Abstract
Alterations in dopamine output within the various subnuclei of the amygdala have previously been implicated in cocaine reinforcement, as well as cocaine-seeking behavior. To elucidate the potential for increased stimulation of D1- and D2-like receptors (D1Rs and D2Rs, respectively) specifically in the central nucleus of the amygdala (CeA) to modulate cue- and cocaine-elicited reinstatement of cocaine-seeking behavior, we infused either the D1R agonist, SKF-38393 (0 – 4.0 μg/side) or the D2R agonist, 7-OH-DPAT (0 – 4.0 μg/side) into the CeA immediately prior to tests for cue and cocaine-primed reinstatement. We also examined the effects of 7-OH-DPAT on cocaine self-administration as a positive behavioral control. 7-OH-DPAT decreased cue and cocaine-primed reinstatement, and reduced the number of cocaine infusions during self-administration; SKF-38393 produced no discernable effects. The results suggest that enhanced stimulation of D2Rs, but not D1Rs, in the CeA is sufficient to inhibit expression of the incentive motivational effects of cocaine priming and cocaine-paired cues. Together with previous findings that D1R blockade attenuates reinstatement of cocaine-seeking behavior, the results suggest that D1R stimulation may be necessary, but not sufficient, to modulate the incentive motivational effects of cues and cocaine priming.
Keywords: Self-administration, Incentive motivation, Reinforcement, Reinstatement, Dopamine, Amygdaloid nuclei, 7-OH-DPAT, SKF-38393
1. Introduction
Currently, there are no effective treatments for preventing relapse to cocaine addiction [75]. Sampling cocaine or exposure to drug-associated stimuli can elicit incentive motivational effects that manifest as craving in humans and which may factor into relapse [23, 31]. Incentive motivational effects produced by these stimuli can be investigated preclinically using the extinction/reinstatement model [29, 34, 65]. In this model, animals are typically trained to press a lever reinforced by cocaine infusion delivered concurrently with discrete light/tone cues. Subsequently, animals undergo extinction training during which lever presses produces no consequences. This responding in the absence of cocaine reinforcement is referred to as cocaine-seeking behavior. Tests for reinstatement of extinguished cocaine-seeking behavior are then performed by re-exposing the animals to cocaine or to response-contingent presentations of the light/tone cues in order to investigate the incentive motivational effects of these stimuli.
To understand the mechanisms that contribute to cocaine craving and relapse, numerous studies have focused on the role of dopamine D1-like and D2-like receptor (D1R and D2R) subtypes in cocaine reinforcement and reinstatement of extinguished cocaine-seeking behavior. D2R agonists shift the cocaine self-administration dose-response curve to the left [7, 16, 17, 18], suggesting increased sensitivity to cocaine reinforcement, while D1R agonists produce a downward shift, suggesting either impaired motor behavior or a general dampening of motivation for cocaine [7, 18]. Blockade of either D1Rs or D2Rs during cocaine self-administration, however, alters responding in a manner consistent with antagonistic effects [15]. Similarly, dopamine receptor partial agonists, which have lower intrinsic activity at the receptor compared to the neurotransmitter, produce slight downward and rightward shifts in the psychostimulant dose-response curve (i.e., dampening reinforcement), and therefore have also received attention as potential therapeutic agents [83, 97]. Interestingly, both stimulation and blockade of D1Rs via systemic administration of agonists and antagonists, respectively, attenuate drug-seeking behavior elicited by cocaine cues or cocaine priming [3, 4, 24, 28, 49, 50, 86, 91, 92, 99]. In humans, both D1R agonism and antagonism result in dampened cocaine-elicited euphoria [42, 85]. Although D1 agonists and antagonists produce similar behavioral outcomes, we have suggested that they do so via distinct processes, with the agonist likely reducing motivation to initiate cocaine-seeking behavior whereas the antagonist likely attenuates the reinforcing effects of the cues and cocaine primes [3, 4]. In contrast to D1R drugs, D2R agonists and antagonists produce opposite effects on drug-seeking behavior. D2R agonists reinstate extinguished cocaine-seeking behavior [27, 29, 35, 49, 91], while D2R antagonists block the ability of cocaine [49, 86, 96] and cocaine-paired cues to reinstate cocaine-seeking behavior [22, 26, 38].
The effects of dopaminergic drugs on cocaine reinforcement and cocaine-seeking behavior are mediated, at least in part, by dopamine receptors within the amygdala. The amygdala is innervated by dopaminergic projections arising from both the ventral tegemental area (VTA) and substantia nigra pars compacta [62], and in turn sends efferent projections to other limbic and cortical structures implicated in addiction-related behaviors [48]. Extracellular dopamine levels within the amygdala are elevated during cocaine self-administration, and in vivo manipulations whereby dopamine is directly perfused into the amygdala can increase or decrease cocaine intake depending on the concentration of dopamine infused [45, 46]. The amygdala is strongly activated in animals and humans, as measured via Fos protein expression and functional imaging, respectively, following cocaine injections or exposure to drug-associated cues [11, 40, 51, 73]. Importantly, dopamine concentrations within the amygdala are elevated following exposure to a drug-associated context or discriminative stimuli predictive of cocaine availability, and dopamine levels in the amygdala correspond to cocaine-seeking behavior [94, 98]. Furthermore, amygdala lesions or dopamine receptor antagonist infusions directly into the amygdala reduce the reinforcing and stimulus effects of cocaine, as well as the conditioned motivational effects of cocaine-related stimuli [8, 10, 13, 19, 45, 68, 90].
More recent work suggests dissociable roles of the basolateral (BlA) and central (CeA) nuclei of the amygdala in mediating cocaine-related behavior. The BlA is nonessential for processing the primary rewarding effects of cocaine as BlA lesions do not affect cocaine self-administration [41, 69, 100] and BlA infusions of psychomotor stimulants fail to condition a place preference [76]. However, BlA lesions do interfere with responding for conditioned reinforcers [12] and disrupt acquisition, extinction, and consolidation of cue-cocaine associations [33, 36]. In contrast, infusion of amphetamine directly into the CeA produces a conditioned place preference, suggesting a role in initiating the rewarding effects of psychostimulants [76]. The CeA is also thought to amplify expression of drug-conditioned behaviors [84] and is likely involved in the increases in cue-elicited drug-seeking behavior that occur over time during abstinence [61, 63]. Although both the CeA and the BlA are activated during expression of drug-conditioned responses [53, 93], selective activation has been observed in the CeA by cocaine priming and in the BlA by cocaine-associated cues [24, 54, 73, 102].
D1Rs contribute to the amygdala subregion-specific regulation of cocaine-related behaviors. For instance, Mashhoon et al. [66] recently demonstrated that the D1 agonist SKF 81297 infused into the caudal (c) BlA specifically increases cocaine-seeking behavior under a second-order schedule of reinforcement when cocaine and cues are available, whereas the agonist infused into the rostral (r) BlA specifically increases cocaine-seeking behavior when only cues are available. Infusion of the D1 antagonist SCH-23390 into these subregions produced opposite effects on these behaviors, suggesting D1R mediation. Interestingly, in addition to attenuating cocaine-seeking behavior elicited by a cocaine-predictive cue, systemic injection of a D1R antagonist blocks Fos protein expression within the BlA that occurs with exposure to discriminative stimuli predictive of drug [24]. Infusion of SCH-23390 into either the BlA or the CeA increases cocaine self-administration rates [2, 13]. Furthermore, infusion of SCH-23390 specifically into the cBlA, and to a lesser extent the CeA, attenuates cue-elicited cocaine-seeking behavior, while infusion of this drug into the rBlA or CeA attenuates cocaine-primed reinstatement [2]. It was surprising to find that SCH-23390 produced similar effects throughout the amygdala given that dopamine receptor subtype distribution is not equal. Indeed, the D1R family is predominately located within the BlA, whereas the CeA predominately contains the D2R family [89]. Some potential limitations of SCH-23390 to examine behavior mediated by D1Rs are that 1) it is highly lipophilic and may therefore spread rapidly from the injection site, 2) it blocks inward rectifying potassium channels [55], and 3) it acts as an agonist at 5-HT2C receptors [70]. Furthermore, its action via blockade of inward rectifying potassium channels may indirectly alter D2 receptor function [55]. These caveats notwithstanding, D1Rs within the amygdala, particularly the BlA, are likely involved in modulating motivation for cocaine.
Given the greater distribution of D2-like receptors within the CeA, the primary focus of the present study was to examine the role of D2R activation within the CeA in mediating cocaine-related behaviors. Notably, of the various subnuclei that compose the amygdaloid complex, the CeA receives the greatest dopaminergic innervation [5, 32], therefore implicating this particular nucleus in dopamine-mediated conditioned responses that involve the amygdala. For instance, mesoamygdaloid D2R activation within the CeA has previously been implicated in the acquisition and expression of conditioned approach behaviors [43, 44, 81].
In the present study, we were interested in examining whether D2R stimulation within the CeA alters the expression of cue- and cocaine-elicited reinstatement of extinguished cocaine-seeking behavior. Therefore, we examined intra-CeA infusions of the selective D2/D3 agonist 7-OH-DPAT [60] prior to tests for cue reinstatement and cocaine-primed reinstatement of extinguished cocaine-seeking behavior. Additionally, given our previous work demonstrating that intra-CeA infusion of SCH-23390 blocks expression of both cue and cocaine-primed reinstatement [2], we were interested in further whether increased stimulation of D1Rs in the CeA modulates these behaviors. Therefore, we also examined the effects of intra-CeA infusion of the D1R agonist SFK-38393 on cue and cocaine-primed reinstatement. Notably, this drug has high efficacy and intrinsic activity at the unique phosphoinositide-linked D1Rs located in the amygdala, unlike its relatively low intrinsic activity at the traditional adenylyl cyclase-linked D1Rs [47, 95]. An important advantage in using 7-OH-DPAT and SKF-38393 to investigate the unique roles of D2- and D1-like receptors, respectively, in modulating reinstatement behavior is that both of these agonists have previously been used to dissociate dopaminergic-related behavioral functions of the CeA from surrounding subnuclei, suggesting they exhibit limited spread [44, 101].
2. Methods
2.1. Animals
Male Sprague-Dawley rats, weighing 225–250 g upon arrival to our laboratory, were individually housed in a climate-controlled colony with a 12-h reverse light/dark cycle. The housing conditions and care of the rats were in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996). Rats were handled for 1–2 min/day for at least 5 days and they weighed at least 275 g by the time they underwent surgery.
2.2. Surgery
Rats received atropine sulfate (10 mg/kg, IP) to decrease bronchial secretions, and 5 min later, they were anesthetized with sodium pentobarbital (50 mg/kg, IP). Catheters were constructed from Silastic tubing (10 cm; inner diameter 0.012 × outer diameter 0.025 inches; Dow Corning, Midland, MI) connected to a bent 22 gauge metal cannula encased within a plastic screw connector (Plastics One, Roanoke, VA) at one end and affixed with a small ball of aquarium sealant ~4 cm from the other end. The catheters were implanted into the jugular vein as described in Neisewander et al. [73]. Rats were then placed into a stereotaxic instrument. The connective tissue overlying the skull was removed and 4 stainless steel jeweler’s screws were implanted into the skull. Small holes were then drilled into the skull, through which stainless steel guide cannulae (23-gauge) were lowered into the CeA bilaterally at the following coordinates relative to bregma [79]: AP= −2.5, ML = ±4.1, DV= −6.5. Dental acrylic was used to permanently affix the metal end of the catheter and the guide cannulae to the screws, which anchored the entire headpiece to the skull. Stainless steel stylets (30-gauge) were placed into the guide cannulae to maintain patency. Catheter patency was maintained by daily flushing with a solution of 0.1 ml bacteriostatic saline containing heparin sodium (10 U/ml; Elkins-Sinn Inc., Cherry Hills, NJ), streptokinase (0.67 mg/ml; Astra USA Inc., Westerborough, MA), and ticarcillin sodium (66.7 mg/ml; SmithKline Beecham Pharmaceuticals, Philadelphia, PA). Proper catheter functioning was verified periodically throughout the experiments by the intravenous administration of 0.05 ml of methohexital sodium (16.6 mg/kg; Jones Pharma Inc., St. Louis, MO), a dose sufficient to briefly produce loss of muscle tone only when administered intravenously.
2.3. Drugs and intracranial infusions
Cocaine hydrochloride (NIDA drug supply system) was dissolved in saline, filtered through a 0.2 μm filter, and administered intravenously (IV) for self-administration and intraperitoneally (IP, 1 ml/kg) for cocaine reinstatement. SKF-38393 and 7-OH-DPAT were purchased from Research Biochemicals, Inc., Natick, MA. 7-OH-DPAT was diluted in 0.1 M PBS (pH ≈ 7.4). SKF-38393 was dissolved in methanol and then diluted with heated, distilled water (pH ≈ 6.0). 7-OH-DPAT was bilaterally infused at an injection volume of 0.2 μl/side over 1 min. SKF-38393 was bilaterally infused into the CeA at an injection volume of 0.3 μl/side over 1.5 min. Vehicle solutions and injection volumes were identical to their respective drug condition. For the intra-CeA infusions, a 30-gauge injector connected to a 10-μl syringe via PE 10 tubing was lowered to extend 2 mm below the guide cannulae tips. After 1 min, rats received drug or vehicle infusions over the designated amount of time. Infusion of the drug was confirmed by movement of an air bubble the correct distance in the injection tubing. Injectors remained in place for an additional 1 min to allow diffusion away from the injector, and then the stylets were replaced. Intracranial infusions produced a transient reduction in activity in some rats. Therefore, rats were allowed to recover to normal activity (maximum of 3 min) before being placed into the chambers for testing.
2.4 Behavioral assays
2.4.1. Cocaine self-administration training
After at least 5 days of recovery from surgery, training began during daily, 2-h sessions that took place during the rats’ dark cycle. To facilitate acquisition [21], rats were initially restricted to 15–20 g of food/day beginning 2 days before training. The operant conditioning chambers were equipped with two levers mounted on the front wall (an ‘active’ and an ‘inactive’ lever), a cue light above the active lever, a tone generator, and a house light mounted on the center of the back wall. Each chamber was housed in a larger, sound-attenuating chamber. Completion of the schedule of reinforcement on the active lever simultaneously activated the house light, illuminated the cue light above the active lever, and activated a tone. After 1 s, a 6-s cocaine infusion (0.75 mg/kg/0.1 ml, IV) was delivered. The infusion pump, cue light and tone were then inactivated simultaneously and the house light remained on signaling a 20-s timeout period, during which active lever presses were recorded but produced no consequences. Based on their individual self-administration performance, rats progressed from a fixed ratio (FR) 1 schedule to a variable ratio (VR) 2, VR 3, and finally VR 5 schedule of reinforcement. The rationale for this progression of reinforcement is that it results in greater resistance to extinction and subsequently greater reinstatement responding during tests for reinstatement [1]. Rats remained food restricted until they achieved 14 infusions/session for two consecutive days on a VR 3 schedule of reinforcement. Thereafter, rats were given access to food ad libitum in the home cage throughout the remainder of the experiment. Rats received a minimum of 20 days of self-administration training and were maintained on the VR 5 schedule with free food access for at least the last 5 days of training prior to testing. Responses on the inactive lever were recorded but were not reinforced.
2.4.2. Cocaine self-administration test phase (7-OH-DPAT experiment only)
Rats were required to reach a stability criterion on the VR 5 schedule of less than 15% variability of infusions/session for 3 consecutive days with no upward or downward trends. Each rat then received two self-administration tests: one following intra-CeA infusion of vehicle, and one following infusion of their assigned dose of 7-OH-DPAT, with order of test condition counterbalanced. Cocaine was available on a VR 5 schedule during the 1-h test sessions and at least 3 self-administration sessions intervened between the test days to re-establish a stable baseline self-administration rate. Following their second test, rats were given three more self-administration sessions prior to the start of extinction training to ensure stabilized operant responding prior to extinction training.
2.4.3. Extinction phase
Beginning the day after self-administration training finished, rats underwent 1-h daily extinction sessions during which they were placed back into the chambers and active and inactive lever presses were recorded but had no consequences. Extinction training continued until animals reached a criterion of either < 20 responses/h or a decrease of 80% of initial response rate during extinction.
2.4.4. Cue and cocaine reinstatement test phases
For cue reinstatement, the same stimulus complex previously paired with cocaine during self-administration training was delivered response-contingently on an FR 1 schedule across the 1-h test session as this reinforcement schedule during reinstatement testing produces robust responding [1]. A non-contingent cue presentation was delivered to rats that did not receive a cue within the first 5 min.
For cocaine reinstatement, a cocaine priming injection (10 mg/kg, IP) was administered immediately before the rat was placed into the chamber. Lever presses were recorded for a 1-h test session, but had no consequences. To control for reinstatement that might have resulted from injection stress, rats were given an IP injection of saline on the extinction day immediately preceding the cocaine reinstatement test (i.e., baseline).
Rats were tested twice for cue reinstatement and twice for cocaine reinstatement, with the order of test type counterbalanced and separated by a minimum of 4 additional extinction sessions. For each test type, rats were pre-treated with vehicle on one day and their assigned dose of drug on the other test day, counterbalanced for order, and separated by at least 4 days of extinction to re-establish baseline responding.
2.5. Experimental design
In separate experiments, rats received bilateral CeA infusions of either 7-OH-DPAT (0.25, 0.5, 1.0, 2.0, 4.0 μg/0.2 μl/side) or SKF-38393 (1.0, 2.0, 4.0 μg/0.3 μl/side). Final n/group ranged from 6 – 9 and is specified for each group in the figure captions. The 0.25 μg dose of 7-OH-DPAT was used in tests for self-administration but was not included in tests for cue and cocaine-primed reinstatement. The between subjects assignment to dosage group for each drug type was counterbalanced for previous cocaine intake during self-administration training. For each rat, the dose assignment was held constant for both cue and cocaine reinstatement testing; however, in the 7-OH-DPAT experiment, dose assignment for self-administration varied from that used during reinstatement and re-assignment to dosage groups was balanced to the extent possible across subsequent reinstatement groups. Reinstatement was operationally defined as a doubling of active lever presses relative to baseline and at least 10 active lever presses on either the vehicle or drug pretreatment test day. Some rats exhibited cue reinstatement but not cocaine reinstatement, and vice versa. These rats were excluded only from the analyses of the test type for which they failed to exhibit reinstatement.
2.6. Histology
Following completion of each experiment, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, IP) and decapitated. Their brains were removed and subsequently sectioned coronally at a thickness of 40 μm. Sections were thaw-mounted onto gelatin-coated slides, stained with cresyl violet, and examined under a microscope by an observer unaware of group assignment in order to examine cannulae placements and excessive brain damage.
2.7. Data analysis
Given the high degree of variability in vehicle responding at each dose of each agonists used, and given that we were primarily interested in within subject effects of vehicle vs. agonist responding at each dose of 7-OH-DPAT and SKF-38393, difference scores between vehicle and agonist responding at each dose of each agonist was calculated. We also analyzed the raw data and found the same outcome; therefore, the data analyses and graphs presented in the manuscript are change from baseline responding given that this is the clearest way to demonstrate the effects. In each experiment, difference scores were calculated by subtracting baseline from test day values. For self-administration, baseline values were the mean number of infusions earned during the sessions preceding the test days. For reinstatement, baseline values were the mean response rate during the extinction sessions preceding the reinstatement tests. These difference scores were analyzed separately for active and inactive lever presses. Difference scores for each test type from each experiment were analyzed using separate ANOVAs with the vehicle and drug test conditions as a within subject factor and dosage group as a between subjects factor. Significant interactions were followed up with post-hoc Tukey’s HSD tests (p<.05). For the 7-OH-DPAT reinstatement tests, due to graded dose-response functions and strong main effects masking an interaction, planned comparison, paired-sample t-tests with Bonferroni correction were used to make the most relevant comparisons between vehicle and drug pretreatment test days for each dosage group.
3. Results
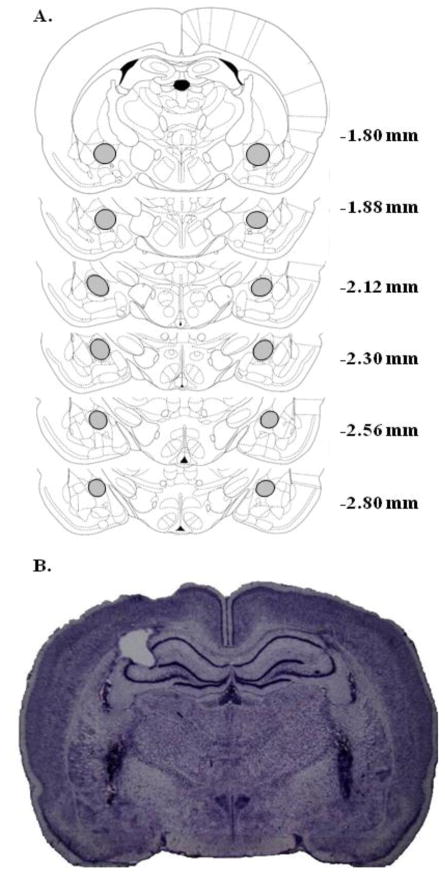
All statistics are reported as mean ± SEM, and all significant effects are presented. Only those rats meeting reinstatement criteria and with both injector tips located in the CeA (see Figure 1) were included using the following criteria for correct placements: the anterior-posterior range of − 1.80 to − 2.80 mm posterior to bregma, the medial-lateral range of approximately + 3.60 to 4.60 mm lateral to the midsagittal suture, and the dorsal-ventral range of approximately −7.50 to −8.50 mm ventral to the flat skull surface (Figure 1; Paxinos and Watson [79]). Average daily number of cocaine infusions obtained during self-administration training did not differ across dosage groups and is shown in Table 1. For the cohort of rats that underwent testing with 7-OH-DPAT (Experiment 1), self-administration took place between 30 – 34 days including the 7-OH-DPAT tests during self-administration. The cohort of rats that were tested with SKF-38393 (Experiment 2) did not receive intra-CeA SKF-38393 infusions during self-administration, and their training lasted for three weeks. Length of extinction training varied for both cohorts of rats, depending on the time it took to stabilize responding both prior to and between tests. Extinction training lasted between 34 – 42 days for both experiments. No significant effects were detected for inactive lever pressing in any of the experiments during self-administration or reinstatement test days (data not shown).
Figure 1.
Coronal sections representing the cannula placement criteria for inclusion in the analyses (A; adapted from Paxinos and Watson, 1998) and representative coronal section stained with cresyl violet demonstrating bilateral cannula placements in the CeA (B). The section was from a rat that was infused with the 4.0 μg/side dose of 7-OH-DPAT. Only rats with bilateral cannula tip placements within the gray highlighted boundaries of the CeA were included in the analyses; rats with either one or both tips outside of this area were excluded.
Table 1.
Reinforcers and response rates (mean ± SEM) during self-administration and extinction
| Drug (μg/side) | Total infusions obtained | Infusions/h on last day of self-administration | Active lever presses/h on last day of self-administration | Active lever presses/h on first day of extinction |
|---|---|---|---|---|
| 7-OH-DPAT | ||||
| 0.5 | 678.1 ± 42.5 | 15.1 ± 1.8 | 75.9 ± 8.7 | 114.3 ± 15.3 |
| 1.0 | 686.8 ± 43.3 | 15.4 ± 1.2 | 78.8 ± 6.3 | 126.7 ± 15.7 |
| 2.0 | 711.4 ± 40.1 | 16.3 ± 1.2 | 82.0 ± 6.9 | 100.5 ± 17.2 |
| 4.0 | 700.1 ± 38.9 | 16.7 ± 1.4 | 83.6 ± 7.1 | 106.0 ± 21.7 |
| SKF-38393 | ||||
| 1.0 | 567.9 ± 44.4 | 13.7 ± 1.8 | 68.9 ± 8.8 | 89.3 ± 11.4 |
| 2.0 | 552.1 ± 47.1 | 12.9 ± 1.5 | 64.4 ± 8.1 | 79.3 ± 13.6 |
| 4.0 | 589.4 ± 56.1 | 12.8 ± 1.9 | 65.1 ± 8.5 | 99.1 ± 12.2 |
Each cocaine infusion contained 0.75 mg/kg/0.1 ml.
3.1.1. Experiment 1a: Effects of 7-OH-DPAT on cocaine self-administration
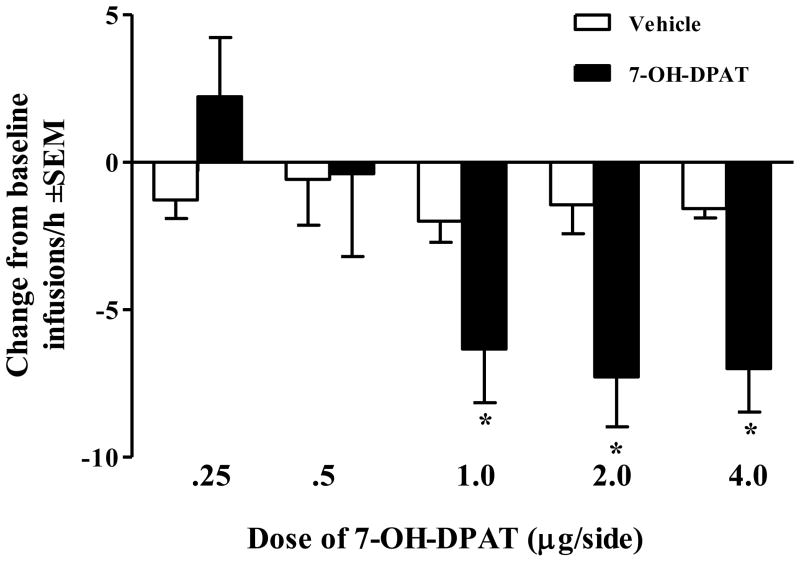
Infusion of 7-OH-DPAT into the CeA produced a significant Drug × Dose interaction (F(4,33) = 3.60, p<.05) on infusions/h. As shown in Figure 2, post-hoc analyses revealed that intra-CeA infusions of 7-OH-DPAT dose-dependently decreased cocaine self-administration at the 3 highest doses tested relative to vehicle infusion (p<.05, Tukey’s HSD). Average baseline self-administration infusions for each 7-OH-DPAT dosage group are provided in the caption of Figure 2.
Figure 2.
Effects of 7-OH-DPAT infused into the CeA on cocaine self-administration, expressed as a difference from baseline self-administration infusions/h. Rats were tested twice, pretreated once with intra-CeA vehicle (white bar) and once with their assigned intra-CeA dose of 7-OH-DPAT (black bar), counterbalanced for order. During the 1-h self-administration test, rats received response-contingent infusions of cocaine (0.75 mg/kg/0.1ml) paired with the light/tone stimulus complex on a VR5 schedule of reinforcement (n per dose = 7, 6, 9, 9, and 7, respectively) and the mean (±SEM) infusions/h on the preceding 1st h of the baseline self-administration sessions were 16.3 ± 2.6, 17.3 ± 2.0, 18.0 ± 1.8, 19.8 ± 2.8, and 16.4 ± 1.4 for each dosage group, respectively. At least 3 days of stable self-administration intervened these tests. Asterisk (*) represents a difference from vehicle pretreatment (p<.05, Tukey’s HSD).
3.1.2. Experiment 1b: Effects of 7-OH-DPAT on cue reinstatement and cocaine reinstatement
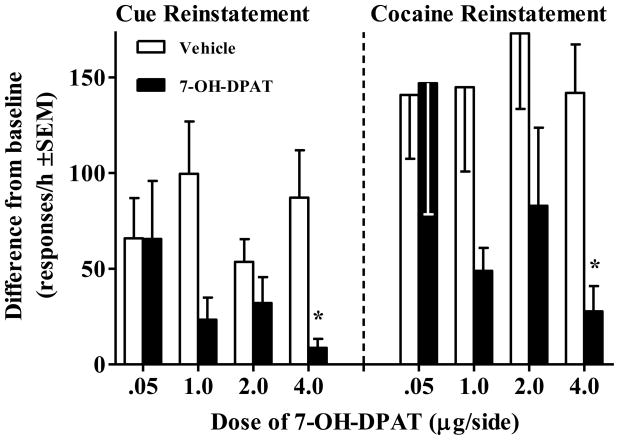
A total of 9 rats failed to demonstrate cue reinstatement and were therefore excluded from the cue reinstatement analysis. The overall ANOVA failed to reveal a Drug × Dose interaction for cue reinstatement (see Figure 3). However, planned comparisons made for each group between response rates following 7-OH-DPAT versus vehicle revealed a significant decrease in cocaine-seeking behavior at the 4.0 μg/side dose [t(6) = 3.48, p<.0125, Bonferroni correction]. There was also a trend toward a decrease in cocaine-seeking behavior at the 1 μg dose (p=0.12). Average baseline responses for the extinction days immediately preceding the cue reinstatement tests did not differ across 7-OH-DPAT dosage groups (see the caption for Figure 3).
Figure 3.
Effects of 7-OH-DPAT infused into the CeA on cue and cocaine reinstatement, expressed as a difference from baseline extinction responses/h. For each type of reinstatement rats were tested twice, pretreated once with intra-CeA infusion of vehicle (white bar) and once with intra-CeA infusion of 7-OH-DPAT (black bar), counterbalanced for order. For cue reinstatement, rats received response-contingent cue presentations on an FR1 schedule of reinforcement (n per dose = 8, 7, 7, and 7, respectively). The mean (±SEM) responses/h on the preceding extinction baseline sessions were 10.4 ± 2.6, 11.3 ± 2.8, 8.9 ± 2.3, and 7.5 ± 2.9 for each dosage group, respectively. For cocaine reinstatement, rats received a cocaine priming injection (10 mg/kg, IP) immediately prior to testing (n per dose = 7, 8, 8, and 8, respectively). Rats received a saline injection immediately prior to the preceding extinction baseline session. The mean (±SEM) responses/h during baseline sessions for each dosage group was 10.0 ± 2.3, 8.6 ± 2.9, 8.2 ± 1.9, and 7.8 ± 2.3, respectively. Asterisk (*) represents a difference from vehicle pretreatment (paired t-test, p<.0125, Bonferroni correction).
A total of 7 rats failed to demonstrate cocaine-primed reinstatement when pre-treated with an intra-CeA vehicle infusion and were therefore excluded from the cocaine reinstatement analysis. The overall ANOVA also failed to reveal a Drug × Dose interaction during tests of cocaine-primed reinstatement (see Figure 3). However, planned comparisons made for each group between response rates following 7-OH-DPAT versus vehicle revealed a significant decrease in cocaine-seeking behavior at the 4.0 μg/side dose [t(7) = 3.81, p<.0125, Bonferroni correction]. There was also a strong trend toward a decrease in cocaine-seeking behavior at the 1 μg dose (p=0.05). Average baseline responses for the extinction days immediately preceding the cocaine reinstatement tests did not differ across 7-OH-DPAT dosage groups (see the caption of Figure 3).
3.2. Experiment 2: Effects of SKF-38393 on cue and cocaine reinstatement
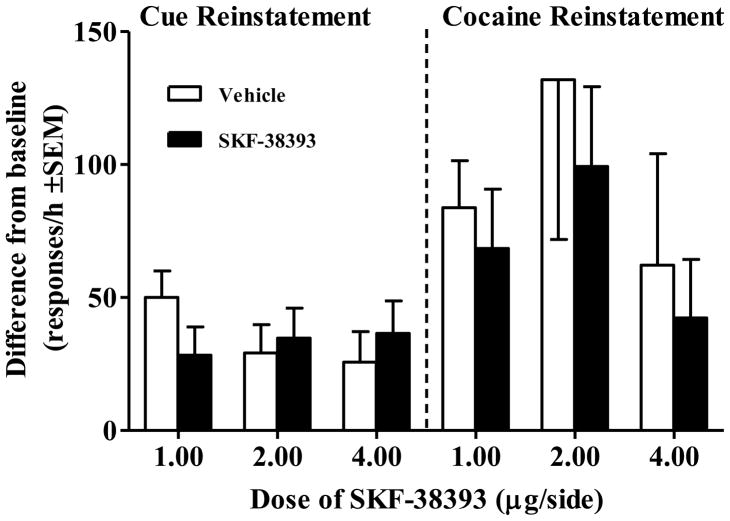
A total of 3 rats failed to demonstrate cue reinstatement and 2 rats failed to demonstrate cocaine-primed reinstatement and were therefore excluded from each respective analysis. Cue and cocaine-primed reinstatement results are summarized in Figure 4. SKF-38393 failed to produce significant differences at any dose tested in either cue- or cocaine-elicited reinstatement. Average baseline responses for the extinction days immediately preceding either the cue reinstatement or cocaine-primed reinstatement tests did not differ across SKF-38393 dosage groups (see the caption of Figure 4).
Figure 4.
Effects of SKF-38393 infused into the CeA on cue and cocaine reinstatement, expressed as a difference from extinction baseline responses/h. For each type of reinstatement rats were tested twice, pretreated once with intra-CeA infusion of vehicle (white bar) and once with intra-CeA infusion of SKF-38393 (black bar), counterbalanced for order. For cue reinstatement, rats received response-contingent cue presentations on an FR1 schedule of reinforcement (n per dose = 8, 7, and 6, respectively). The mean (±SEM) responses/h during extinction baseline for each dosage group were 12.8 ± 1.4, 13.0 ± 1.9, and 12.2 ± 1.5, respectively. For cocaine reinstatement, rats received a cocaine priming injection (10 mg/kg, IP) immediately prior to testing (n = 8 per group). Rats received a saline injection immediately prior to the preceding extinction baseline session. The mean (±SEM) responses/h during baseline for each dosage group were 12.7 ± 1.4, 12.1 ± 1.9, and 10.9 ± 2.7, respectively.
3.3. Histological considerations
Some gliosis was observed near the cannulae tracts within the CeA, but there was no excessive damage with either agonist. Furthemore it is unlikely that multiple microinjections alone caused a change in amygdala function since no changes in behavior were observed with SKF and low doses of 7-OH-DPAT.
Discussion
The present findings suggest that increasing stimulation of D2Rs within the CeA is sufficient to modulate cocaine self-administration as well as cue- and cocaine-elicited reinstatement of extinguished cocaine-seeking behavior. In contrast, increasing D1R stimulation within the CeA is not sufficient to modulate cue or cocaine-elicited drug-seeking behavior.
Contrary to our prediction that increased stimulation of D2Rs would enhance reinstatement effects, 7-OH-DPAT attenuated both cue- and cocaine-elicited reinstatement, as well as cocaine self-administration. The effect of intra-CeA 7-OH-DPAT on cocaine self-administration is not surprising given that similar effects have been observed following systemic administration [14, 16, 18]. Therefore, the CeA may represent a centrally-located site of action of peripherally administered D2-like receptor agonists. Additionally, consistent with the idea that intra-CeA 7-OH-DPAT may be modulating the rewarding effects of cocaine, it has been shown that administration of amphetamine into the CeA supports conditioned place preference, suggesting this brain region plays a role in initiating the rewarding effects of psychostimulants [76]. 7-OH-DPAT shifts the cocaine self-administration curve to the left, such that animals respond as though they are receiving a higher dose of cocaine [16]. The dose of cocaine used during self-administration tests in the present study (i.e., 0.75 mg/kg) lies on the descending limb of cocaine dose-response functions; therefore, a shift to the left at this dose would be reflected by a decrease in responding for cocaine, as was observed here. This pattern of responding has been interpreted as reward enhancement [16, 78], suggesting that intra-CeA infusions of 7-OH-DPAT produced a cocaine-like effect. In further support of this conclusion, Hurd and Ponten [46] demonstrated that perfusion of dopamine (45 nM) directly into the BlA and CeA reduces self-administration of a high dose of cocaine (1.5 mg/kg/infusion), an effect that they interpret as an increase in the magnitude of cocaine reinforcement.
Alternatively, given our observed reduction in cocaine-primed reinstatement, 7-OH-DPAT effects on cocaine self-administration may instead reflect a generalized decrease in motivation. In the absence of a full examination of intra-CeA infusions of 7-OH-DPAT across a range of cocaine doses, we can only speculate that 7-OH-DPAT reduces cocaine self-administration via enhancement of cocaine reward while it attenuates cocaine-primed reinstatement via a decrease in the incentive motivational effects of cocaine priming. These opposite effects of intra-CeA infusions of 7-OH-DPAT on cocaine self-administration versus cocaine-primed reinstatement likely reflect dissociable mechanisms underlying reinforcement (as measured in tests for cocaine self-administration) versus motivation (as measured in tests for reinstatement of extinguished cocaine-seeking behavior; see Pentkowski et al. [80] for review). One important indicator that intra-CeA infusion of 7-OH-DPAT did not produce non-specific alterations in lever responding is the lack of differences in inactive lever presses on test days across groups. Furthermore, although we did not test the effects of the drugs in the absence of a reinstating stimulus, previous studies examining intra-CeA microinjection of 7-OH-DPAT, as well as SKF-38393, have failed to observe effects of these drugs on locomotor behavior [43, 44, 101] or sucrose consumption [43] using a similar dose range, therefore suggesting that it is unlikely these drugs alter motor responses or general motivation. Furthermore, intra-amygdala infusion of 7-OH-DPAT following overtraining of a conditioned stimulus-unconditioned stimulus relationship has no effect on expression of subsequent conditioned approach behavior [81], suggesting 7-OH-DPAT effects are not due to memory impairment of drug-cue associations.
In light of previous microdialysis research demonstrating that increases in amygdala dopamine correspond with time-dependent increases in cocaine-seeking behavior [94], it is somewhat puzzling that infusion of a dopamine agonist into the CeA would attenuate, rather than facilitate, reinstatement. Although the effective high dose of 7-OH-DPAT used in the present study should result in activation of both pre- and post-synaptically located D2-like receptors, it is possible that pre-synaptic autoreceptor stimulation attenuated dopamine release elicited by the cocaine prime and/or cues, thereby decreasing cocaine-seeking behavior. Also, it is possible that the agonist effect could be a result of an increase in the ratio of D3 to D2 receptor stimulation by 7-OH-DPAT relative to endogenous dopamine, with D3 receptor stimulation opposing the increase in cocaine-seeking behavior associated with D2 receptor stimulation [35]. Furthermore, it is possible that dopamine in the amygdala is not the mechanism underlying the time-dependent increases in cocaine-seeking behavior observed previously despite corresponding changes in these measures [94]. Alternatively, the correlation between increased dopamine within the amygdala and increased cocaine-seeking behavior could be driven by dopamine levels in the BlA specifically, and not by levels in the CeA. Finally, it is possible that some degree of D1R and D2R co-stimulation within the amygdala is necessary to express robust cocaine-seeking behavior (see discussion below).
The CeA is also thought to play a role in conditioned approach behavior [77], and may amplify reward signals resulting in greater behavioral expression of reward-related behavior [84]. Hitchcott and colleagues demonstrated that dopaminergic mechanisms within the amygdala contribute to the acquisition and expression of stimulus-reward associations [43, 81]. Expression of these associations can be measured via an approach response toward a conditioned stimulus (CS) that has previously been paired with a rewarding stimulus (i.e., unconditioned stimulus, US), or via instrumental responding by which the CS is presented response-contingently. Infusion of 7-OH-DPAT into the CeA dose-dependently attenuates the expression of a conditioned approach response to a CS previously associated with sucrose, but does not have an effect on instrumental responding for the CS [44]. Although conditioned approach to the light/tone cues was not explicitly measured in the present study, it likely contributed to initiation of cocaine-seeking behavior. Interestingly, our findings seem at odds with Hitchcott and Phillips [44] given that we found that 7-OH-DPAT infused into the CeA was effective in decreasing CS (i.e., cue)-elicited lever pressing. This discrepancy might be due to differences in the unconditioned stimuli used in the two studies (i.e., cocaine versus sucrose), suggesting that 7-OH-DPAT may selectively attenuate the conditioned reinforcing effects of drug-paired cues. Furthermore, Kruzich and See [53], using tetrodotoxin inactivation of the CeA, demonstrated that this region is involved in the expression of cue-induced reinstatement of cocaine-seeking behavior. The present results support this latter finding, and suggest that D2R stimulation within the CeA may specifically blunt the expression of drug-seeking behavior.
7-OH-DPAT has pharmacological actions at both D2 and D3 receptors [59]; therefore, it is unclear which specific receptor subtype underlies the behavioral effects observed in the present study. The prevalence of D2-like receptors within the CeA may actually be best represented as a predominance of D3 receptors in light of finding that the highest concentration of amygdala D3 receptors lies in the CeA coupled with the finding that there are generally low levels of D2 receptor mRNA found within this subregion [64, 71]. The selective D3 antagonist, SB-277011, blocks the primary rewarding effects of cocaine, as well as cocaine-seeking behavior elicited by a cocaine prime; however, selective D3 receptor modulation alone does not appear to affect cocaine intake on continuous reinforcement schedules [37, 96]. Furthermore, a number of studies have demonstrated that D3 antagonism, as well as D3 partial agonism, decreases cue- and cocaine-elicited cocaine seeking behavior [20, 30, 38, 39, 82, 96], as well as cocaine cue-conditioned locomotion [57]. This latter effect was accompanied by an increase in immediate early gene expression within the amygdala, although a dissociation was not made between the CeA and BlA [57]. Additionally, Gal and Gyertyan [38] demonstrated that a D2-preferring antagonist, as well as a D3 partial agonist/D2 antagonist, was effective in attenuating cue-induced cocaine-seeking behavior, suggesting that D2 and D3 receptor subtypes may both play an important, and perhaps complementary, role in mediating incentive motivational effects of drug-paired stimuli. Taken together, these findings suggest a complex pattern of changes in cocaine-seeking behavior via altered tonic stimulation of D2-like receptors that may be explained by region specificity and/or whether the predominating effect involves D2 or D3 receptors.
Based on our previous research demonstrating that the D1 antagonist SCH-23390 infused into the CeA attenuates cue and cocaine-primed reinstatement [2], we predicted that the D1 agonist SKF-38393 would have the opposite effect of enhancing both types of reinstatement similar to the findings of Mashhoon et al. [66] who reported opposite effects of a D1R agonist and antagonist infused into BlA. Even though SKF-38393 does not alter reinstatement of cocaine-seeking behavior when administered systemically [4], we expected that localized infusions into the CeA would enhance reinstatement similar to the effects of D1 agonist infusions into the nucleus accumbens (NAc) shell [6, 87, 88]. Indeed, the D1 agonist SKF-81297 fails to reliably reinstate extinguished drug-seeking behavior following systemic administration [4, 49, 52], whereas infusion into NAc shell reinstates cocaine-seeking behavior [6, 87, 88]. Given that D1Rs exhibit region-specific functional selectivity, we chose to use SKF-38393 for this study because it has high intrinsic activity at D1Rs in the amygdala [95] even though it is characterized as a partial agonist (i.e., low intrinsic activity) at the more typical adenylate cyclase-linked D1Rs [58]. The range of intra-CeA SKF-38393 doses used has been shown to modulate morphine- and cocaine-conditioned place preference when infused into either the amygdala or the prefrontal cortex [9, 101]. Therefore, it seems unlikely that the failure to observe effects with SKF-38393 was due to inappropriate choice of doses. Furthermore, we have tested effects of a single dose (1 μg/side) of SKF-81297, a high efficacy D1 agonist in adenylate cyclase assays that has been shown to reinstate drug-seeking behavior when infused into the NAc shell [87], and found that it also fails to alter cue or cocaine-primed reinstatement when infused into the CeA (unpublished observations). Thus, the present finding that stimulation of D1 receptors in the CeA had no effect on cue or cocaine-primed reinstatement whereas blockade attenuates both forms of reinstatement [2] suggests that stimulation of these receptors may be necessary but not sufficient for expression of the behavior. It is also important to note that the relatively greater concentration of D2Rs compared to D1Rs within the CeA may additionally explain why D1 stimulation alone produces no effects whereas D2 stimulation reduces cocaine-seeking [89]. In addition, our previous findings demonstrating the effects D1R blockade in the CeA using SCH-23390 may have been due to the non-specific effects that SCH-23390 exerts on other G protein-coupled, inwardly rectifying potassium channels [55] as opposed to D1R antagonism.
The close proximity of the BlA and CeA nuclei warrants caution when interpreting pharmacological manipulations aimed specifically at these regions. It is important to note that the agonists used in the present study show region-specific effects at the doses used, and therefore, it is unlikely that our effects are due to spread to a neighboring region. Mashhoon et al. [66] recently demonstrated that D1 receptor activation within the rBlA using SKF-81297 increased cocaine-seeking behavior; therefore, if the D1 agonist used in the present study had diffused into the BlA, we would have expected to see similar positive effects. Furthermore, Hitchcott and Phillips [44] infused 7-OH-DPAT into both the CeA and BlA and found dissociable involvement of these nuclei in the expression of Pavlovian and instrumental-related behaviors, suggesting that this drug remains isolated to the region in which it is infused. Finally, it is important again to note that the relatively low distribution of D2-like receptors within the BlA [89] mitigates the idea that 7-OH-DPAT effects were due to spread to this region.
Previous research suggests that D1Rs sometimes play a permissive role in mediating behaviors involving D2Rs and/or that stimulation of D1 and D2Rs can interact synergistically in dopamine-mediated behavior [25]. For instance, D2 agonists alone produce mild stereotypy that is intensified by D1 agonists, even though D1 agonists do not produce stereotypy when given alone [67, 56, 74]. Furthermore, sniffing and chewing behaviors produced by D2 agonists can be reversed by either co-administration of a D1 or D2R antagonist [72]. In regard to cocaine-seeking behavior, intra-NAc infusion of either a D1 or D2/D3 agonist reinstates cocaine-seeking behavior, and the effects of both agonists are reversed by either D1R- or D2R-selective antagonists [6, 87, 88]. The finding that each antagonist attenuated reinstatement induced by either agonist suggests that reinstatement of drug-seeking behavior requires dopaminergic activity at both D1-like and D2-like receptor subtypes in the NAc [6]. In order to determine if D1 and D2Rs play a similar permissive/cooperative role in modulating cocaine-seeking behavior within the CeA, it will be interesting for future studies to examine whether 7-OH-DPAT effects cans be reversed by a D1R antagonist. Furthermore, it would be important to examine the effects of co-administration of sub-threshold doses of D1R and DR2 agonists to see if similar reduction of reinstatement observed in the present study from the highest dose of 7-OH-DPAT alone could be reproduced.
In conclusion, our previous findings [2], in combination with the present findings, suggest that both the D1-like and D2-like dopamine receptor families in the CeA play a role in modulating incentive motivation for cocaine, perhaps by enhancing behavior expression related to motivation. Interestingly, despite producing opposite effects on cocaine self-administration, the D1 antagonist used in our previous study and the D2 agonist used in the present study produced similar effects on cocaine-seeking behavior. While stimulation of D2Rs in the CeA is sufficient to attenuate cue and cocaine-primed reinstatement, stimulation of D1Rs in this region appears necessary but not sufficient for modulating these behaviors. Future studies will be needed to better characterize which D2-like receptor subtypes mediate the effects of the intra-CeA infusions of 7-OH-DPAT, as well as the effects of D2-like receptor manipulations on the acquisition/consolidation of cocaine-cue associations. Further elucidating the dopaminergic mechanisms involved in cocaine reinforcement and reinstatement may aid in developing pharmacological treatments for cocaine addiction.
Acknowledgments
We thank Jenny Browning and Arturo Zavala for their expert technical assistance. This research was supported by NIDA grants DA11064 and DA06095.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acosta JI, Thiel KJ, Sanabria F, Browning JR, Neisewander JL. Effect of schedule of reinforcement on cue-elicited reinstatement of cocaine-seeking behavior. Behav Pharmacol. 2008;19:129–36. doi: 10.1097/FBP.0b013e3282f62c89. [DOI] [PubMed] [Google Scholar]
- 2.Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31:363–74. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- 3.Alleweireldt AT, Kirschner KF, Blake CB, Neisewander JL. D1-receptor drugs and cocaine-seeking behavior: investigation of receptor mediation and behavioral disruption in rats. Psychopharmacology (Berl) 2003;168:109–17. doi: 10.1007/s00213-002-1305-x. [DOI] [PubMed] [Google Scholar]
- 4.Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2002;159:284–93. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- 5.Asan E. Ultrastructural features of tyrosine-hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell Tissue Res. 1997;288:449–69. doi: 10.1007/s004410050832. [DOI] [PubMed] [Google Scholar]
- 6.Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- 7.Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47 (Suppl 1):256–73. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience. 2006;137:699–706. doi: 10.1016/j.neuroscience.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 9.Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–82. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown EE, Fibiger HC. Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharmacology (Berl) 1993;113:123–30. doi: 10.1007/BF02244344. [DOI] [PubMed] [Google Scholar]
- 11.Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112–21. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns LH, Robbins TW, Everitt BJ. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of D-amphetamine. Behav Brain Res. 1993;55:167–83. doi: 10.1016/0166-4328(93)90113-5. [DOI] [PubMed] [Google Scholar]
- 13.Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- 14.Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–6. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- 15.Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther. 1994;270:209–18. [PubMed] [Google Scholar]
- 16.Caine SB, Koob GF. Pretreatment with the dopamine agonist 7-OH-DPAT shifts the cocaine self-administration dose-effect function to the left under different schedules in the rat. Behav Pharmacol. 1995;6:333–347. [PubMed] [Google Scholar]
- 17.Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P. D3 receptor test in vitro predicts decreased cocaine self-administration in rats. Neuroreport. 1997;8:2373–7. doi: 10.1097/00001756-199707070-00054. [DOI] [PubMed] [Google Scholar]
- 18.Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. J Pharmacol Exp Ther. 1999;291:353–60. [PubMed] [Google Scholar]
- 19.Callahan PM, Bryan SK, Cunningham KA. Discriminative stimulus effects of cocaine: antagonism by dopamine D1 receptor blockade in the amygdala. Pharmacol Biochem Behav. 1995;51:759–66. doi: 10.1016/0091-3057(95)00027-t. [DOI] [PubMed] [Google Scholar]
- 20.Campiani G, Butini S, Trotta F, Fattorusso C, Catalanotti B, Aiello F, et al. Synthesis and pharmacological evaluation of potent and highly selective D3 receptor ligands: inhibition of cocaine-seeking behavior and the role of dopamine D3/D2 receptors. J Med Chem. 2003;46:3822–39. doi: 10.1021/jm0211220. [DOI] [PubMed] [Google Scholar]
- 21.Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. J Pharmacol Exp Ther. 1981;217:241–7. [PubMed] [Google Scholar]
- 22.Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–9. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- 23.Childress A, Ehrman R, McLellan AT, O’Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988;81:74–80. [PubMed] [Google Scholar]
- 24.Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–81. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark D, White FJ. D1 dopamine receptor--the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse. 1987;1:347–88. doi: 10.1002/syn.890010408. [DOI] [PubMed] [Google Scholar]
- 26.Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–15. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- 27.De Vries TJ, Schoffelmeer AN, Binnekade R, Raaso H, Vanderschuren LJ. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- 28.De Vries TJ, Schoffelmeer AN, Binnekade R, Vanderschuren LJ. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology (Berl) 1999;143:254–60. doi: 10.1007/s002130050944. [DOI] [PubMed] [Google Scholar]
- 29.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–43. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 30.Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–38. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- 31.Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–9. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 32.Fallon JH, Ciofi P. Distribution of Monoamines within the Amygdala. In: Aggleton JP, editor. The Amygdala. New York: Wiley-Liss; 1992. pp. 97–114. [Google Scholar]
- 33.Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006;23:2809–13. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology (Berl) 1998;135:151–60. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs RA, Tran-Nguyen LT, Weber SM, Khroyan TV, Neisewander JL. Effects of 7-OH-DPAT on cocaine-seeking behavior and on re-establishment of cocaine self-administration. Pharmacol Biochem Behav. 2002;72:623–32. doi: 10.1016/s0091-3057(02)00731-1. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs RA, Weber SM, Rice HJ, Neisewander JL. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res. 2002;929:15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- 37.Gal K, Gyertyan I. Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Res Bull. 2003;61:595–601. doi: 10.1016/s0361-9230(03)00217-x. [DOI] [PubMed] [Google Scholar]
- 38.Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, et al. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–5. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22:473–9. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- 42.Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology (Berl) 1999;143:102–10. doi: 10.1007/s002130050925. [DOI] [PubMed] [Google Scholar]
- 43.Hitchcott PK, Bonardi CM, Phillips GD. Enhanced stimulus-reward learning by intra-amygdala administration of a D3 dopamine receptor agonist. Psychopharmacology (Berl) 1997;133:240–8. doi: 10.1007/s002130050397. [DOI] [PubMed] [Google Scholar]
- 44.Hitchcott PK, Phillips GD. Double dissociation of the behavioural effects of R(+) 7-OH-DPAT infusions in the central and basolateral amygdala nuclei upon Pavlovian and instrumental conditioned appetitive behaviours. Psychopharmacology (Berl) 1998;140:458–69. doi: 10.1007/s002130050790. [DOI] [PubMed] [Google Scholar]
- 45.Hurd YL, McGregor A, Ponten M. In vivo amygdala dopamine levels modulate cocaine self-administration behaviour in the rat: D1 dopamine receptor involvement. Eur J Neurosci. 1997;9:2541–8. doi: 10.1111/j.1460-9568.1997.tb01683.x. [DOI] [PubMed] [Google Scholar]
- 46.Hurd YL, Ponten M. Cocaine self-administration behavior can be reduced or potentiated by the addition of specific dopamine concentrations in the nucleus accumbens and amygdala using in vivo microdialysis. Behav Brain Res. 2000;116:177–86. doi: 10.1016/s0166-4328(00)00271-0. [DOI] [PubMed] [Google Scholar]
- 47.Jin LQ, Wang HY, Friedman E. Stimulated D(1) dopamine receptors couple to multiple Galpha proteins in different brain regions. J Neurochem. 2001;78:981–90. doi: 10.1046/j.1471-4159.2001.00470.x. [DOI] [PubMed] [Google Scholar]
- 48.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 49.Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–7. [PubMed] [Google Scholar]
- 50.Khroyan TV, Platt DM, Rowlett JK, Spealman RD. Attenuation of relapse to cocaine seeking by dopamine D1 receptor agonists and antagonists in non-human primates. Psychopharmacology (Berl) 2003;168:124–31. doi: 10.1007/s00213-002-1365-y. [DOI] [PubMed] [Google Scholar]
- 51.Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–41. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- 52.Koeltzow TE, Vezina P. Locomotor activity and cocaine-seeking behavior during acquisition and reinstatement of operant self-administration behavior in rats. Behav Brain Res. 2005;160:250–9. doi: 10.1016/j.bbr.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, et al. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuzhikandathil EV, Oxford GS. Classic D1 dopamine receptor antagonist R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzaze pine hydrochloride (SCH23390) directly inhibits G protein-coupled inwardly rectifying potassium channels. Mol Pharmacol. 2002;62:119–26. doi: 10.1124/mol.62.1.119. [DOI] [PubMed] [Google Scholar]
- 56.LaHoste GJ, Marshall JF. Dopamine supersensitivity and D1/D2 synergism are unrelated to changes in striatal receptor density. Synapse. 1992;12:14–26. doi: 10.1002/syn.890120103. [DOI] [PubMed] [Google Scholar]
- 57.Le Foll B, Frances H, Diaz J, Schwartz JC, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–26. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- 58.Leonard SK, Anderson CM, Lachowicz JE, Schulz DW, Kilts CD, Mailman RB. Amygdaloid D1 receptors are not linked to stimulation of adenylate cyclase. Synapse. 2003;50:320–33. doi: 10.1002/syn.10272. [DOI] [PubMed] [Google Scholar]
- 59.Levant B, Bancroft GN, Selkirk CM. In vivo occupancy of D2 dopamine receptors by 7-OH-DPAT. Synapse. 1996;24:60–4. doi: 10.1002/(SICI)1098-2396(199609)24:1<60::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 60.Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, et al. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N, N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A. 1992;89:8155–9. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM, et al. Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci. 2008;28:13248–57. doi: 10.1523/JNEUROSCI.3027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loughlin SE, Fallon JH. Dopaminergic and non-dopaminergic projections to amygdala from substantia nigra and ventral tegmental area. Brain Res. 1983;262:334–8. doi: 10.1016/0006-8993(83)91029-6. [DOI] [PubMed] [Google Scholar]
- 63.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–9. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 64.Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, Watson SJ. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci. 1990;10:2587–600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–82. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- 66.Mashhoon Y, Tsikitas LA, Kantak KM. Dissociable effects of cocaine-seeking behavior following D1 receptor activation and blockade within the caudal and rostral basolateral amygdala in rats. Eur J Neurosci. 2009;29:1641–53. doi: 10.1111/j.1460-9568.2009.06705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mashurano M, Waddington JL. Stereotyped behaviour in response to the selective D-2 dopamine receptor agonist RU 24213 is enhanced by pretreatment with the selective D-1 agonist SK&F 38393. Neuropharmacology. 1986;25:947–9. doi: 10.1016/0028-3908(86)90027-4. [DOI] [PubMed] [Google Scholar]
- 68.McGregor A, Roberts DC. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res. 1993;624:245–52. doi: 10.1016/0006-8993(93)90084-z. [DOI] [PubMed] [Google Scholar]
- 69.Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–48. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- 70.Millan MJ, Newman-Tancredi A, Quentric Y, Cussac D. The “selective” dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors. Psychopharmacology (Berl) 2001;156:58–62. doi: 10.1007/s002130100742. [DOI] [PubMed] [Google Scholar]
- 71.Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci U S A. 1994;91:11271–5. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murray AM, Waddington JL. Further evidence for two directions of D-1:D-2 dopamine receptor interaction revealed concurrently in distinct elements of typical and atypical behaviour: studies with the new enantioselective D-2 agonist LY 163502. Psychopharmacology (Berl) 1989;98:245–50. doi: 10.1007/BF00444699. [DOI] [PubMed] [Google Scholar]
- 73.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nolan EB, Harrison LM, Lahoste GJ, Ruskin DN. Behavioral synergism between D(1) and D(2) dopamine receptors in mice does not depend on gap junctions. Synapse. 2007;61:279–87. doi: 10.1002/syn.20371. [DOI] [PubMed] [Google Scholar]
- 75.O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–31. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 76.O’Dell LE, Sussman AN, Meyer KL, Neisewander JL. Behavioral effects of psychomotor stimulant infusions into amygdaloid nuclei. Neuropsychopharmacology. 1999;20:591–602. doi: 10.1016/S0893-133X(98)00083-9. [DOI] [PubMed] [Google Scholar]
- 77.Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000;12:405–13. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- 78.Parsons LH, Weiss F, Koob GF. Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci. 1998;18:10078–89. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- 80.Pentkowski NS, Acosta JI, Browning JR, Hamilton EC, Neisewander JL. Stimulation of 5-HT(1B) receptors enhances cocaine reinforcement yet reduces cocaine-seeking behavior. Addict Biol. 2009;14:419–30. doi: 10.1111/j.1369-1600.2009.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phillips GD, Hitchcott PK. Blockade of the acquisition, but not expression, of associative learning by pre-session intra-amygdala R(+) 7-OH-DPAT. Psychopharmacology (Berl) 2009;203:161–73. doi: 10.1007/s00213-008-1382-6. [DOI] [PubMed] [Google Scholar]
- 82.Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, et al. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–5. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- 83.Pulvirenti L, Koob GF. Dopamine receptor agonists, partial agonists and psychostimulant addiction. Trends Pharmacol Sci. 1994;15:374–9. doi: 10.1016/0165-6147(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 84.Robledo P, Robbins TW, Everitt BJ. Effects of excitotoxic lesions of the central amygdaloid nucleus on the potentiation of reward-related stimuli by intra-accumbens amphetamine. Behav Neurosci. 1996;110:981–90. doi: 10.1037//0735-7044.110.5.981. [DOI] [PubMed] [Google Scholar]
- 85.Romach MK, Glue P, Kampman K, Kaplan HL, Somer GR, Poole S, et al. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166) Arch Gen Psychiatry. 1999;56:1101–6. doi: 10.1001/archpsyc.56.12.1101. [DOI] [PubMed] [Google Scholar]
- 86.Schenk S, Gittings D. Effects of SCH 23390 and eticlopride on cocaine-seeking produced by cocaine and WIN 35,428 in rats. Psychopharmacology (Berl) 2003;168:118–23. doi: 10.1007/s00213-002-1276-y. [DOI] [PubMed] [Google Scholar]
- 87.Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci. 2006;23:219–28. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- 88.Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006;142:451–61. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 89.Scibilia RJ, Lachowicz JE, Kilts CD. Topographic nonoverlapping distribution of D1 and D2 dopamine receptors in the amygdaloid nuclear complex of the rat brain. Synapse. 1992;11:146–54. doi: 10.1002/syn.890110208. [DOI] [PubMed] [Google Scholar]
- 90.See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–10. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- 91.Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–9. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- 92.Self DW, Karanian DA, Spencer JJ. Effects of the novel D1 dopamine receptor agonist ABT-431 on cocaine self-administration and reinstatement. Ann N Y Acad Sci. 2000;909:133–44. doi: 10.1111/j.1749-6632.2000.tb06679.x. [DOI] [PubMed] [Google Scholar]
- 93.Thomas KL, Arroyo M, Everitt BJ. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. Eur J Neurosci. 2003;17:1964–72. doi: 10.1046/j.1460-9568.2003.02617.x. [DOI] [PubMed] [Google Scholar]
- 94.Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- 95.Undie AS, Weinstock J, Sarau HM, Friedman E. Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J Neurochem. 1994;62:2045–8. doi: 10.1046/j.1471-4159.1994.62052045.x. [DOI] [PubMed] [Google Scholar]
- 96.Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, et al. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology. 2007;32:2238–47. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–6. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weissenborn R, Deroche V, Koob GF, Weiss F. Effects of dopamine agonists and antagonists on cocaine-induced operant responding for a cocaine-associated stimulus. Psychopharmacology (Berl) 1996;126:311–22. doi: 10.1007/BF02247382. [DOI] [PubMed] [Google Scholar]
- 100.Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121:747–57. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
- 101.Zarrindast MR, Rezayof A, Sahraei H, Haeri-Rohani A, Rassouli Y. Involvement of dopamine D1 receptors of the central amygdala on the acquisition and expression of morphine-induced place preference in rat. Brain Res. 2003;965:212–21. doi: 10.1016/s0006-8993(02)04201-4. [DOI] [PubMed] [Google Scholar]
- 102.Zavala AR, Browning JR, Dickey ED, Biswas S, Neisewander JL. Region-specific involvement of AMPA/Kainate receptors in Fos protein expression induced by cocaine-conditioned cues. Eur Neuropsychopharmacol. 2008;18:600–11. doi: 10.1016/j.euroneuro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]