Abstract
BACKGROUND:
The respiratory pattern is often modified or even blocked during flexibility exercises, but little is known about the cardiovascular response to concomitant stretching and the Valsalva maneuver (VM) in healthy subjects.
OBJECTIVES:
This study evaluated the heart rate (HR), systolic blood pressure (SBP), and rate-pressure product (RPP) during and after large and small muscle group flexibility exercises performed simultaneously with the VM.
METHODS:
Asymptomatic volunteers (N = 22) with the following characteristics were recruited: age, 22 ± 3 years; weight, 73 ± 6 kg; height, 175 ± 5 cm; HR at rest, 66 ± 9 BPM; and SBP at rest, 113 ± 10 mmHg. They performed two exercises: four sets of passive static stretching for 30 s of the dorsi-flexion (DF) of the gastrocnemius and the hip flexion (HF) of the ischio-tibialis. The exercises were performed with (V+) or without (V-) the VM in a counterbalanced order. The SBP and HR were measured, and the RPP was calculated before the exercise session, at the end of each set, and during a 30-min post-exercise recovery period.
RESULTS:
The within-group comparisons showed that only the SBP and RPP increased throughout the sets (p<0.05), but no post-exercise hypotension was detected. The between-group comparisons showed that greater SBP increases were related to the VM and to a larger stretched muscle mass. Differences for a given set were identified for the HR (the HFV+ and HFV- values were higher than the DFV+ and DFV- values by approximately 12 BPM), SBP (the HFV+ value was higher than the DFV+ and DFV- values by approximately 12 to 15 mmHg), and RPP (the HFV+ value was higher than the HFV- value by approximately 2000 mmHGxBPM, and the HFV+ value was higher than the DFV+ and DFV- values by approximately 4000 mmHGxBPM).
CONCLUSION:
Both the stretched muscle mass and the VM influence acute cardiovascular responses to multiple-set passive stretching exercise sessions.
Keywords: Flexibility, Cardiovascular physiology, Health, Exercise, Physical fitness
INTRODUCTION
Mechanical stress caused by flexibility training can affect hemodynamic responses.1 It has been shown that stretched muscle fibers activate mechanoreceptors, which elicit cardiovascular adjustments through parasympathetic withdrawal and sympathetic activation.2–6 In this context, previous studies with animal models have demonstrated that the muscle tension produced while stretching increases cardiovascular responses, particularly the heart rate (HR).7 Other studies have demonstrated that small muscle fiber receptors also react to stretching in humans,4,8 with a significant impact on the initial HR acceleration. In addition, sustained contractions of large muscle groups increase the peripheral vascular resistance and therefore influence the cardiac output and blood pressure (BP).9 Considering this accumulated evidence, it is reasonable to hypothesize that the muscle mass affected by a stretching exercise may also be a determinant of cardiovascular responses to flexibility training.
Another issue to be considered is the increase in cardiac preload due to the Valsalva maneuver (VM).10 Depending on the body position, it is not unusual for respiration to be blocked while stretching. The expiratory effort and increase in thoracic pressure during the VM reduce the venous return and cardiac output, which provokes baroreflex responses that can increase the BP.11 The combination of prolonged static contractions and the VM is known to amplify the increase in BP and the pressure load on the heart.12,13 Sedentary or less flexible subjects may perform the VM during stretching exercises due to difficulty in reaching and sustaining extreme ranges of motion. In addition, maintaining an adequate workload position for several seconds may demand sustained static (isometric) contractions of reasonable intensity. Despite these considerations, studies on the acute cardiovascular responses to flexibility training are lacking. Assuming that important levels of static contraction may occur during this kind of exercise, it is possible that cardiovascular responses are great enough to be a concern in exercise programs designed for special populations, such as cardiac patients.
It would be also interesting from a clinical perspective to investigate the potential of flexibility exercises to induce post-exercise hypotension. There is substantial evidence showing that BP decreases following dynamic resistance exercises.14,15 It is also well accepted that cardiovascular responses are influenced by training variables, such as the exercise execution time (or number of repetitions), workload, muscle mass recruited, type of contraction, and respiratory blockage.9,12,15,16 However, studies of cardiovascular post-exercise responses have focused mainly on aerobic and strength training bouts. To the best of our knowledge, no previous research has observed the influence of these variables on BP following flexibility training sessions.
Therefore, the purpose of this study was to evaluate, in young healthy subjects with poor flexibility levels, HR, systolic BP (SBP), and the rate-pressure product (RPP) during and after passive stretching exercises involving different muscle masses performed with and without the VM. We hypothesized that stretching exercises involving larger muscle masses and the VM would induce greater cardiovascular responses as compared to exercises involving smaller muscle masses and avoiding the VM. Additionally, post-exercise hypotension was expected to occur, at least after stretching larger muscle groups.
MATERIALS AND METHODS
Subjects
All of the subjects in this study declared themselves to have “very limited flexibility” and to have not stretched regularly for at least two years. Additionally, they needed to exhibit a low level of flexibility in hip and ankle flexion movements (less than 90° and 30°, respectively).17 The following were additional exclusion criteria: a) use of tobacco or drugs that could affect cardiovascular responses; b) bone, joint, or muscle problems or any clinical condition that could limit exercise performance. A total of 22 healthy male subjects without previous experience in flexibility training volunteered for the study and satisfied the inclusion and exclusion criteria. The subjects had the following characteristics: age, 22±3 years; weight, 73±6 kg; height, 175±5 cm; resting HR, 66±9 BPM; resting SBP, 113±10 mmHg; hip flexion maximal range of motion, 69±9 degrees. The experimental approach had institutional ethical board approval, and all of the subjects signed an informed consent form prior to participation in the study.
Experimental Design
All of the subjects were instructed to avoid caffeine, alcohol, and any kind of physical exercise in the 24 hr prior to the exercise session. The subjects were matched according their flexibility to assure that the exercises represented a similar relative workload. This procedure was adopted due to a possible relationship between flexibility levels and cardiovascular responses. Flexibility was assessed by a universal goniometer, and a poor flexibility level was ratified by means of the cut-off points previously mentioned.
The stretching exercises were performed passively, according to the static method, in four sets of 30 s separated by 15-s intervals. Two movements that aimed to stretch a larger muscle group (the ischio-tibialis muscle) and a smaller muscle group (the gastrocnemius muscle) were selected. The exercises were performed over the maximal range of motion with and without the VM, which was carried out during the last 15 s of the stretching stimulus.
Each subject completed four exercise sessions separated by 48 hrs in a counterbalanced cross-over design: a) hip flexion with the subject lying prone, the knees extended, with the VM (HFV+); b) hip flexion with the subject lying prone, the knees extended, without the VM (HFV-); c) dorsiflexion with the subject lying prone, the knees extended, with the VM (DFV+); d) dorsiflexion with the subject lying prone, the knees extended, without the VM (DFV-). The left and right limbs were stretched alternately in each session (unilateral exercise) to minimize possible effects of the ischio-tibial muscle stretching on the gastrocnemius flexibility (passive resistance of the posterior muscle groups). All exercises were performed with the assistance of an experienced evaluator.
The HR and SBP were assessed at rest and during and after the exercise session (immediately after and every 10 min for 30 min after the exercise session). For the rest assessment, the subjects remained comfortably seated in a quiet environment for 5 min prior to the assessment. The measurements during the exercise were taken at the end of each set. For the post-exercise assessments, the subjects remained seated, relaxed and quiet for 30 min.
Blood Pressure and Heart Rate Measurement
The SBP was measured by a semi-automatic device (HEM-431 CINT OmronTM, Vernon Hills, IL, USA), following published recommendations.18 The HR was assessed beat-by-beat using an R-R recorder (PolarTM S810, Kempele, Finland). A pilot study with 12 healthy, active, low flexibility male volunteers was implemented to determine the reliability of the SBP assessment during exercise. To estimate the test-retest reliability, the same evaluator measured the SBP three times at the end of the same stretching exercise. The intra-class correlation coefficient was considered satisfactory (ICC = 0.81, p = 0.032), and a one-way ANOVA model did not detect differences between the measurements (p = 0.08).
Statistical Analyses
Normality of the data was confirmed by a univariate analysis. Hence, a three-way ANOVA model with repeated measures for the third factor (exercise × VM × temporal comparison) and the Tukey post-hoc test (where indicated) were used to compare the HR, SBP, and RPP at rest, during the exercise session, and during the post-exercise recovery period. For all analyses, significance was set at P ≤ 0.05. The same statistical software was used for all calculations (Statistica 6.0, StatsoftTM, Tulsa, OK, USA).
RESULTS
The statistical power of the within-between-group interaction test in the repeated measures ANOVA model was determined using the GPower version 3.1.2 software (Universität Kiel, Kiel, Germany). The following parameters were used to calculate the statistical power: effect-size = 2.5; type I error probability = 0.05; number of groups = 4; number of measurements = 8; correlation among repeated measures = 0.5; and nonsphericity correction = 1. The actual power for N = 22 was 0.797, which is considered to be acceptable for a type II error.
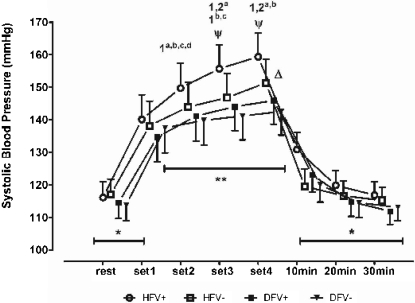
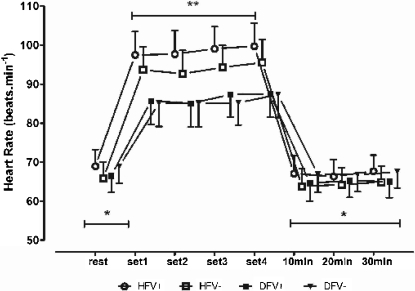
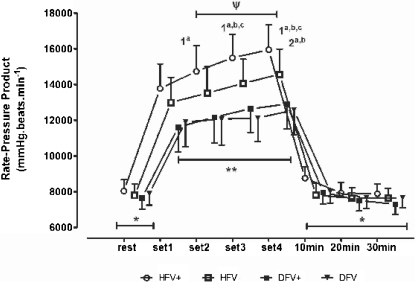
Figures 1 to 3 show the results for the within-group and between-group comparisons. The number of sets done influenced the SBP, but not the HR (P < 0.03). In fact, the SBP increased significantly between the 1st and 2nd sets in all of the stretching protocols, between the 1st and 3rd sets in the HFV+, HFV-, and DFV+ protocols, and between the 2nd and 4th sets in the HFV+ and HFV- protocols. No post-exercise hypotension was observed in the 30 min following the end of the exercise session, regardless of the stretching protocol (P > 0.08). The BP responses influenced the RPP, which increased significantly between the 1st and 2nd sets of exercise in the HFV+ protocol [P < 0.017] and between the 1st and 3rd sets (P < 0.021) in the HFV+, HFV-, and DFV+ protocols. The RPP also increased between the 2nd and 4th sets in the HFV+ and HFV- protocols (P < 0.04). In both the ischio-tibialis and gastrocnemius stretching exercises, there was a clear tendency for the cardiovascular responses to increase when the VM was performed.
Figure 1.
Systolic blood pressure at rest, at the end of each stretching set and 30 min post-exercise. Within-group comparison, -. *, significant differences in all sets (a: HFV+, hip flexion with Valsalva; b: HFV-, hip flexion without Valsalva; c: DFV+, dorsiflexion with Valsalva; d: DFV-, dorsiflexion without Valsalva) (p<.05). The superscripted alpha-numeric codes indicate significant differences for the indicated set of exercise protocols specified by the letters (p<.05). Between-group comparison, -. **, significant differences for HFV+ as compared to DFV+ and DFV- (1st through 4th sets). ψ, a significant difference between HFV+ and HFV- (3rd and 4th sets). Δ:a significant difference between HFV- and DFV- (4th set). The vertical bars denote 0.95 confidence intervals.
Figure 2.
The heart rate at rest, at the end of each stretching set, and 30 min post-exercise. Within-group comparison, -. *, significant differences in all sets (a: HFV+, hip flexion with Valsalva; b: HFV-, hip flexion without Valsalva; c: DFV+, dorsiflexion with Valsalva; d: DFV-, dorsiflexion without Valsalva) (p<.05). Between-group comparison, -. **, significant differences for HFV+ and HFV- as compared to DFV+ and DF- across all sets. The vertical bars denote 0.95 confidence intervals.
Figure 3.
The rate-pressure product at rest, at the end of each stretching set, and 30 min post-exercise. Within-group comparison, -. *, significant differences in all sets (a: HFV+, hip flexion with Valsalva; b: HFV-, hip flexion without Valsalva; c: DFV+, dorsiflexion with Valsalva; d: DFV-, dorsiflexion without Valsalva) (p<.05). The superscripted alpha-numeric codes indicate significant differences in the indicated set of exercise protocols specified by the letters (p<.05). Between-group comparison, -. **, significant differences for HFV+ and HFV- as compared to DFV+ and DFV- (1st through 4th sets). ψ, a significant difference between HFV+ and HFV- (2nd through 4th sets). The vertical bars denote 0.95 confidence intervals.
The between-group comparison allowed for a more specific analysis of the influence of stretched muscle mass and the VM on the cardiovascular responses for an equivalent training volume in each exercise (e.g., the number of sets and time of stimulation). The SBP increased throughout all of the sets for the HFV+ and HFV- protocols, but not for the DFV+ and DFV- protocols. Furthermore, the SBP values assessed for the HFV+ protocol were significantly higher than those obtained for the DFV+ and DFV- protocols. This pattern was observed when comparing the HFV- and the DFV+ protocols and the HFV- and the DFV- protocols. No differences in the HR for any set were detected between the HFV+ and HFV protocols or the DFV+ and DFV-protocols. By contrast, the HR was systematically higher with the hip flexion than with the dorsiflexion, regardless of the inclusion of the VM. As expected from the results obtained for the SBP and HR, the RPP was significantly higher throughout all of the sets for the HFV+ and HFV- protocols as compared to the DFV+ and DFV- protocols. Moreover, the RPP was higher in the 2nd to 4th sets of the HFV+ protocol than in the same sets of the HFV- protocol. Therefore, the RPP was affected by both the VM and the muscle mass stretched.
DISCUSSION
The present study aimed to observe the cardiovascular responses to multiple sets of stretching exercises involving different muscle masses and the VM. The primary findings revealed that there were significant and cumulative increases in the SBP and RPP throughout the passive stretching sets and that such increases were higher when the exercises were performed with the VM. By contrast, the HR remained stable throughout the sets, although it was significantly greater than the resting condition value. No post-exercise hypotension was detected, regardless of the stretching protocol.
The physiological mechanisms underlying cardiovascular responses during flexibility exercises are not completely known. In the present study, the maximal range of motion was reached and held passively, and the stretched muscle was certainly contracted because of the muscle spindle reflex. The sustained muscle tension may have favored the HR and SBP increases due to the activation of muscle and tendon mechanoreceptors.6,19 The sustained static contraction combined with stretching to the maximal range of motion may also have occluded muscle vessels, leading to an increase in the SBP.3,20 Moreover, it has been shown that, in simultaneous muscle stretching and contraction (which is typical of the static stretching method, due to the muscle spindle reflex), type III fibers and metaboreceptor activation may induce vagal inhibition and baroreflex stimulation and contribute to an increase in the overall cardiovascular response.21,22
It has been established that the training level can reduce the hemodynamic response to resistive exercises.23 Moreover, the acute HR response depends on the duration of the muscle contraction, being higher at the end of prolonged exercise than for shorter exercise.24 In the present study, the HR was assessed during exercise sessions performed with a static component sustained for 30 s, with four repetitions. Because the subjects had low flexibility levels, the contraction intensity can be considered as at least moderate, representing a non-negligible muscle workload. Additionally, the HR and BP in each set increased proportionally to the duration of the muscle contraction. This issue evokes the importance of controlling the stimulation duration to prevent undesirable cardiac stress.
The mass of the stretched muscle influenced the HR, regardless of the presence of the VM. The values for the HFV+ and HFV- protocols were systematically higher than those of the DFV+ and DFV- protocols. It is possible to imagine that, in stiff subjects, stretching exercises increase the intramuscular pressure and therefore the peripheral resistance and ejection volume.25 In addition, the execution of the VM may enhance the intra-abdominal and intra-thoracic pressures, which would influence the BP.26 It has been previously demonstrated that static contractions with 40%-60% of the maximal voluntary strength can restrain blood flow and elevate the BP response, particularly if the muscle contraction is prolonged.27
It is well accepted that successive sets of resistive exercises lead to a progressive and cumulative rise in the SBP.28 The same tendency was observed in the present study for multiple sets of stretching exercises, even though they were performed passively. Such a result is not consistent with the findings of previous studies. Cornelius et al.25 did not observe alterations in the SBP during flexibility exercise using proprioceptive neuromuscular facilitation and short static contractions. The respiration was not controlled, however, which raises the possibility that the VM had been performed. Moreover, the static contraction period was considerably shorter than in the present study (4 to 5 s). Gladwell and Coote8 observed the HR and SBP during a sustained passive stretch of the triceps surae for 1 min and found a significant increase in the HR but not in the SBP, while our findings indicate that both the SBP and HR increase. A possible explanation for such disparity lies in type III afferent fibers being sensitive to stretching and contraction and therefore being stimulated by muscle tissue deformation and static tension.
The SBP was influenced by the VM, at least in the exercises that engaged a larger muscle mass. In all of the sets, there were significant differences between the HFV+ and HFV- protocols but not between the DFV+ and DFV- protocols. These results suggest that the influence of respiratory blocking on the cardiovascular response during flexibility exercises is greater when larger muscle groups are stretched. O'Connor et al.26 proposed that the VM combined with static exercises may significantly increase the BP in normotensive subjects. It is also possible that the VM interferes with the transition from rest to exercise, perhaps contributing to the BP increase.29 Our findings are consistent with this idea; the combination of incorporating a larger muscle mass and the VM produced greater increases in the BP, regardless the number of sets done.
The RPP is usually applied to estimate the cardiac workload in aerobic and strength exercises,15 but data on flexibility training are not available. There is accumulated evidence indicating that the muscle mass exercised influences the cardiac workload during resistive training (the larger the muscle mass, the greater the workload imposed on the heart).9,12,15 It has also been shown that in static flexibility training, the RPP may reach levels similar to those observed during dynamic resistive exercises performed with a high intensity and a relatively low number of repetitions.13,30 Our results agree with these findings because the RPP observed in the HFV+ protocol is similar to the values previously reported for resistance exercises performed with 80% of 1 RM.23 Based on these findings, and considering that subjects with coronary disease may have very low ischemic thresholds, further research comparing resistive and flexibility exercises performed by the lower and upper limbs in these patients would be valuable for determining the relative cardiac workloads in different stretching protocols.
Previous research has demonstrated that bouts of resistance exercise may induce post-exercise hypotension,14,16 but the effects of stretching exercise sessions have not been investigated. In the present study, no post-exercise hypotension was observed, regardless of the muscle group stretched. A possible explanation may be the small volume of work involved, considering that each stretching session was only 2 min. The mechanisms underlying the post-exercise hypotension phenomenon are not fully understood. Changes in the neural control of the circulatory system and the release of vasodilatory metabolites are probably connected with the phenomenon, however.31 In this sense, the volume, intensity, and type of exercise may influence the magnitude and duration of the hypotension.9,14–16 Hence, it is possible that the short exercise periods and relatively small muscle masses recruited by the study's stretching protocols represented a small exercise volume that was not capable of inducing the hypotensive response.
The present study has some limitations. The VM intensity was not controlled, so the expiratory pressure was set individually. During the flexibility exercises, the range of motion was also not predefined, and the intensity of the reflex static contractions during the stretching was not quantified. In this context, the measurement of electromyographic activity could provide essential information about muscle activity. The exercise protocol did aim to reproduce actual training practices, however, to increase the potential impact for practitioners who need to make appropriate decisions when designing exercise programs.
In summary, the muscle masses involved and the VM influenced the BP responses during the stretching exercises. By contrast, there was no effect on the HR or BP in the post-exercise recovery period. The number of sets performed had a cumulative incremental effect on the SBP but not on the HR, regardless of the muscle masses involved. The cardiac workload (as reflected by the RPP) was influenced by the interaction of all of these variables. It was higher in situations that combined a larger stretched muscle mass, a higher number of sets, and the VM. Although the present findings were obtained from young, healthy subjects, they may have important practical implications for exercise prescriptions in other training contexts; the question certainly warrants future research. For example, it is conceivable that an increase in cardiovascular responses during flexibility training sessions should be avoided in subjects with a high risk of adverse cardiovascular events, given that flexibility exercises are commonly executed after aerobic or resistive training, when the HR and RPP may be still elevated.
CONCLUSIONS
In conclusion, the muscle mass stretched and the VM during passive static flexibility exercises influence acute cardiovascular responses, particularly the SBP.
ACKNOWLEGEMENTS
This study was partially supported by grants from the Brazilian Council for Research and Technological Development (CNPq) and the Carlos Chagas Foundation for Research Support in Rio de Janeiro (FAPERJ).
REFERENCES
- 1.Rassier DE, Macintosh BR, Herzog W. Length dependence of active force production in skeletal muscle. J Appl Physiol. 1999;86:1445–57. doi: 10.1152/jappl.1999.86.5.1445. [DOI] [PubMed] [Google Scholar]
- 2.Drew RC, Bell MP, White MJ. Modulation of spontaneous baroreflex control of heart rate and indexes of vagal tone by passive calf muscle stretch during graded metaboreflex activation in humans. J Appl Physiol. 2008;104:716–23. doi: 10.1152/japplphysiol.00956.2007. 10.1152/japplphysiol.00956.2007 [DOI] [PubMed] [Google Scholar]
- 3.Fisher JP, Bell MP, White MJ. Cardiovascular responses to human calf muscle stretch during varying levels of muscle metaboreflex activation. Exp Physiol. 2005;90:773–81. doi: 10.1113/expphysiol.2005.030577. 10.1113/expphysiol.2005.030577 [DOI] [PubMed] [Google Scholar]
- 4.Gladwell VF, Fletcher J, Patel N, Elvidge LJ, Lloyd D, Chowdhary S, et al. The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J Physiol. 2005;567:713–21. doi: 10.1113/jphysiol.2005.089243. 10.1113/jphysiol.2005.089243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher WJ, White MJ. Training-induced adaptations in the central command and peripheral reflex componts of the pressor response to isometric exercise of the human tríceps surae. J Physiol. 1999;520:621–8. doi: 10.1111/j.1469-7793.1999.00621.x. 10.1111/j.1469-7793.1999.00621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol. 2005;99:1891–6. doi: 10.1152/japplphysiol.00629.2005. 10.1152/japplphysiol.00629.2005 [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Kawada T, Kamiya A, Takaki H, Shishido T, Sunagawa K, et al. Muscle mechanoreflex augments arterial baroreflex-mediated dynamic sympathetic response to carotid sinus pressure. Am J Physiol Heart Circ Physiol. 2008;295:1081–9. doi: 10.1152/ajpheart.00023.2008. 10.1152/ajpheart.00023.2008 [DOI] [PubMed] [Google Scholar]
- 8.Gladwell VF, Coote JH. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol. 2002;540:1095–102. doi: 10.1113/jphysiol.2001.013486. 10.1113/jphysiol.2001.013486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald J, MacDougall J, Hogben C. The effects of exercising muscle mass on post exercise hypotension. J Hum Hypertens. 2000;14:317–20. doi: 10.1038/sj.jhh.1000999. 10.1038/sj.jhh.1000999 [DOI] [PubMed] [Google Scholar]
- 10.Korner PI, Tonkin AM, Uther JB. Reflex and mechanical circulatory effects of graded Valsalva maneuvers in normal man. J Appl Physiol. 1976;40:434–40. doi: 10.1152/jappl.1976.40.3.434. [DOI] [PubMed] [Google Scholar]
- 11.Khayat RN, Przybylowski T, Meyer KC, Skatrud JB, Morgan BJ. Role of sensory input from the lungs in control of muscle sympathetic nerve activity during and after apnea in humans. J Appl Physiol. 2004;97:635–40. doi: 10.1152/japplphysiol.00241.2004. 10.1152/japplphysiol.00241.2004 [DOI] [PubMed] [Google Scholar]
- 12.MacDougall JD.Blood pressure responses to resistive, static and dynamic exercise Fletcher G F, editor Cardiovascular Response to exercise. Mount Kisco: Futura Publishing Company; 1994155–173. [Google Scholar]
- 13.Longhurst JC, Stebbins CL. The power athlete. Cardiol Clin. 1997;15:413–29. doi: 10.1016/s0733-8651(05)70349-0. 10.1016/S0733-8651(05)70349-0 [DOI] [PubMed] [Google Scholar]
- 14.Polito MD, Farinatti PTV. The effects of muscle mass and number of sets during resistance exercise on post-exercise hypotension. J Strength Cond Res. 2009;23:2351–7. doi: 10.1519/JSC.0b013e3181bb71aa. 10.1519/JSC.0b013e3181bb71aa [DOI] [PubMed] [Google Scholar]
- 15.Simão R, Fleck SJ, Polito M, Monteiro W, Farinatti P. Effects of resistance training intensity, volume, and session format on the postexercise hypotensive response. J Strength Cond Res. 2005;19:853–8. doi: 10.1519/R-16494.1. [DOI] [PubMed] [Google Scholar]
- 16.Jones H, Taylor CE, Lewis NC, George K, Atkinson G. Post-exercise blood pressure reduction is greater following intermittent than continuous exercise and is influenced less by diurnal variation. Chronobiol Int. 2009;26:293–306. doi: 10.1080/07420520902739717. 10.1080/07420520902739717 [DOI] [PubMed] [Google Scholar]
- 17.Kurz T. Island Pond: Stadion; 1994. Stretching Scientifically: a guide to flexibility training 3rd ed. [Google Scholar]
- 18.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals. Part 1: Blood pressure measurement in humans. A statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 19.Drew RC, McIntyre DB, Ring C, White MJ. Local metabolite accumulation augments passive muscle stretch-induced modulation of carotid-cardiac but not carotid-vasomotor baroreflex sensitivity in man. Exp Physiol. 2008;93:1044–57. doi: 10.1113/expphysiol.2008.042234. 10.1113/expphysiol.2008.042234 [DOI] [PubMed] [Google Scholar]
- 20.Higginbotham MB. Cardiac performance during sub maximal and maximal exercise in health persons. Heart Failure. 1988;4:68–76. [Google Scholar]
- 21.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res. 2002;12:429–39. doi: 10.1007/s10286-002-0059-1. 10.1007/s10286-002-0059-1 [DOI] [PubMed] [Google Scholar]
- 22.Baum K, Selle K, Leyk D, Essfeld D. Comparison of blood pressure and heart rate responses to isometric exercise and passive muscle stretch in humans. Eur J Appl Physiol Occup Physiol. 1995;70:240–5. doi: 10.1007/BF00238570. 10.1007/BF00238570 [DOI] [PubMed] [Google Scholar]
- 23.Fleck SL, Dean LS. Resistance-training experience and the pressor response during resistance exercise. J Appl Physiol. 1987;1:116–20. doi: 10.1152/jappl.1987.63.1.116. [DOI] [PubMed] [Google Scholar]
- 24.Benn SJ, McCartney N, McKelvie RS. Circulatory responses to weight lifting, walking, and stair climbing in older males. J Am Geriatr Soc. 1996;44:121–5. doi: 10.1111/j.1532-5415.1996.tb02426.x. [DOI] [PubMed] [Google Scholar]
- 25.Cornelius WL, Jensen RL, Odell ME. Effects of PNF stretching phases on acute arterial blood pressure. Can J Appl Physiol. 1995;20:222–9. doi: 10.1139/h95-016. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor P, Sforzo GA, Frye P. Effect of breathing instruction on blood pressure responses during isometric exercise. Phys Ther. 1989;69:757–61. doi: 10.1093/ptj/69.9.757. [DOI] [PubMed] [Google Scholar]
- 27.Fleck SJ. Cardiovascular adaptations to resistance training. Med Sci Sports Exerc. 1988;20:146–51. doi: 10.1249/00005768-198810001-00010. 10.1249/00005768-198810001-00010 [DOI] [PubMed] [Google Scholar]
- 28.Gotshall R, Gootman J, Byrnes W, Fleck SJ, Valovich T. Noninvasive characterization of the blood pressure during exercise testing can be misleadiding. JEP-on line. 1999;4:1–6. [Google Scholar]
- 29.Soucek M, Frana P, Kara T, Sitar J, Halamek J, Jurak P, et al. The effect of short-term isometric muscle contraction and the Valsalva maneuver on systemic and pulmonary hemodynamics in patients with severe heart failure. Clin Cardiol. 2009;32:32–9. doi: 10.1002/clc.20390. 10.1002/clc.20390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeBrusk RF, Valdez R, Houston N, Haskell W. cardiovascular responses to dynamic and static effort soon after myocardial infarction: application to occupational work assessment. Circulation. 1978;58:368–75. doi: 10.1161/01.cir.58.2.368. [DOI] [PubMed] [Google Scholar]
- 31.Lockwood JM, Pricher MP, Wilkins BW, Holowatz LA, Halliwill JR. Postexercise hypotension is not explained by a prostaglandin-dependent peripheral vasodilation. J Appl Physiol. 2005;98:447–53. doi: 10.1152/japplphysiol.00787.2004. 10.1152/japplphysiol.00787.2004 [DOI] [PubMed] [Google Scholar]