells/μl | 1.1%/mo | ± 10% | |||
| CD4 >350 cells/μl | 0.8%/mo | ± 10% | |||
| Cumulative risks of toxicity and failure | Year 1 | Year 3 | Range | Source | |
| tenofovir + lamivudine + efavirenz | |||||
| Virologic failure | 12% | 24% | ± 4% | Gallant, Arribas[10, 33] | |
| Lipoatrophy | 6% | 17% | ± 3% | Haubrich[11] | |
| Renal failure | 1% | 1% | ± 1% | Gallant[11] | |
| Myocardial infarction | 0.1% | 0.1% | ± 0.1% | Gallant[12, 33, 34] | |
| tenofovir + lamivudine + nevirapine | Year 1 | Year 3 | Range | Source | |
| Virologic failure | 18% | 31% | ± 6% | Gallant, Nachega, Smith[15, 33, 35] | |
| Lipoatrophy | 6% | 17% | ± 3% | Haubrich[11] | |
| Renal failure | 1% | 1% | ± 1% | Gallant[11] | |
| Hepatotoxicity | 6.3% | 6.3% | ± 5% | Amoroso[14] | |
| zidovudine + lamivudine + efavirenz | Year 1 | Year 3 | Range | Source | |
| Virologic failure | 17% | 31% | ± 7% | Gallant[33], Arribas[10] | |
| Lipoatrophy | 23% | 54% | ± 15% | Arribas, Haubrich[10, 11] | |
| Anemia | 6% | 6% | ± 3% | Gallant[33] | |
| Myocardial infarction | 0.2% | 0.2% | ± 0.2% | Gallant[12, 33, 34] | |
| zidovudine + lamivudine + nevirapine | Year 1 | Year 3 | Range | Source | |
| Virologic failure | 25% | 46% | ± 12% | Gallant, Arriba, Nachega, Smith [10, 15, 33, 35] | |
| Lipoatrophy | 23% | 54% | ± 15% | Arribas, Haubrich[10, 11] | |
| Anemia | 6% | 6% | ± 3% | Gallant[33] | |
| Myocardial infarction | 0.1% | 0.1% | ± 0.1% | Gallant[12, 33, 34] | |
| Hepatotoxicity | 6.3% | 6.3% | ± 5% | Amoroso[14] | |
| stavudine + lamivudine + nevirapine | Year 1 | Year 3 | Range | Source | |
| Virologic failure | 18% | 31% | ± 6% | Gallant, Nachega, Smith[15, 33, 35] | |
| Lipoatrophy | 30% | 66% | ± 20% | Haubrich[11] | |
| Neuropathy | 25% | 25% | ± 10% | Gallant[11] | |
| Myocardial infarction | 0.3% | 0.3% | ± 0.3% | Gallant[12, 33, 34] | |
| Hepatotoxicity | 6.3% | 6.3% | ± 5% | Amoroso[14] | |
| Lactic acidosis | 0.5% | 0.5% | ± 0.2% | John, Boubaker [36, 37] | |
| Quality of life weights and duration of toxicities | |||||
| HIV without HAART | 0.84 | 0.84-1 | Cleary[38] | ||
| HIV with HAART | 0.91 | 0.91-1 | Cleary[38] | ||
| Lipoatrophy | 0.87 | Irreversible | 0.8-0.95 | Rosen[23, 39] | |
| Anemia | 0.58 | 4 mo | 0.5-0.66 | Kimel[40] | |
| Renal failure | 0.7 | 4 mo | 0.6-0.8 | Rosen[23, 39] | |
| Neuropathy | 0.87 | Irreversible after 1 year | 0.8-0.95 | Gallant[12] | |
| Lactic acidosis | 0.5 | 3 mo | 0.4-0.6 | Rosen[23, 39] | |
| MI | 0.6 | 2 mo | 0.4-0.8 | Assumption | |
| Hepatotoxicity | 0.7 | 2 mo | 0.5-0.9 | Assumption | |
| Utilization and cost variables | |||||
| Annual number of inpatient days without a severe opportunistic disease | Badri et al[6, 41] | ||||
| CD4>350 (on/off HAART) | 0.14 / 1.9 | ± 50% | |||
| CD4 201-350 (on/off HAART) | 0.39 / 3 | ± 50% | |||
| CD4<=200 (on/off HAART) | 0.26 / 7.7 | ± 50% | |||
| Annual number of inpatient days with a severe opportunistic disease | Badri et al[6, 41] | ||||
| CD4>350 (on/off HAART) | 0.37 / 5.7 | ± 50% | |||
| CD4 201-350 (on/off HAART) | 0.52 / 10.8 | ± 50% | |||
| CD4<=200 (on/off HAART) | 1.8 / 17.7 | ± 50% | |||
| Annual number of outpatient visits | Badri et al[6, 41] | ||||
| CD4>350 (on/off HAART) | 4.3 / 4.1 | ± 50% | |||
| CD4 201-350 (on/off HAART) | 3.9 / 5.0 | ± 50% | |||
| CD4<=200 (on/off HAART) | 4.7 / 6.6 | ± 50% | |||
| Monitoring Costs (2009 USD) | |||||
| Inpatient Day | $182 | ± 50% | Badri; Cleary[6, 38] | ||
| Outpatient visit | $26 | ± 50% | Badri; Cleary[6, 38] | ||
| Cost per CD4 test | $15 | ± 50% | Badri, Zijeneh[6, 42] | ||
| HAART Costs (2009 USD) | WHO[43] | ||||
| tenofovir + lamivudine + efavirenz | $675 | $100-675 | |||
| tenofovir + lamivudine + nevirapine | $538 | $100-538 | |||
| zidovudine + lamivudine + efavirenz | $384 | $100-384 | |||
| zidovudine + lamivudine + nevirapine | $247 | $100-247 | |||
| stavudine + lamivudine + nevirapine | $121 | $100-121 | |||
| Second line HAART | $769 | $100-769 | |||
| Toxicity costs | |||||
| Lipoatrophy | $121 | $60-242 | Rosen[23] | ||
| Anemia | $136 | $68-272 | Cantor[44] | ||
| Renal failure | $213 | $106-8,477 | Rosen[23] | ||
| Neuropathy | $130 | $65-260 | Rosen[23] | ||
| Lactic acidosis | $192 | $96-7,999 | Rosen[23] | ||
| MI | $300 | $150-600 | Assumption | ||
| Hepatotoxicity | $300 | $150-600 | Assumption | ||
Effectiveness is also affected by regimen toxicities. We considered 7 toxicities: lipoatrophy, severe anemia, renal failure, peripheral neuropathy, lactic acidosis, hepatotoxicity, and myocardial infarctions related to changes in cholesterol. The toxicities we consider affected quality of life and commonly lead to a regimen substitution. Where possible, we estimate the types of toxicities and incidence rate for each regimen from long-term follow-up studies of clinical trials.[10-12] We use that source because of the strict case definitions and careful monitoring. Where clinical trial data was not available, we use African observational data.[13-17] Quality of life weights for each toxicity were multiplied with the baseline HIV weight to estimate the quality of life of having both HIV and the toxicity. Our assumptions about rates of toxicities, the changes in quality of life, and the substitution algorithm are described in greater detail in the Methods Appendix and shown in the Methods Appendix Table 1.
Table 1.
Study strategies and associated virologic efficacy and toxicity profile
| |
Initial regimen |
Virologic failure at 1, 2, and 3 years |
One-year risk of toxicities |
Management following toxicity |
|---|---|---|---|---|
| 1 | TDF + 3TC + EFV | 1 year – 12%[25] | a. Lipoatrophy – 6% (3-9%)[33] | a. Lipoatrophy – no change |
| 2 year – 20%[45] | b. Renal failure – 1% (0-2%)[26] | b. Renal failure – switch to AZT+3TC+EFV | ||
| 3 year – 24%[32] | c. Myocardial infarction (MI) – 0.1% (0%-0.2%)[25, 26, 46] | c. Non-fatal MI – no change | ||
| 2 | TDF + 3TC + NVP | 1 year – 18%[25, 29, 30] | a. Lipoatrophy – 6% (3-9%)[33] | a. Lipoatrophy – no change |
| 2 year – 36%[29, 30, 45] | b. Renal failure – 1% (0-2%)[26] | b. Renal failure – switch to AZT+3TC+EFV | ||
| 3 year – 31%[29, 30, 32] | c. MI – 0% (0%-0.1%)[25, 26, 46, 47] | c. Non-fatal MI – no change | ||
| d. Hepatotoxicity – 6.3% (4-8%)[35] | d. Hepatotoxicity – switch to TDF+3TC+EFV | |||
| 3 | AZT + 3TC + EFV | 1 year – 17%[25] | a. Lipoatrophy – 23% (15-30%)[32, 33] | a. Lipoatrophy - switch to TDF+3TC+EFV |
| 2 year – 26%[45] | b. Anemia – 6% (4-8%)[25] | b. Anemia - switch to TDF+3TC+EFV | ||
| 3 year – 31%[32] | c. MI – 0.2% (0.1%-0.3%)[25, 46] | c. Non-fatal MI - switch to TDF+3TC+EFV | ||
| 4 | AZT + 3TC + NVP | 1 year – 25%[25, 29, 30] | a. Lipoatrophy – 23% (15-30%)[32, 33] | a. Lipoatrophy - switch to TDF+3TC+NVP |
| 2 year – 39%[29, 30, 45] | b. Anemia – 6% (4-8%)[25] | b. Anemia - switch to TDF+3TC+NVP | ||
| 3 year – 46%[29, 30, 32] | c. MI – 0.1% (0%-0.2%)[25, 46, 47] | c. Non-fatal MI - switch to TDF+3TC+NVP | ||
| d.Hepatotoxicity – 6.3% (4-8%)[35] | d. Hepatotoxocity – switch to AZT+3TC+EFV | |||
| 5 | d4T + 3TC + NVP | 1 year – 18%[26, 29, 30] | a. Lipoatrophy - 30% (20-40%)[26, 33, 36] | a. Lipoatrophy - switch to TDF+3TC+NVP |
| 2 year – 36%[26, 29, 30] | b. Non-fatal MI - switch to TDF+3TC+NVP | |||
| 3 year – 31%[26, 29, 30] | b. MI - 0.3% (0.1%-0.5%)[26, 46, 47] | c. Peripheral neuropathy - switch to TDF+3TC+NVP | ||
| c. Peripheral neuropathy - 25% (15-35%)[34, 37] | ||||
| d. Lactic acidosis - switch to TDF+3TC+NVP | ||||
| d. Lactic acidosis - 0.5% (0.1-1.5%)[38, 39] | e. Hepatotoxicity – switch to AZT+3TC+EFV | |||
| e. Hepatotoxicity – 6.3% (4-8%)[35] |
All patients have access to CD4 monitoring for treatment initiation and routine monitoring. We use immunologic criteria outlined in the WHO guidelines and recent clinical trials – a drop to a CD4 count of less than 100 cells/μl – to estimate timing of virologic failure and the need to switch to second-line therapy consisting of ritonavir-boosted lopinavir.[1, 18]
Disease Progression
The disease progression model is a state transition simulation of the course of HIV that tracks essential patient information and estimates mortality and quality of life in monthly increments. The foundations of our disease progression model are described elsewhere and in the Methods Appendix.[19, 20] HIV disease progression is determined by CD4 counts, viral load, and opportunistic diseases. Viral load determines the rate of decline of CD4 counts in the absence of effective ART. Increases in CD4 counts after onset of effective ART are determined by the CD4 at the time of treatment initiation and the duration of therapy. We estimate risk of death based on age- and gender-specific mortality rates, HIV-specific mortality rates, and the presence or absence of opportunistic diseases. We use information on rates of development of ten AIDS-defining opportunistic diseases from the Cape Town area.[21]
Outcomes and Sensitivity Analyses
We collect outcome information on life expectancy, quality-adjusted life expectancy, and costs. For the primary analysis, we adopt a societal perspective, discounted all costs and benefits at 3% annually, and adhered to the recommendations of the Panel on Cost-Effectiveness in Health and Medicine.[22] We report cost-effectiveness as the incremental cost divided by the incremental effectiveness between pairs of strategies. Strategies which were more costly and less effective than another strategy (or combination of strategies) were determined to be “dominated” and not compared in the primary cost-effectiveness analysis.
We conducted sensitivity analyses on the rates of failure, toxicity rates, quality of life weights, and cost of the antiretroviral agents. The bounds of our assumptions are shown in Table 1. In addition, we performed a probabilistic sensitivity analysis where we varied all the variables simultaneously and repeated the analysis 1,000 times. This allowed us to estimate the confidence in our results if the true value of each variable is anywhere within the uncertainty bounds shown. For example, we estimate the likelihood that a strategy which appears dominant in the base case – one which is more effective and less costly than another strategy – may not be dominant.
Results
Choice of antiretroviral agents
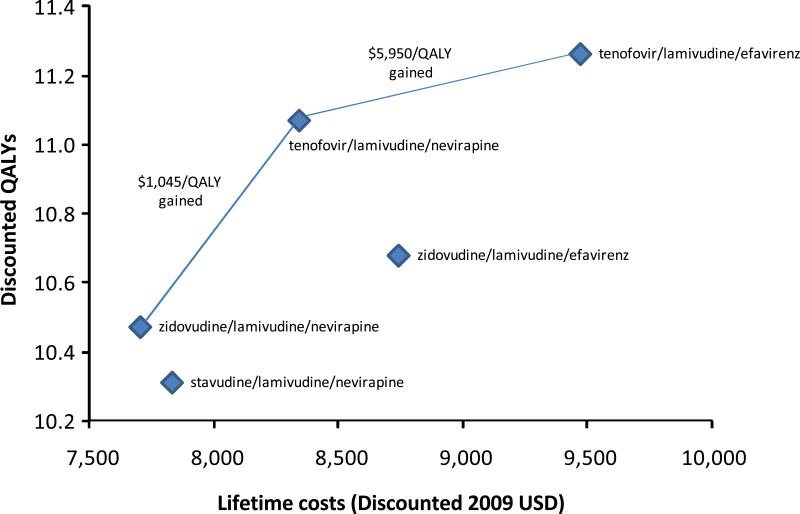
In a South African setting, quality-adjusted life expectancy based on the choice of agents for first-line ART differed by nearly 12 months between the most effective and least effective regimens (Table 2 and Figure 2). The most effective regimen, tenofovir/lamivudine/efavirenz, was associated with a projected quality-adjusted life expectancy from the time of presentation to care of 11.3 years. The least effective regimen stavudine/lamivudine/nevirapine was estimated to yield a discounted quality-adjusted life expectancy of 10.3 years. The effectiveness of the regimens is also reflected in the frequency of opportunistic diseases: individuals who started ART with tenofovir/lamivudine/efavirenz had, on average, 2.0 opportunistic diseases over their lifetime, while those who started with stavudine/lamivudine/nevirapine had 2.2. We estimate overall life expectancy – undiscounted and without quality of life adjustments – for the most effective regimen at over 18.4 years. Estimates of quality-adjusted and unadjusted life expectancy for each of the five regimens are shown in Table 2.
Table 2.
Life expectancy, costs, and cost-effectiveness of strategies in primary analysis
| Initial regimen | Quality-adjusted LE (discounted years) | Life expectancy (discounted years) | Average number of ODs per person | Total lifetime costs (discounted 2009USD) | Incremental cost-effectiveness ratio ($ per QALY) |
|---|---|---|---|---|---|
| zidovudine + lamivudine + nevirapine | 10.47 | 12.39 | 2.22 | 7,711 | Base |
| stavudine + lamivudine + nevirapine | 10.31 | 12.51 | 2.17 | 7,824 | Dominated |
| tenofovir + lamivudine + nevirapine | 11.08 | 12.54 | 2.18 | 8,348 | 1,045 |
| zidovudine + lamivudine + efavirenz | 10.69 | 12.72 | 2.07 | 8,748 | Dominated |
| | |||||
| tenofovir + lamivudine + efavirenz | 11.27 | 12.82 | 2.03 | 9,479 | 5,950 |
Figure 2. Health and Cost Outcomes for First-line Antiretroviral Strategies.
Lifetime discounted costs in 2009 US dollars and quality-adjusted life expectancy for the five first-line antiretroviral regimens. Strategies that could be considered cost-effective are connected with a line that indicates the incremental cost-effectiveness ration of moving from one strategy to the next. Two strategies – stavudine/lamivudine/nevirapine and zidovudine/lamivudine/efavirenz – are unlikely to be cost effective. The results support the decision to exclude stavudine/lamivudine/nevirapine from the list of recommended regimens, and suggest an important role for tenofovir-based regimens.
Costs and Cost-Effectiveness
Lifetime costs for all five strategies varied between 7,711 and 9,478 discounted 2009 USD (Table 2 and Figure 2). Using undiscounted values of costs and life expectancy, this amounts to between $713 and $810 in total direct annual costs for people living with HIV and remaining in care in South Africa. The initial regimen zidovudine/lamivudine/nevirapine was the least expensive, while tenofovir/lamivudine/efavirenz was the most expensive. Figure 1 shows the lifetime costs and quality-adjusted life expectancy of the primary analysis in discounted 2009 USD and QALYs.
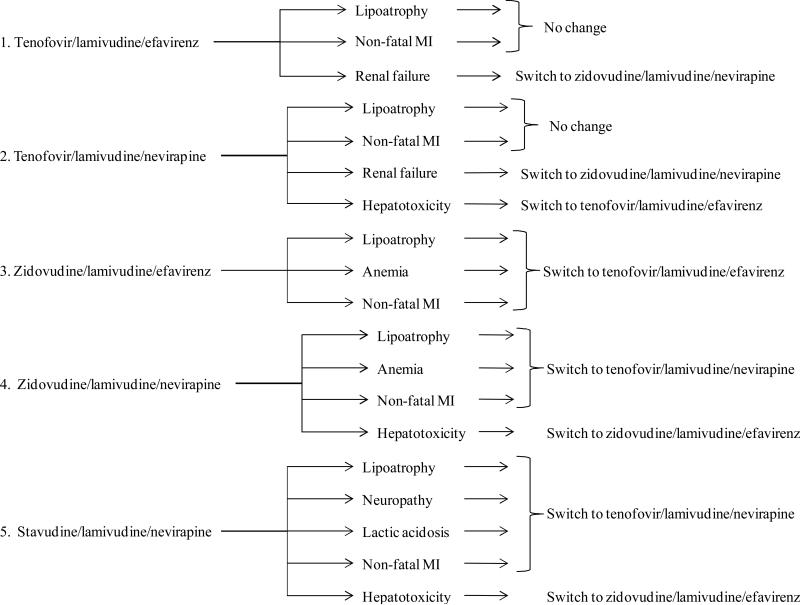
Figure 1. Study regimens with associated toxicities and regimen substitutions.
The five first-line strategies evaluated in this study are shown with each of the associated toxicities we evaluated. Toxicities resulted in a substitution to another 1st line regimen, if another regimen is thought to have a superior toxicity profile. The rates of toxicities and virologic failure of each regimen are shown in Table 1.
We find that only three first-line ART strategies could be considered cost-effective: zidovudine/lamivudine/nevirapine, tenofovir/lamivudine/nevirapine, and tenofovir/lamivudine/efavirenz. At least one of these strategies was more effective and less costly than the other two strategies (stavudine/lamivudine/nevirapine and zidovudine/lamivudine/efavirenz). When adjusting for quality of life, the strategy containing stavudine/lamivudine/nevirapine in initial regimen was more expensive and less effective that the strategy containing zidovudine/lamivudine/nevirapine, the increased expense primarily due to the costs of managing stavudine-associated toxicities. Compared with zidovudine/lamivudine/nevirapine, the strategy containing tenofovir/lamivudine/nevirapine in first-line provided 7.3 additional quality-adjusted months of life at an additional cost of $636, an incremental cost-effectiveness ratio of $1,045 per QALY. The most effective strategy, tenofovir/lamivudine/efavirenz, was associated with additional 2.3 months of quality-adjusted months of life and an incremental cost-effectiveness ratio of $5,949 per QALY compared with tenofovir/lamivudine/nevirapine. We estimate that one of the strategies recommended by the WHO – zidovudine/lamivudine/efavirenz – is more costly and less effective than a strategy with tenofovir/lamivudine/nevirapine as initial therapy. In cases where nevirapine is not appropriate (such as simultaneous treatment of HIV and tuberculosis), the strategy with zidovudine/lamivudine/efavirenz is the least costly strategy, and the strategy with tenofovir/lamivudine/efavirenz has an incremental cost-effectiveness ratio of $1,251 per QALY in comparison.
Sensitivity analyses
We performed sensitivity analyses to answer two important questions: do our estimates of each regimen's virologic failure change the results? And under what conditions do our estimates of the quality of life associated with toxicities matter for the outcomes? We considered these the most uncertain estimates and the ones most likely to affect our comparisons.
We first focused on our estimates of virologic failure. We varied the failure rate associated with zidovudine/lamivudine/nevirapine. This had important implications because higher failure rates associated with zidovudine/lamivudine/nevirapine could make the most common regimen in current use (stavudine/lamivudine/nevirapine) cost-effective. We found that a first-line regimen with stavudine/lamivudine/nevirapine remained more costly and less effective than a first-line regimen containing zidovudine/lamivudine/nevirapine even if the rates of virologic failure of zidovudine/lamivudine/nevirapine were twice as high (Table 1). These findings are driven by the toxicities associated with stavudine and the costs of managing these toxicities that more than offset the decreased benefits of zidovudine/lamivudine/nevirapine.
Rates of virologic failure were also important for determining the incremental cost-effectiveness of tenofovir/lamivudine/efavirenz compared with tenofovir/lamivudine/nevirapine. When we varied the rates of failure of tenofovir/lamivudine/nevirapine over a broad range (from identical to tenofovir/lamivudine/efavirenz to twice the rate), the incremental cost-effectiveness ratio ranged from $2,927 per QALY when the rates of failure with tenofovir/lamivudine/nevirapine were twice that of tenofovir/lamivudine/efavirenz to $27,900 per QALY when the rates of failure were identical. At acceptable cost-effectiveness thresholds for South Africa (less than gross domestic product per capita, $5,800), tenofovir/lamivudine/efavirenz remained cost-effective compared with tenofovir/lamivudine/nevirapine as long as virologic failure (at any point) was at least 1.5 times more likely with tenofovir/lamivudine/nevirapine.
We then examined when quality-of-life adjustments changed the results. We estimate that an initial regimen of stavudine/lamivudine/nevirapine could be a first-line consideration if the relative quality of life with lipoatrophy were 0.95 compared to life without lipoatrophy (up from 0.87 in the primary analysis). However, the incremental cost-effectiveness ratio of stavudine/lamivudine/nevirapine compared with zidovudine/lamivudine/nevirapine was $19,967 per QALY at that quality of life, well above established thresholds for cost-effectiveness in less developed countries. No other toxicity, when varied over a broad range of assumptions, changed the relative rank order of strategies in terms of effectiveness, suggesting the results are robust to changes in any single estimate of toxicity.
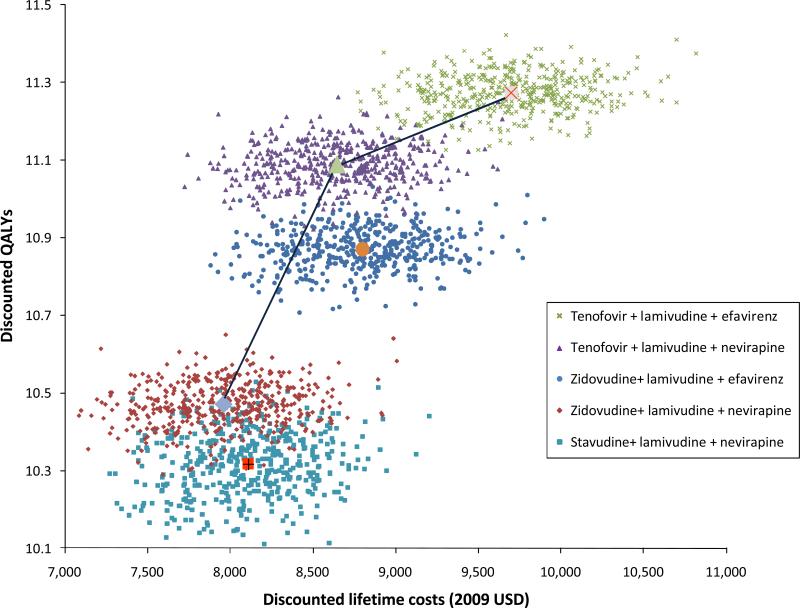
In probabilistic sensitivity analysis, we varied all parameters simultaneously to estimate the overall uncertainty in the results. During 96% of the simulations, a first-line regimen containing zidovudine/lamivudine/nevirapine provided greater benefits and cost less than stavudine/lamivudine/nevirapine. Under those assumptions when stavudine/lamivudine/nevirapine was less costly than zidovudine/lamivudine/nevirapine, the mean incremental cost-effectiveness ratio of starting with zidovudine/lamivudine/nevirapine was $200 per QALY gained (Figure 3). We are also able to calculate an uncertainty range for the incremental cost-effectiveness ratios. In our estimates, 95% of the incremental cost-effectiveness ratios comparing a first-line regimen with tenofovir/lamivudine/nevirapine to zidovudine/lamivudine/nevirapine fell in the range of $36 per QALY gained to $2926 per QALY gained. Similarly, 95% of the time tenofovir/lamivudine/efavirenz cost between $1787 and $9449 per QALY gained compared with tenofovir/lamivudine/nevirapine. The incremental cost-effectiveness ratios for tenofovir/lamivudine/efavirenz compared with tenofovir/lamivudine/nevirapine was less than the South African gross domestic product per person in 52% of our simulations.
Figure 3. Results of a Probabilistic Sensitivity Analysis.
This presents the results of repeated simulations allowing for simultaneous uncertainty in all model parameters. The small markers represent the results of individual simulations, while the large central markers represent the results from the primary analysis. The figure shows the dominance of zidovudine/lamivudine/nevirapine over stavudine/lamivudine/nevirapine is less certain than the other comparative results.
Discussion
We present a comparison of the effectiveness and cost-effectiveness of strategies recommended by the World Health Organization for initial ART regimens in resource-constrained settings. Our analysis has three main conclusions: (1) it supports the decision by the WHO to eliminate stavudine/lamivudine/nevirapine from the guidelines for first-line regimens; (2) it calls into question the recommendation to have a first-line regimen that includes zidovudine/lamivudine/efavirenz when a first-line regimen that includes tenofovir/lamivudine/nevirapine is available for widespread use; and (3) it suggests that a first-line regimen with tenofovir/lamivudine/nevirapine would be cost-effective for South Africa, while the cost-effectiveness of a first-line regimen with tenofovir/lamivudine/efavirenz is less favorable at current drug prices. Below we discuss these conclusions in detail and highlight the implications for treatment campaigns and drug development.
The finding that a first-line regimen containing stavudine/lamivudine/nevirapine is more costly and less effective than a first-line regimen containing zidovudine/lamivudine/nevirapine supports the WHO's recommendations to eliminate stavudine/lamivudine/nevirapine from the recommended first-line regimens. The removal of stavudine from all recommended first-line regimens was a primary motive for the WHO's revised guidelines. The WHO cites concerns over toxicities, and our analysis estimates the decrease in quality-adjusted life-years associated with that regimen. This was not a foregone conclusion for two reasons. First, we used efficacy data that suggests stavudine is similar to tenofovir while zidovudine is inferior to tenofovir. Indeed, we find that without accounting for quality of life, stavudine/lamivudine/nevirapine is more effective than zidovudine/lamivudine/nevirapine. However, this advantage is reversed after quality of life adjustments for stavudine-associated toxicities. Second, we find that stavudine/lamivudine/nevirapine in initial regimen is also more expensive than zidovudine because of the higher costs associated with managing toxicities and because of the earlier switch to more expensive regimens observed after the initiation of stavudine-related toxicities.
We also find that a first-line regimen with zidovudine/lamivudine/efavirenz is not cost-effective: it is more costly and less effective than a regimen containing tenofovir/lamivudine/nevirapine. We estimated these regimens have relatively similar rates virologic failure, but the effect of toxicities associated with zidovudine/lamivudine/efavirenz (primarily lipoatrophy and anemia) render that combination less effective. Reducing the annual cost of efavirenz can make a first-line regimen with zidovudine/lamivudine/efavirenz less expensive than tenofovir/lamivudine/nevirapine, but even if efavirenz cost as much nevirapine, it is likely that zidovudine/lamivudine/efavirenz would remain cost-ineffective.
Our finding that a first-line regimen with tenofovir/lamivudine/nevirapine is cost-effective by WHO criteria for at least some developing countries is congruent with previous analyses, although our ability to extrapolate beyond South Africa is limited.[5, 23] In developed countries, the most common formulation of tenofovir is in combination with emtricitabine and efavirenz in a fixed-dose combination. The combination of tenofovir, lamivudine, and nevirapine, by comparison, is relatively unfamiliar and understudied, but increasing recent data supports its efficacy compared with established regimens.[24, 25] Previous studies suggest that nevirapine can be used in once-daily drug combinations.[26] A fixed dose pill with that combination could be an important contribution to the current options for developing countries, especially if a head-to-head trial comparing those regimens confirms our estimates of improved effectiveness with tenofovir/lamivudine/nevirapine.
In extensive sensitivity analysis we show that one of the regimens recommended by the WHO – first line with zidovudine/lamivudine/efavirenz – remains cost-ineffective over a broad range of assumptions. A combination of zidovudine, lamivudine, and efavirenz was very popular in developed countries for several years (as Combivir and Sustiva), and the familiarity and historical reputation of this regimen may make it attractive for many settings in developing countries. However, our analysis shows that it is unlikely to be cost-effective when tenofovir is also available.
In preparing this analysis, data on virologic efficacy and toxicity rates was occasionally limited to clinical trials or experience in developed settings. Cohort and observational evidence suggests that virologic efficacy of all the drugs evaluated in this study may be similar between developed countries and sub-Saharan Africa.[27] However, toxicity rates are less reliable: a recent study suggested that zidovudine-related anemia in African settings was more frequent than in developed countries.[18] In addition, while rates of lipoatrophy on stavudine were obtained from a study that used strict case definitions in Rwanda, the quality of life of the associated symptons are derived from a US-based catalog.[16] The generalizability of our study to other countries in southern Africa is limited since we used cost and utilization that represent the Cape Town area, where cost and access to health care are generally above average for the region. We attempted to account for this in our probabilistic sensitivity where we varied cost and utilization parameters widely. Our analyses assume that patients developing toxicities on zidovudine- or stavudine-containing regimens switch to a tenofovir-containing regimen. In reality, only a few African settings have guidelines in place and the capacity to switch individuals to less toxic and more expensive regimens. Finally, we focused on individuals who are followed in HIV clinics, have access to care, and adhere to their treatment regimen. We did not account for the effects of linkage to care, adherence, and loss to follow-up.
In summary, we compare the effectiveness and cost-effectiveness of the regimens recommended for first-line treatment of HIV in resource-limited settings and show that eliminating stavudine from the formulary is justifiable based on cost-effectiveness considerations, and that a regimen containing tenofovir/lamivudine/nevirapine is likely to be cost-effective in settings where it is accessible and acceptable. We also show that one of the recommended regimens – zidovudine/lamivudine/efavirenz – is unlikely to be cost-effective, and consideration should be given to its removal from the recommendations for the general population as a way to focus attention and experience to other, preferred regimens.
Acknowledgments
Funding sources
Dr. Bendavid is supported by the National Institute of Allergy and Infectious Diseases (K01-AI084582). Dr. Grant receives support from Bristol Myers Squibb and GlaxoSmithKline; Dr. Talbot receives support from the Gilead Foundation; Dr. Owens receives support from Department of Veterans Affairs, and the National Institute on Drug Abuse (R01 DA15612-01); and Dr. Zolopa receives support from Gilead Sciences, BristolMyersSquibb, and Viiv Pharmaceuticals.
Methods Appendix
This appendix provides a detailed description of the methods used in the article Cost-Effectiveness of Antiretroviral Regimens in the World Health Organization's Treatment Guidelines: A South African Analysis (Bendavid, et al.)
Overview
We developed a simulation model of HIV disease that followed the natural history of HIV-infected individuals from the time of presentation to care until death. Taking a societal perspective, the model follows the costs and benefits of five treatment strategies in sub-Saharan Africa (four recommended by the World Health Organization – WHO – and the ART combination in most common use in sub-Saharan Africa). The model estimates discounted and undiscounted quality-adjusted life expectancy from the time of presentation to care until the end of life in 2009 US dollars. The comparative value of alternative treatment strategies is expressed in terms of incremental cost-effectiveness ratios of each strategy compared with the next less effective strategy.
Model Structure
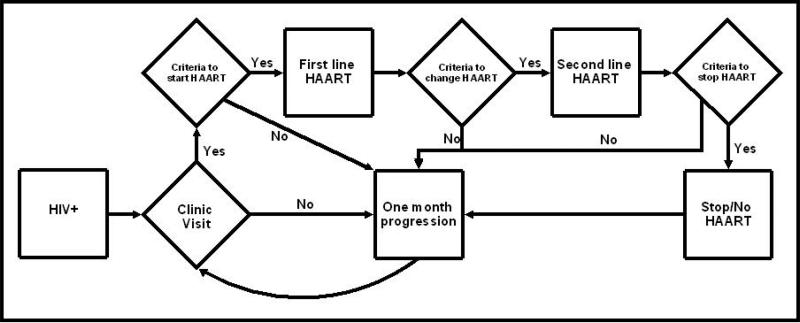
The model follows patients in one month intervals from the time at which they first make contact with the medical system for their HIV infection until death. Figure 1 shows a schematic flow diagram of patient care tracked in the model. At presentation and at each clinic visit, patients who are not on ART are evaluated whether they meet criteria to start ART. ART is initiated when CD4 cell count drops below 350 cells/μl, as suggested in the recent WHO guidelines. Patients who are on first-line ART also arrive to the clinic in regular intervals, and are evaluated for continued efficacy and possible toxicities of first-line ART. Patients are switched from first-line ART to second-line ART for two reasons: suspected treatment failure (by immunologic or clinical criteria) and drug toxicity severe enough to necessitate a change in regimen. Patients on second-line ART are also evaluated at regular intervals for signs of medication toxicity and treatment of opportunistic diseases (ODs). Second-line treatment is stopped in patients who have severe medication toxicity or are otherwise unable to tolerate the medications. However, patients who are on second-line ART and have evidence of virologic failure are maintained on ART due to the independent survival advantages of non-suppressive regimens compared with ART cessation.[1, 2]
Figure 1. Model flow of routine patient care management.
Squares represent states or processes, and diamonds represent decision nodes. For example, newly diagnosed HIV+ patients are seen in clinic, and evaluated whether they meet criteria to start ART. If they meet criteria, they are started on first-line ART, and if they do not meet criteria, the model evaluates them again next month. The model does not show the development of acute clinical events such as severe opportunistic diseases or some medication toxicities, which may occur at any time.
Patients may present at any clinical stage of disease and with any laboratory parameters. That is, on entry, a patient may or may not be ill with an opportunistic disease and may have any CD4 count or viral load. The distribution of CD4 counts at entry was taken from published cohort studies in the Cape Town region, and their risk of presenting with an OD was dependent on their CD4 count at presentation.[3, 4] Individuals with CD4 counts under 350 cells/μl are placed on treatment at the beginning of the simulation. Those with higher CD4 counts are observed until their CD4 count dropped below the threshold; in practice, very few individuals present with CD4 counts higher than 350 cells/μl. We assume that no patients have transmitted drug resistance (TDR). Although TDR does impact the treatment efficacy of NNRTI-based regimens, the rates of TDR reported from areas of Africa using the consensus definition of TDR have generally been low and thus are unlikely to have a major impact on treatment efficacies beyond the observed rates of virologic failure.[5, 6] Estimated rates of virologic suppression for each regimen are displayed in Table 1.
The model evaluates all patients in one month intervals. While the model tracks all patient parameters including CD4 count, viral load, ART regimen, medication toxicities, and development of ODs – most parameters are only available to providers during regular clinic visits. That is, while the model tracks an individual's health status monthly, that patient's data is only available for treatment decisions if it is measured and if that patient presents to clinic that month.
If a patient experiences a severe OD, we assume they present for acute medical care rather than to a routine clinic visit that month. The risk of developing a severe OD was dependent on the current CD4 count. We calculated the risk of a severe OD based on the risk of developing most WHO Stage 4 diseases (CMV infection, cryptococcal meningitis, Toxoplasmosis, Pneumocystis pneumonia, extrapulmonary TB, wasting, esophageal candidiasis, and chronic diarrhea) plus the risk of pulmonary TB based on experience in Cape Town.[3, 4, 7] We used data from patient cohorts that received cotrimoxazole prophylaxis.
A patient's risk of death from an OD is proportional to the CD4 count at the time of illness. The costs incurred reflect care for the OD. If a patient survives the acute illness, he/she returns to routine care. Patients were followed until death from HIV or other causes (background age-specific mortality rate).[8-11] Thus, we follow the lifetime costs and benefits of HIV care delivery for a group of simulated patients using clinical and utilization data of cohorts.
Disease Progression
We follow the disease progression of patients from the time of presentation based on the following parameters: age, CD4 count, viral load, ART regimen, ART duration, history of OD, virologic failure, and medication toxicity. We monitored all parameters for all patients monthly, but the information was only available to providers every 6 months or sooner for acute clinical events (such as onset of an OD or medication toxicity).
Upon entry to care, each patient is assigned an initial CD4 count, viral load, and age from a distribution that is calibrated to Cape Town study cohorts.[4, 12-14] Each patient's risk of clinical events is determined by his/her CD4 count. The CD4 count was modeled as a continuous variable that varied based on the viral load, ART, and occurrence of treatment failure. In patients whose viral load was not suppressed, the rate of CD4 decline was determined by their current CD4 count and viral load.[15, 16] Given the uncertainty about the exact relationship between viral load and CD4 change, we allow two non-linear determinants of CD4 decline: random variability that loosens the correlation between viral load and CD4 count decline, and a slower rate of CD4 decline, both guided by published data.[15-17]
Once a patient is started on a successful first-line ART regimen, his/her CD4 rises to a peak that depends primarily on the CD4 count at the time of treatment initiation. While some data support an age-related effect of CD4 rise, the strongest reproducible predictor of CD4 rise on effective ART is the CD4 count at the time of treatment initiation.[18-21] Published data on CD4 rise were extracted using the graph digitizing program DigitizeIt v.1.5.8 (Braunschweig, Germany), and monthly CD4 increments were determined based on time elapsed from treatment initiation.
The principal activity of ART is suppression of viral replication, and we use viral suppression to undetectable levels as the principal marker that allows CD4 to rise after treatment initiation. While on successful treatment, viral load is undetectable at a threshold of less than 400 copies/ml.
Treatment failure is modeled as failure to suppress virologic replication and a return of the viral load to detectable levels. Patients with virologic failure who are continued on ART have a lower viral “set point,” and their rate of CD4 decline is consequently slower.[2] Clinically, virologic failure is inferred through CD4 monitoring, and we use immunologic criteria outlined in the WHO guidelines and recent clinical trials – a drop to a CD4 count of less than 100 cells/μl – to estimate timing of virologic failure and the need to switch to second-line therapy.[7, 22, 23]
Treatment Strategies
Regimens
We compared the effectiveness and costs of five alternative ART first-line regimens (four recommended by the WHO and the ART combination in most common use in sub-Saharan Africa):
Tenofovir + lamivudine + efavirenz
Tenofovir + lamivudine + nevirapine
Zidovudine + lamivudine + efavirenz
Zidovudine + lamivudine + nevirapine
Stavudine + lamivudine + nevirapine
All the regimens have a similar purpose – to suppress viral replication and enable immunologic recovery – but they differ substantively on two primary domains: success rates of achieving virologic suppression and their respective toxicity profile. Table 1 shows the estimates of each regimen's rates of virologic suppression and toxicity profile used in the model.
Virologic Suppression
We estimate rates of virologic suppression from comparative trials. Using data from clinical trials performed mostly in developed country settings raises questions about generalizability to an African setting. We use this data for two primary reasons. First, it is the best available comparative data for these regimens. Literature with estimates of virologic suppression from uncontrolled cohorts suggests that data are scant and unreliable, as it fluctuates widely within regimens based on the patient population, virologic assay, and case definitions for virologic failure. Second, recent literature suggests that suppression rates are similar between subtype B and non-subtype-B, despite the genotypic differences, supporting observations that ART effectiveness is similar between developed and developing settings.[24]
We rely heavily on two long-standing clinical trials in establishing rates of virologic suppression. One compares regimens containing tenofovir to regimens containing stavudine, while the other compares regimens containing tenofovir to regimens containing zidovudine.[25, 26] Those studies show that tenofovir and stavudine are similarly efficacious, while tenofovir is more efficacious than zidovudine. Since both studies were performed using a similar protocol with the same group of investigators, we assume by transitivity that stavudine is more efficacious than zidovudine. Both studies use efavirenz as the NNRTI of choice. We estimated the rates of virologic failure with nevirapine were about 1.5 times higher than those with efavirenz, based on several studies that suggest a consistent estimate of efavirenz's superior ability to maintain viral suppression in combination with a variety of NRTIs.[27-31]
Toxicities and Regimen Changes
We include the effect of seven dominant toxicities associated with ART: lipoatrophy, renal failure, anemia, hepatotoxicity, myocardial infarctions, peripheral neuropathy, and lactic acidosis. While other toxicities are known to be associated with ART, we chose to examine those toxicities that are most common and significant in terms of their effect on quality of life. Where possible, we estimate the types of toxicities and incidence rate for each regimen from long-term follow-up studies of clinical trials.[26, 32, 33] We use these sources because of the strict case definitions and careful monitoring. Where clinical trial data was not available, we use African observational data.[30, 34-37] In particular, we rely on cohorts that identify toxicities that led to regimen change as the clinically relevant endpoint. For a few toxicities we rely on observational data from non-African cohorts.[38, 39] Table 1 shows the toxicities associated with each regimen and 1-year frequency of each toxicity. The cumulative risk of all the toxicities except for lipoatrophy plateaus after a year, and individuals on regimens associated with each toxicity who remain on the regimen for at least a year without experiencing the toxicity are no longer at risk. The most common toxicity, associated with all the regimens, is lipoatrophy. It occurs most frequently with stavudine-based regimens and least frequently with tenofovir-based regimens. We estimate a declining rise in the risk of lipoatrophy up to three years, when the cumulative risk plateaus.
Therapeutic decisions following toxicities aim to minimize the risk of future toxicity burden. Practically, most toxicities that are associated with zidovudine or stavudine prompt a switch to a tenofovir-containing regimen. Most toxicities associated with nevirapine prompt a switch to an efavirenz-containing regimen. The main exception is renal failure with tenofovir, which prompts a switch to a zidovudine-containing regimen. These therapeutic decisions are shown in Figure 1 of the main manuscript. These regimen substitutions with toxicities are based primarily on the WHO formulary.
Consistent with WHO guidelines and with standard practice in many parts of sub-Saharan Africa, we modeled a second-line ART for those who experience toxicities on multiple first-line regimens or who are thought to fail first-line ART. Second-line ART included a combination of NRTIs that depended on the initial regimen and a boosted protease inhibitor.[7]
Benefits and Costs
Benefits are measured in life years (LYs) and quality-adjusted life years (QALYs) from the time of presentation. We compared both discounted and undiscounted life years and QALYs among the various treatment strategies. Where possible, we used quality of life estimates from the same clinical trials that reported the toxicity incidence. We had this information for neuropathy. We use a study on switching from stavudine to tenofovir in South Africa for most other quality of life estimates. That study, in turn, uses a general quality of life catalog to estimate many of the associated weights. Because of the uncertainty associated with the QALY weights, and their importance in shaping the results, we varied the quality of life weight estimates for each toxicity widely (Table 2 in main manuscript).
We consider direct costs of care from a societal perspective in this study. We included the costs of inpatient care, outpatient care, provision of ART, laboratory monitoring, and treating toxicities. Inpatient and outpatient clinic costs are taken from a detailed costing study of HIV care in South Africa.[40, 41] The differences in cost of care between South Africa and other sub-Saharan countries poses a legitimate concern to the study's generalizability. Consequently, we varied the costs widely based on measured variations in cost of medical care provided in the WHO-CHOICE database.[42]
We obtained the cost of each regimen from the WHO Global Price Reporting Mechanism database.[43] That database provides price data of antiretroviral drugs obtained directly from national AIDS programs and other major purchasers, and publishes detailed transactional information, including quantities purchased, dosages, and the amount paid. Some antiretroviral drugs have fixed-dose combinations, which generally reduce the price of the regimen. For example, staduvine, lamivudine, and nevirapine come in a fixed-dose combination, as do zidovudine and lamivudine. Regimens with fixed-dose combinations are cheaper than regimens with the individual drugs procured independently. We obtained the lowest price for each regimen reported in the Global Price Reporting Mechanism for South Africa in 2008. Notably, the prices of all ART regimens converged in sub-Saharan Africa by 2008, and the prices paid for ART were nearly identical between South Africa and other African countries.[44]
Sensitivity Analysis
Our sensitivity analysis includes several one-way and multi-way analyses, as well as a probabilistic sensitivity analysis. The uncertainty bounds for each parameter are shown in Table 1 of the main manuscript. We pay particular attention to those data elements for which we have less certainty and which change significantly between alternative strategies. These include the rates of failure, toxicity rates, quality of life weights with toxicities, and cost of the antiretroviral agents. We perform a probabilistic sensitivity analysis where we vary all the variables simultaneously and repeat the analysis 1,000 times. Each variable is drawn from a probability distribution and the entire analysis is re-run. Probabilities for events or health states were sampled using beta distributions with alpha and beta parameters determined by the point estimate (mean) and variance; and costs were sampled using gamma distributions with the mode at the point estimate. Beta distributions are defined by two shape parameters, α and β, that were estimated for each variable to approximate the mean and variance as follows:
Gamma distributions are defined by a shape and scale parameters, k and θ:
This allows us to estimate the confidence in our results if the true value of each variable is anywhere within the uncertainty bounds shown. For example, we estimate the likelihood that a strategy which appears dominant in the base case – one which is more effective and less costly than another strategy – may not be dominant.
Footnotes
Conflict of Interest Statement and Role of Funding Agencies
We the authors (PG, AT, AZ, DKO, EB) declare that we have no conflicts of interest except for the possible conflict of interest from receipt of funding from the entities mentioned in the Funding Sources. Dr. Bendavid had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funding agencies had no part in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Contributors
Eran Bendavid: I declare that I participated in originating the concept, in conducting the data collection, and data analysis. I did most of the writing of the paper, including the final revision. I had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rapid Advice: Antiretroviral Therapy for HIV Infection in Adults and Adolescents. World Health Organization; Geneva: Nov, 2009. [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adult and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. 2009 January 25; 2010. Available from: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 3.Gazzard B. British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV medicine. 2008;9(8):563. doi: 10.1111/j.1468-1293.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 4.Hammer SM, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. Jama. 2008;300(5):555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 5.Bender M, et al. Cost-Effectiveness of Tenofovir as First-Line Antiretroviral Therapy in India. Clinical Infectious Diseases. 2010;50:416–425. doi: 10.1086/649884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badri M, et al. When to initiate highly active antiretroviral therapy in sub-Saharan Africa? A South African cost-effectiveness study. Antivir Ther. 2006;11(1):63–72. [PubMed] [Google Scholar]
- 7.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368(9543):1254–9. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 8.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359(9323):2059–64. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 9.ART-LINC. ART-CC Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. The Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 10.Arribas J, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2008;47(1):74. doi: 10.1097/QAI.0b013e31815acab8. [DOI] [PubMed] [Google Scholar]
- 11.Haubrich R, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23(9):1109. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallant J, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2):191. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins C, et al. Antiretroviral durability and tolerability in HIV-infected adults living in urban Kenya. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2007;45(3):304. doi: 10.1097/QAI.0b013e318050d66c. [DOI] [PubMed] [Google Scholar]
- 14.Amoroso A. CROI. Los Angeles: 2007. ART-Associated Toxicities Leading to a Switch in Medication: Experience in Uganda, Kenya, and Zambia. [Google Scholar]
- 15.Nachega J, et al. Efavirenz versus nevirapine-based initial treatment of HIV infection: clinical and virological outcomes in Southern African adults. AIDS. 2008;22(16):2117. doi: 10.1097/QAD.0b013e328310407e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Griensven J, et al. High prevalence of lipoatrophy among patients on stavudine-containing first-line antiretroviral therapy regimens in Rwanda. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101(8):793–798. doi: 10.1016/j.trstmh.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Toure S, et al. 3rd HIV/AIDS Implementers’ Meeting. Kigali, Rwanda: 2007. Main reasons of modification of the first-line antiretroviral regimen in adult patients who initiated HAART in the International Family Health Initiative (ACONDA/ISPED/EGPAF) in Abidjan, Cote d'Ivoire. [Google Scholar]
- 18.Mugyenyi P, et al. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010;375(9709):123–31. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bendavid E, et al. Cost-effectiveness of HIV monitoring strategies in resource-limited settings – a Southern African analysis. Archives of Internal Medicine. 2008;168(17):1910–1918. doi: 10.1001/archinternmed.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendavid E, et al. Expanding Antiretroviral Options in Resource-Limited Settings-A Cost-Effectiveness Analysis. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2009;52(1):106–113. doi: 10.1097/QAI.0b013e3181a4f9c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes CB, et al. CD4 Decline and Incidence of Opportunistic Infections in Cape Town, South Africa: Implications for Prophylaxis and Treatment. J Acquir Immune Defic Syndr. 2006;42(4):464–469. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein MC, et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 23.Rosen S, et al. Cost and cost-effectiveness of switching from stavudine to tenofovir in first-line antiretroviral regimens in South Africa. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2008;48(3):334. doi: 10.1097/QAI.0b013e31817ae5ef. [DOI] [PubMed] [Google Scholar]
- 24.Soriano V, et al. International AIDS Society. Cape Town, South Africa: 2009. Prospective comparison of nevirapine and atazanavir/ritonavir both combined with tenofovir DF/emtricitabine in treatment-na ve HIV-1 infected patients: ARTEN study week 48 results [abstract LB PEB07]. [Google Scholar]
- 25.Labarga P, et al. Safety and efficacy of tenofovir/emtricitabine plus nevirapine in HIV-infected patients. [Letter]. AIDS. 2010;24:000–000. doi: 10.1097/QAD.0b013e3283322895. [DOI] [PubMed] [Google Scholar]
- 26.Van Leth F, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. The lancet. 2004;363(9417):1253–1263. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 27.Kantor R, et al. Impact of HIV-1 subtype and antiretroviral therapy on protease and reverse transcriptase genotype: results of a global collaboration. PLoS Med. 2005;2(4):e112. doi: 10.1371/journal.pmed.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorrington R, et al. The demographic impact of HIV/AIDS in South Africa: National and provincial indicators for 2006. Centre for Actuarial Research, South African Medical Research Council and Actuarial Society of South Africa; Cape Town: 2006. [Google Scholar]
- 29.US Census Bureau International Data Base. 2010 January 15; Available from: http://www.census.gov/ipc/www/idb/tables.html.
- 30.Mellors JW, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Annals of internal medicine. 1997;126(12):946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez B, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296(12):1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 32.Ledergerber B, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364(9428):51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 33.Gallant J, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. New England Journal of Medicine. 2006;354(3):251. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 34. [2010 February 3];Framingham Risk Calculator. Available from: http://hp2010.nhlbihin.net/atpiii/calculator.asp.
- 35.Smith C, et al. The rate of viral rebound after attainment of an HIV load< 50 copies/mL according to specific antiretroviral drugs in use: results from a multicenter cohort study. The journal of infectious diseases. 2005;192(8):1387–1397. doi: 10.1086/466534. [DOI] [PubMed] [Google Scholar]
- 36.John M, et al. Chronic hyperlactatemia in HIV-infected patients taking antiretroviral therapy. AIDS. 2001;15(6):717. doi: 10.1097/00002030-200104130-00007. [DOI] [PubMed] [Google Scholar]
- 37.Boubaker K, et al. Hyperlactatemia and antiretroviral therapy: the Swiss HIV Cohort Study. Clinical infectious diseases. 2001;33(11):1931–1937. doi: 10.1086/324353. [DOI] [PubMed] [Google Scholar]
- 38.Cleary S, et al. Cost-Effectiveness Of Antiretroviral Treatment For HIV-Positive Adults In A South African Township. Health Systems Trust; Cape Town: 2004. [Google Scholar]
- 39.Nyman J, et al. Quality-of-life weights for the US population: self-reported health status and priority health conditions, by demographic characteristics. Medical care. 2007;45(7):618. doi: 10.1097/MLR.0b013e31803dce05. [DOI] [PubMed] [Google Scholar]
- 40.Kimel M, et al. Does epoetin alfa improve health-related quality of life in chronically ill patients with anemia? Summary of trials of cancer, HIV/AIDS, and chronic kidney disease. Value in Health. 2008;11(1):57. doi: 10.1111/j.1524-4733.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 41.Badri M, et al. Cost-effectiveness of highly active antiretroviral therapy in South Africa. PLoS Med. 2006;3(1):e4. doi: 10.1371/journal.pmed.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zijenah LS, et al. Affordable flow cytometry for enumeration of absolute CD4+ T-lymphocytes to identify subtype C HIV-1 infected adults requiring antiretroviral therapy (ART) and monitoring response to ART in a resource-limited setting. Journal of translational medicine. 2006;4:33–39. doi: 10.1186/1479-5876-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization: Global price reporting mechanism. 2009 August 13; Available from: http://www.who.int/hiv/amds/gprm/en/
- 44.Cantor S, et al. Costs of blood transfusion: a process-flow analysis. Journal of Clinical Oncology. 1998;16(7):2364. doi: 10.1200/JCO.1998.16.7.2364. [DOI] [PubMed] [Google Scholar]
- 1.Deeks SG, et al. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. The New England journal of medicine. 2001;344(7):472–480. doi: 10.1056/NEJM200102153440702. [DOI] [PubMed] [Google Scholar]
- 2.Ledergerber B, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364(9428):51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 3.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368(9543):1254–9. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 4.Holmes CB, et al. CD4 Decline and Incidence of Opportunistic Infections in Cape Town, South Africa: Implications for Prophylaxis and Treatment. J Acquir Immune Defic Syndr. 2006;42(4):464–469. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JA, et al. Minority HIV-1 drug resistance mutations are present in antiretroviral treatment-naive populations and associate with reduced treatment efficacy. PLoS Med. 2008;5(7):e158. doi: 10.1371/journal.pmed.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartolo I, et al. Antiretroviral drug resistance surveillance among treatment-naive human immunodeficiency virus type 1-infected individuals in Angola: evidence for low level of transmitted drug resistance. Antimicrob Agents Chemother. 2009;53(7):3156–8. doi: 10.1128/AAC.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapid Advice: Antiretroviral Therapy for HIV Infection in Adults and Adolescents. World Health Organization; Geneva: Nov, 2009. [PubMed] [Google Scholar]
- 8.Bradshaw D, et al. Initial burden of disease estimates for South Africa, 2000. S Afr Med J. 2003;93(9):682–688. [PubMed] [Google Scholar]
- 9.Bradshaw D, et al. South African cause-of-death profile in transition--1996 and future trends. S Afr Med J. 2002;92(8):618–623. [PubMed] [Google Scholar]
- 10.World Health Organization . National Burden of Disease Studies: A Practical Guide. Geneva: 2001. [Google Scholar]
- 11.World Health Organization Life Tables for WHO Member States. 2009 July 15; Available from: http://www.who.int/whosis/database/life_tables/life_tables.cfm.
- 12.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359(9323):2059–64. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 13.Coetzee D, et al. Promoting adherence to antiretroviral therapy: the experience from a primary care setting in Khayelitsha, South Africa. AIDS. 2004;18(Suppl 3):S27–S31. doi: 10.1097/00002030-200406003-00006. [DOI] [PubMed] [Google Scholar]
- 14.Coetzee D, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. Aids. 2004;18(6):887–95. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 15.Mellors JW, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Annals of internal medicine. 1997;126(12):946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Lau B. CROI. Los Angeles: 2007. Predictive Value of Plasma HIV RNA Levels for Rate of CD4 Decline and Clinical Disease Progression. [Google Scholar]
- 17.Rodríguez B, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296(12):1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann GR, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163(18):2187–95. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann GR, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41(3):361–372. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 20.Battegay M, et al. Immunological recovery and antiretroviral therapy in HIV-1 infection. The Lancet infectious diseases. 2006;6(5):280–287. doi: 10.1016/S1473-3099(06)70463-7. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, et al. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis. 2006;6:59. doi: 10.1186/1471-2334-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antiretroviral Therapy For HIV Infection in Adults And Adolescents: Recommendations for a public health approach (2006 revision) World Health Organization; Geneva: [PubMed] [Google Scholar]
- 23.Mugyenyi P, et al. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010;375(9709):123–31. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantor R, et al. Impact of HIV-1 subtype and antiretroviral therapy on protease and reverse transcriptase genotype: results of a global collaboration. PLoS Med. 2005;2(4):e112. doi: 10.1371/journal.pmed.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallant J, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. New England Journal of Medicine. 2006;354(3):251. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 26.Gallant J, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2):191. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 27.Rates of disease progression according to initial highly active antiretroviral therapy regimen: a collaborative analysis of 12 prospective cohort studies. J Infect Dis. 2006;194(5):612–22. doi: 10.1086/506362. [DOI] [PubMed] [Google Scholar]
- 28.Braithwaite RS, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS. 2007;21(12):1579–89. doi: 10.1097/QAD.0b013e3281532b31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith C, et al. The rate of viral rebound after attainment of an HIV load< 50 copies/mL according to specific antiretroviral drugs in use: results from a multicenter cohort study. The journal of infectious diseases. 2005;192(8):1387–1397. doi: 10.1086/466534. [DOI] [PubMed] [Google Scholar]
- 30.Nachega J, et al. Efavirenz versus nevirapine-based initial treatment of HIV infection: clinical and virological outcomes in Southern African adults. AIDS. 2008;22(16):2117. doi: 10.1097/QAD.0b013e328310407e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Berg-Wolf M, et al. Virologic, Immunologic, Clinical, Safety, and Resistance Outcomes from a Long-Term Comparison of Efavirenz-Based Versus Nevirapine-Based Antiretroviral Regimens as Initial Therapy in HIV-1-infected Persons. HIV clinical trials. 2008;9(5):324–336. doi: 10.1310/hct0905-324. [DOI] [PubMed] [Google Scholar]
- 32.Arribas J, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2008;47(1):74. doi: 10.1097/QAI.0b013e31815acab8. [DOI] [PubMed] [Google Scholar]
- 33.Haubrich R, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23(9):1109. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawkins C, et al. Antiretroviral durability and tolerability in HIV-infected adults living in urban Kenya. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2007;45(3):304. doi: 10.1097/QAI.0b013e318050d66c. [DOI] [PubMed] [Google Scholar]
- 35.Amoroso A. CROI. Los Angeles: 2007. ART-Associated Toxicities Leading to a Switch in Medication: Experience in Uganda, Kenya, and Zambia. [Google Scholar]
- 36.van Griensven J, et al. High prevalence of lipoatrophy among patients on stavudine-containing first-line antiretroviral therapy regimens in Rwanda. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101(8):793–798. doi: 10.1016/j.trstmh.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Toure S, et al. 3rd HIV/AIDS Implementers’ Meeting. Kigali, Rwanda: 2007. Main reasons of modification of the first-line antiretroviral regimen in adult patients who initiated HAART in the International Family Health Initiative (ACONDA/ISPED/EGPAF) in Abidjan, Cote d'Ivoire. [Google Scholar]
- 38.Boubaker K, et al. Hyperlactatemia and antiretroviral therapy: the Swiss HIV Cohort Study. Clinical infectious diseases. 2001;33(11):1931–1937. doi: 10.1086/324353. [DOI] [PubMed] [Google Scholar]
- 39.John M, et al. Chronic hyperlactatemia in HIV-infected patients taking antiretroviral therapy. AIDS. 2001;15(6):717. doi: 10.1097/00002030-200104130-00007. [DOI] [PubMed] [Google Scholar]
- 40.Govender V, et al. The Costs and Perceived Quality of Care for People Living with HIV/AIDS in the Western Cape Province in South Africa. Partnerships for Health Reform; Bethesda, MD: 2000. [Google Scholar]
- 41.Cleary SM, McIntyre D, Boulle AM. The cost-effectiveness of Antiretroviral Treatment in Khayelitsha, South Africa - a primary data analysis. Cost effectiveness and resource allocation. 2006;4:1–14. doi: 10.1186/1478-7547-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CHOosing Interventions that are Cost Effective (WHO-CHOICE) 2008 September 27; Available from: http://www.who.int/choice/en/
- 43.World Health Organization: Global price reporting mechanism. 2009 August 13; Available from: http://www.who.int/hiv/amds/gprm/en/
- 44.Bendavid E, et al. The Role of Drug Prices and Foreign Assistance in Expanding HIV Treatment in Africa. 2010. In submission.
- 45.Pozniak A, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes-a 96-week analysis. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;43(5):535. doi: 10.1097/01.qai.0000245886.51262.67. [DOI] [PubMed] [Google Scholar]
- 46. [2010 February 3];Framingham Risk Calculator. Available from: http://hp2010.nhlbihin.net/atpiii/calculator.asp.
- 47.van Leth F, et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS medicine. 2004;1:64–74. doi: 10.1371/journal.pmed.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]