Abstract
OBJECTIVE:
To evaluate the effect of spironolactone on ventricular stiffness in spontaneously hypertensive adult rats subjected to high salt intake.
INTRODUCTION:
High salt intake leads to cardiac hypertrophy, collagen accumulation and diastolic dysfunction. These effects are partially mediated by cardiac activation of the renin-angiotensin-aldosterone system.
METHODS:
Male spontaneously hypertensive rats (SHRs, 32 weeks) received drinking water (SHR), a 1% NaCl solution (SHR-Salt), or a 1% NaCl solution with a daily subcutaneous injection of spironolactone (80 mg.kg-1) (SHR-Salt-S). Age-matched normotensive Wistar rats were used as a control. Eight weeks later, the animals were anesthetized and catheterized to evaluate left ventricular and arterial blood pressure. After cardiac arrest, a double-lumen catheter was inserted into the left ventricle through the aorta to obtain in situ left ventricular pressure-volume curves.
RESULTS:
The blood pressures of all the SHR groups were similar to each other but were different from the normotensive controls (Wistar = 109±2; SHR = 118±2; SHR-Salt = 117±2; SHR-Salt-S = 116±2 mmHg; P<0.05). The cardiac hypertrophy observed in the SHR was enhanced by salt overload and abated by spironolactone (Wistar = 2.90±0.06; SHR = 3.44±0.07; SHR-Salt = 3.68±0.07; SHR-Salt-S = 3.46±0.05 mg/g; P<0.05). Myocardial relaxation, as evaluated by left ventricular dP/dt, was impaired by salt overload and improved by spironolactone (Wistar = -3698±92; SHR = -3729±125; SHR-Salt = -3342±80; SHR-Salt-S = -3647±104 mmHg/s; P<0.05). Ventricular stiffness was not altered by salt overload, but spironolactone treatment reduced the ventricular stiffness to levels observed in the normotensive controls (Wistar = 1.40±0.04; SHR = 1.60±0.05; SHR-Salt = 1.67±0.12; SHR-Salt-S = 1.45±0.03 mmHg/ml; P<0.05).
CONCLUSION:
Spironolactone reduces left ventricular hypertrophy secondary to high salt intake and ventricular stiffness in adult SHRs.
Keywords: Hypertension, Salt Intake, Cardiac Hypertrophy, Ventricular Stiffness, Aldosterone Antagonism
INTRODUCTION
High salt intake promotes the elevation of blood pressure, cardiac hypertrophy, the impairment of left ventricular relaxation, endothelial dysfunction and kidney injury.1–9 Spontaneously hypertensive rats (SHRs) are the most frequently used animal model of essential hypertension in humans. This rat strain is more susceptible to cardiac damage when a high salt diet is introduced early in life.10,11 Increased salt intake induces cardiac fibrosis and hypertrophy that may contribute to impair cardiac function independent of blood pressure.1,12,13 For example, salt overload increases left ventricular hypertrophy two-fold, as evaluated by cardiac weight, in 7-week-old SHRs.14 Similarly, Ziegelhöffer-Mihalovicova et al.15 have shown that 12-week-old SHRs develop cardiac hypertrophy when subjected to high salt intake. High-salt diets decrease plasma aldosterone levels, but increase its production in the heart.16,17 Aldosterone contributes to ventricular remodeling in some pathological conditions by increasing collagen synthesis2,4,18,19 and by modifying endothelial and vascular functions.20
Mineralocorticoid antagonists induce beneficial changes in left ventricular remodeling and prevent or partially reverse cardiac fibrosis and pathological hypertrophy that contribute to the development of diastolic heart failure.18,21,22 For example, in young normotensive Wistar rats, a high-salt diet increases interstitial and perivascular fibrosis in the heart and also induces myocyte hypertrophy. These effects are suppressed by spironolactone treatment, which suggests that aldosterone may mediate salt-induced myocardial damage.23 Accordingly, aldosterone receptor blockade reduces cardiac fibrosis and vascular remodeling in salt-loaded stroke-prone SHRs,22 reduces left ventricular hypertrophy and improves coronary flow in young SHRs subjected to high salt intake.14
However, the majority of studies that have investigated the effect of salt overload in hypertensive rats were carried out in young animals.2,3,4,16,22 These animals had a short exposure time to the pressure overload; consequently, left ventricle remodeling may have been incomplete.24–26 This scenario may explain the confusion surrounding the origin of left ventricular hypertrophy and stiffness. These changes may be secondary to pressure or salt overload. However, the effects of salt overload in fully remodeled adult hypertensive rats remain unknown. Therefore, the aim of this study was to investigate the effects of high-salt intake in an adult animal model with established hypertensive heart disease and to test the effect of spironolactone on left ventricular hypertrophy and stiffness.
MATERIALS AND METHODS
Animals and experimental groups
Animals were acquired from the Federal University of Espírito Santo. All of the protocols in this study were in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996) and the Ethical Principles in Animal Experimentation of the Brazilian College of Animal Experimentation (COBEA) and were approved by the institutional Committee of Ethics on Animal Research (N. 012/2010).
Adult male SHRs (32 weeks) were randomly assigned to receive only drinking water (hypertensive control, SHR, n = 8), 1% NaCl solution in drinking water (SHR-Salt, n = 9), or 1% NaCl solution with daily subcutaneous spironolactone (80 mg.kg-1) administration (SHR-Salt-S, n = 9). Age-matched Wistar rats were used as normotensive controls. The spironolactone dose (80 mg.kg-1) has been previously demonstrated to have beneficial effects on the heart without significantly altering blood pressure in SHRs.27
Hemodynamic evaluation
After 8 weeks in the study protocol, the animals were anesthetized with ketamine (70 mg.kg-1, i.p., Agener União, Brazil) and xylazine (10 mg.kg-1, ip, Bayer, Brazil) for left ventricle catheterization. Briefly, the right common carotid artery was separated from connective tissue and catheterized with a fluid-filled polyethylene catheter (P50). The catheter was connected to a pressure transducer (TRI 21, Letica Scientific Instruments, Spain) and then to a digital system (Powerlab/4SP ML750, ADInstruments, Australia). After arterial systolic and diastolic blood pressure were recorded, the catheter was advanced to the left ventricle to obtain the following parameters: heart rate (HR), left ventricular systolic pressure (LVSP), end-diastolic pressure (LVEDP), and the maximum rate of pressure rise (+dP/dt) and fall (-dP/dt).
Measurement of the in situ left ventricular pressure-volume relationship
After hemodynamic measurements, the heart was arrested with 3 M KCl (0.2 ml, iv), and a double lumen catheter (P50 inserted into P200) was inserted into the left ventricle through the aorta to determine the in situ left ventricle diastolic pressure-volume relationship, as previously described.28 Briefly, the atrio-ventricular groove was occluded, and a small incision was made in the free wall of the right ventricle to hinder any compressor effect. A sodium chloride solution (0.9%) was infused with an infusion pump (BI 200, Insight Equipments, Brazil) at a constant rate of 0.60 mL.min-1 into the P200. Pressure was continuously monitored through the P50. Three curves were recorded in each heart over ten minutes. The obtained curves were segmented and then separately analyzed. Pressure follows a linear pattern from 0 to 5 mmHg during volume infusion, and the slope is indicative of left ventricular dilatation. For the 5 to 30 mmHg segment, the curve was adjusted to a monoexponential model. Thus, to determine the stiffness constant during the 5-30 mmHg interval, a logarithmic transformation was performed on the pressure scale to create a linear fit. The slope of this regression represents the stiffness constant.
After pressure-volume readings were recorded, the heart was excised, and the chambers were separated and weighed. The lungs and kidney were also weighed.
Statistical analysis
Data are shown as mean ± standard error of the mean (SEM). Comparisons between means were assessed using one-way analysis of variance (ANOVA) followed by the Fischer post-hoc test, when appropriate. Linear regression was used to acquire the slope of each segment (dilatation and stiffness constants) of the pressure-volume curve. All statistical analyses were performed with the SPSS 13.0 package (Chicago, IL, USA). Statistical significance was taken as P<0.05.
RESULTS
Morphometric evaluation
All of the animals survived the entire treatment period. At baseline, all of the SHR groups had similar body weights but were significantly different from the age-matched Wistar rats (Wistar = 585±6 g; SHR = 310±7 g; SHR-Salt = 315±5 g; SHR-Salt-S = 317±4 g; P<0.05). However, after 8 weeks, the mean body weight of the SHR-Salt group was higher than in the other SHR groups (Wistar = 596±6 g; SHR = 330±5 g; SHR-Salt = 343±6 g; SHR-Salt-S = 331±4 g; P<0.05).
Table 1 shows the morphometric parameters observed after 8 weeks of treatment. Cardiac hypertrophy, as evaluated by crude ventricular weight, was higher in SHR-Salt group, and normalized in the SHR-SALT-S group. In addition, when cardiac weight was corrected for body weight, the ventricular hypertrophy observed in the salt-loaded SHRs was abated by spironolactone treatment. No differences were found among the groups in the other morphological parameters (i.e., the weight of lungs and kidney, see Table 1).
Table 1.
Morphometric parameters measured after eight weeks of treatment.
| Wistar | SHR | SHR-Salt | SHR-Salt-S | |
| Animals (n) | 9 | 8 | 9 | 9 |
| Ventricles (g) | 1.42±0.03 | 1.14±0.03* | 1.24±0.03*† | 1.15±0.02*‡ |
| Ventricles/BW (mg/g) | 2.90±0.06 | 3.44±0.07* | 3.68±0.07*† | 3.46±0.05*‡ |
| Lungs (g) | 3.10±0.09 | 2.59±0.10* | 2.62±0.11* | 2.72±0.10* |
| Lungs/BW (mg/g) | 6.10±0.2 | 7.85±0.3* | 7.64±0.3* | 8.21±0.3* |
| Lungs H2O(%) | 80.8±0.3 | 81.2±0.3 | 80.4±0.4 | 80.3±0.3 |
| Kidney (g) | 2.91±0.06 | 2.52±0.05* | 2.61±0.05* | 2.56±0.04* |
| Kidney/BW (mg/g) | 6.71±0.11 | 7.65±0.12* | 7.61±0.06* | 7.72±0.09* |
Data are presented as the mean±SEM
P<0.05 vs Wistar; † P<0.05 vs SHR; ‡ P<0.05 vs SHR-Salt
Hemodynamic evaluation
Hemodynamic variables are displayed in Table 2. Heart rate did not vary significantly among the groups. Arterial and left ventricular pressure were measured in anesthetized animals. In this condition, the blood pressure shows lower values as compared to awake animals, mainly in SHRs. The systolic blood pressure was higher in all SHR groups than in the Wistar control group, though there were no significant differences among the SHR groups (Wistar = 109±2 mmHg; SHR = 118±2 mmHg; SHR-Salt = 117±2 mmHg; SHR-Salt-S = 116±2 mmHg). The diastolic blood pressure was similar in all groups (Table 2).
Table 2.
Hemodynamic parameters observed in anesthetized animals after eight weeks of treatment.
| Wistar | SHR | SHR-Salt | SHR-Salt-S | |
| Animals (n) | 9 | 8 | 9 | 9 |
| HR (BPM) | 192±8 | 190±7 | 183±7 | 187±7 |
| SBP (mmHg) | 109±2 | 118±2* | 117±2* | 116±2* |
| DBP (mmHg) | 76±3 | 79±2 | 75±3 | 77±2 |
| LVSP (mmHg) | 113±2 | 121±2* | 123±2* | 119±2* |
| LVEDP (mmHg) | 4.1±0.3 | 6.3±0.3* | 6.8±0.6* | 6.6±0.5* |
| +dP/dt (mmHg/s) | 4854±101 | 4794±86 | 4737±103 | 4536±114 |
| -dP/dt (mmHg/s) | -3698±92 | -3729±125 | -3342±80*† | -3647±104† |
Data are presented as the mean±SEM. * P<0.05 vs Wistar; † P<0.05 vs SHR; ‡ P<0.05 vs SHR-Salt
HR: heart rate, SBP: systolic blood pressure, DBP: diastolic blood pressure, LVSP: left ventricular systolic pressure, LVEDP: left ventricular end-diastolic pressrue, dP/dt: maximum rate of pressure rise and fall.
Left ventricular catheterization showed that the left ventricular systolic pressure was higher in the SHR groups than in the Wistar rats and that neither salt load nor spironolactone changed this parameter. However, the maximum left ventricle relaxation rate, -dP/dt, was reduced in the SHR-Salt group, and spironolactone hindered this effect (Table 2).
Pressure-volume curve
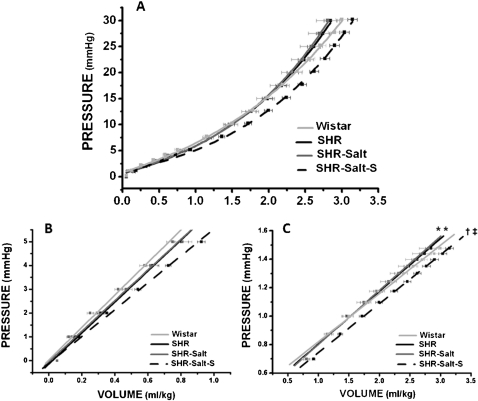
The pressure-volume curve (Figure 1A) was divided into two segments: an initial linear segment that represents the dilatation constant (K1) (Figure 1B) and an exponential segment that indicates left ventricular stiffness (K2) (Figure 1C).
Figure 1.
Left ventricular in situ pressure-volume curve normalized for body weight (A). The chamber dilatation was obtained from the first segment (0 to 5 mmHg) of the curve (B). The chamber stiffness was obtained from the second segment (5 to 30 mmHg) of the curve (C) when plotted on a log scale. Data are presented as the mean±SEM. * P<0.05 vs Wistar; † P<0.05 vs SHR; ‡ P<0.05 vs SHR-Salt
Left ventricular dilatation was similar in all groups, but a trend toward higher values in the SHR-Salt-S group relative to the Wistar rats (Wistar = 0.71±0.05 mmHg/ml; SHR = 0.68±0.04 mmHg/ml; SHR-Salt = 0.69±0.05 mmHg/ml; SHR-Salt-S = 0.65±0.03 mmHg/ml; P = 0.09; Figure 1B) was observed, which suggests that neither salt overload nor spironolactone significantly changed the left ventricular volume. However, compared to the Wistar rats, the untreated SHR group showed increased ventricular stiffness, but interestingly, high salt intake did not alter this parameter. Moreover, in spironolactone-treated rats, left ventricular stiffness was reduced to a level similar to that found in Wistar rats (Wistar = 1.40±0.04 mmHg/ml; SHR = 1.60±0.05 mmHg/ml; SHR-Salt = 1.67±0.12 mmHg/ml; SHR-Salt-S = 1.45±0.03 mmHg/ml; P<0.05; Figure 1C).
DISCUSSION
Our study shows that chronic high salt intake enhances cardiac hypertrophy in adult SHRs. However, left ventricular stiffness was not affected by salt overload. Interestingly, treatment with spironolactone reduced left ventricular hypertrophy and improved left ventricular compliance.
It is well known that SHRs have high blood pressure at two months of age. As a result, cardiac hypertrophy develops early and reaches a stable value around three months of age, remaining constant throughout the rest of the life of the animal.25,26 Our study shows that high salt intake increases cardiac hypertrophy in adult SHRs that had well-established blood pressure levels and compensatory cardiac hypertrophy compared to normotensive rats. Conversely, we did not observe an increase in the blood pressure of adult SHRs subjected to high salt intake. It is important to note that the hemodynamic evaluation was carried out under anesthesia, which is known to considerably reduce blood pressure levels.29,30
Some reports have shown that high salt intake increases left ventricular hypertrophy.1,31,32 Additionally, others have shown that this effect is related to salt-induced hypertension.1,32 However, this view is not unanimous. Matavelli et al.16 have shown that independent of high blood pressure, a high-salt diet leads to left ventricular hypertrophy as well as some other detrimental hemodynamic effects in SHRs. Moreover, in normotensive rats, a high-salt diet does not increase blood pressure even when there is significant cardiac hypertrophy.33 Our data are in agreement with the hypothesis that cardiac hypertrophy is induced by salt overload independent of increased blood pressure, even when hypertension-induced hypertrophy is already fully established.
The hypertrophic response mediated by high salt intake was ameliorated by the co-administration of spironolactone. This result is similar to other reports in which high-salt diet-induced cardiac hypertrophy in normotensive rats was inhibited by spironolactone.23,33 Comparatively, cardiac hypertrophy in SHRs subjected to high salt intake was not reversed by an angiotensin-converting enzyme inhibitor, a beta-blocker or a calcium antagonist.15 This supports the idea that cardiac hypertrophy mediated by salt overload may be induced, at least in part, directly by tissue aldosterone production, independently from the action of angiotensin II. However, it was demonstrated by Varagic et al.32 that blocking the AT1 receptor reduces cardiac hypertrophy without affecting blood pressure in young salt-loaded SHRs. In salt-overloaded rats, eplerenone reduces left ventricular hypertrophy without affecting blood pressure.34 Similarly, in hypertensive humans, an aldosterone antagonist improves diastolic function independent of any reduction in blood pressure or cardiac mass, indicating that this drug may be acting directly on the myocardium.35 Furthermore, the reduction in cardiac hypertrophy was not related to any potential antihypertensive effect of spironolactone because, as we have shown, blood pressure levels were similar in all the SHR groups.
Left ventricular hypertrophy is an increase in myocardial mass that leads to a structural rearrangement of the components of the ventricular wall and may result in ventricular stiffening, thereby compromising systolic and diastolic function.36,37 Analysis of the passive pressure-volume curve confirms that SHRs have increased left ventricular wall stiffness when compared to normotensive rats. Surprisingly, adult SHRs (8 months) subjected to high salt intake did not show an additional increase in left ventricular stiffness. This may be because the heart is already remodeled and has stable levels of left ventricular hypertrophy and interstitial fibrosis.38
In young SHRs, it has been reported that salt overload leads to interstitial and perivascular fibrosis.1,2,32 In turn, fibrosis is related to enhanced left ventricular stiffness, which leads to impairment in left ventricle relaxation.38 In this study, we observed that high salt intake led to a reduction of cardiac -dP/dt, suggesting that relaxation was impaired. It has recently been reported that salt overload leads to diastolic dysfunction in rats with metabolic syndrome.7 However, it has been shown that in 8 week-old SHRs a high-salt diet increases ventricular fibrosis.4 However, in 6 month-old SHRs, a high-salt diet does not alter type I or III collagen mRNA expression in the left ventricle.15 We did not observe an increase in ventricular stiffness in adult SHRs subjected to high salt intake. This response reinforces our hypothesis that the adult SHRs used in this study have well-established left ventricular remodeling (i.e., cardiac hypertrophy and extracellular matrix remodeling). Ten month-old SHRs display extensive cardiac hypertrophy and well-established life of the animal.38 The reduction in -dP/dt observed could be associated with the first stages of diastolic dysfunction because hemodynamic parameters were unchanged, indicating that the rats have compensated for pressure overload.
We have demonstrated that spironolactone-treated SHRs had preserved left ventricular -dP/dt and better compliance when compared to other SHR groups. It is well known that aldosterone directly activates collagen production and deposition in the heart.39 This effect increases left ventricular stiffness and leads to diastolic dysfunction.40 It has been demonstrated that spironolactone reduces myocardial fibrosis in cardiac hypertrophy in animals and humans.41 In 1-year-old SHRs, eplerenone reduces left ventricular fibrosis and improves coronary hemodynamics.42 In the clinical setting, spironolactone improves diastolic dysfunction and reduces left ventricular stiffness in elderly or dilated cardiomyopathic patients.43,44 Thus, it is possible that the anti-fibrotic and anti-hypertrophic effects of spironolactone could be the main cause for the amelioration of left ventricular compliance observed in SHRs.
The absence of non-invasive blood pressure measurements is a limitation of our study. Additionally, we cannot rule out the possibility that high salt intake and/or spironolactone significantly changed blood pressure because we did not test a spironolactone-treated group that was not exposed to high salt intake. Moreover, the possible hypotensive effect of spironolactone cannot be discarded even though it has been reported that the dose of spironolactone (80 mg.kg-1) used in our study is not associated with a reduction in blood pressure.27
CONCLUSIONS
Using an adult hypertensive-rat model, we demonstrated that a high-salt diet leads to left ventricular hypertrophy and impairs left ventricular relaxation. In addition, mineralocorticoid receptor blockade with spironolactone ameliorates salt-induced cardiac dysfunction and hypertrophy and also reduces the left ventricular stiffness observed in adult SHRs. These results reinforce the idea that high-salt diets are deleterious to cardiovascular function and that aldosterone is, at least in part, involved in these effects.
ACKNOWLEDGMENT
This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa do Espírito Santo (FAPES/PRONEX-35884886).
REFERENCES
- 1.Ahn J, Jasmina V, Michel S, Dinko S, Edward DF. Cardiac structural and functional responses to salt loading in SHR. Am J Physiol Heart Circ Physiol. 2004;287:H767–H772. doi: 10.1152/ajpheart.00047.2004. 10.1152/ajpheart.00047.2004 [DOI] [PubMed] [Google Scholar]
- 2.Varagic J, Frohlich ED, Diez J, Susic D, Ahn J, Gonzalez A, et al. Myocardial fibrosis, impaired coronary hemodynamics, and biventricular dysfunction in salt-loaded SHR. Am J Physiol Heart Circ Physiol. 2006;290:H1503–H1509. doi: 10.1152/ajpheart.00970.2005. 10.1152/ajpheart.00970.2005 [DOI] [PubMed] [Google Scholar]
- 3.Frohlich ED, Chien Y, Sesoko S, Pegram BL. Relationship between dietary sodium intake, hemodynamics, and cardiac mass in SHR and WKY rats. Am J Physiol Regul Integr Comp Physiol. 1993;264:R30–R34. doi: 10.1152/ajpregu.1993.264.1.R30. [DOI] [PubMed] [Google Scholar]
- 4.Yu HCM, Burrell LM, Black J, Wu LL, Dilley RJ, Cooper ME, et al. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1998;98:2621–8. doi: 10.1161/01.cir.98.23.2621. [DOI] [PubMed] [Google Scholar]
- 5.Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr. 2006;25:247–55. doi: 10.1080/07315724.2006.10719574. [DOI] [PubMed] [Google Scholar]
- 6.Cook NR. Salt intake, blood pressure and clinical outcomes. Curr Opin Nephrol Hypertens. 2008;17:310–4. doi: 10.1097/MNH.0b013e3282f4b720. 10.1097/MNH.0b013e3282f4b720 [DOI] [PubMed] [Google Scholar]
- 7.Matsui H, Ando K, Kawarazaki H, Nagae A, Fujita M, Shimosawa T, et al. Salt excess causes left ventricular diastolic dysfunction in rats with metabolic disorder. Hypertension. 2008;52:287–94. doi: 10.1161/HYPERTENSIONAHA.108.111815. 10.1161/HYPERTENSIONAHA.108.111815 [DOI] [PubMed] [Google Scholar]
- 8.Jernigan NL, LaMarca B, Speed J, Galmiche L, Granger JP, Drummond HA. Dietary salt enhances benzamil-sensitive component of myogenic constriction in mesenteric arteries. Am J Physiol Heart Circ Physiol. 2008;294:409–20. doi: 10.1152/ajpheart.00571.2007. 10.1152/ajpheart.00571.2007 [DOI] [PubMed] [Google Scholar]
- 9.dos Santos L, Gonçalves MV, Vassallo DV, Oliveira EM, Rossoni LV. Effects of high sodium intake diet on the vascular reactivity to phenylephrine on rat isolated caudal and renal vascular beds: endothelial modulation. Life Sci. 2006;78:2272–9. doi: 10.1016/j.lfs.2005.09.028. 10.1016/j.lfs.2005.09.028 [DOI] [PubMed] [Google Scholar]
- 10.Azar S, Kabat V, Bingham C. Environmental factor(s) during suckling exert long-term upon blood pressure and body weight in spontaneously hypertensive and normotensive rats. J Hypertens. 1991;9:309–27. doi: 10.1097/00004872-199104000-00003. 10.1097/00004872-199104000-00003 [DOI] [PubMed] [Google Scholar]
- 11.Karr-Dullien V, Bloomquist E. The influence of prenatal salt on the development of hypertension by spontaneously hypertensive rats (SHR) Proc Soc Exp Biol Med. 1979;160:421–5. doi: 10.3181/00379727-160-40462. [DOI] [PubMed] [Google Scholar]
- 12.Heimann JC, Drumond S, Alves AT, Barbato AJ, Dichtchekenian V, Marcondes M. Left ventricular hypertrophy is more marked in salt-sensitive than in salt-resistant hypertensive patients. J Cardiovasc Pharmacol. 1991;17:S122–S124. doi: 10.1097/00005344-199117002-00028. 10.1097/00005344-199117002-00028 [DOI] [PubMed] [Google Scholar]
- 13.Antonios TF, MacGregor GA. Salt intake: potential deleterious effects excluding blood pressure. J Hum Hypertens. 1995;9:511–15. [PubMed] [Google Scholar]
- 14.Susic D, Jasmina V, Frohlich E. Cardiovascular effects of inhibition of rennin-angiotensin-aldosterone system components in hypertensive rats given salt excess. Am J Physiol Heart Circ Physiol. 2010;298:1177–81. doi: 10.1152/ajpheart.00866.2009. 10.1152/ajpheart.00866.2009 [DOI] [PubMed] [Google Scholar]
- 15.Ziegelhöffer-Mihalovicova B, Arnold N, Marx G, Tannapfel A, Zimmer HG, et al. Effects of salt loading in various therapies on cardiac hypertrophy and fibrosis in young spontaneously hypertensive rats. Life Sci. 2006;79:838–46. doi: 10.1016/j.lfs.2006.02.041. 10.1016/j.lfs.2006.02.041 [DOI] [PubMed] [Google Scholar]
- 16.Matavelli LC, Zhou X, Varagic J, Susic D, Frohlich ED. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:814–9. doi: 10.1152/ajpheart.00671.2006. 10.1152/ajpheart.00671.2006 [DOI] [PubMed] [Google Scholar]
- 17.Takeda Y, Yoneda T, Demura M, Miyamori I, Mabuchi H. Cardiac aldosterone production in genetically hypertensive rats. Hypertension. 2000;36:495–500. doi: 10.1161/01.hyp.36.4.495. [DOI] [PubMed] [Google Scholar]
- 18.Mill JG, Milanez MC, Resende MM, Gomes MGS, Leite CM. Spironolactone prevents cardiac collagen proliferation after myocardial infarction in rats. Clin Exp Pharmacol Physiol. 2003;30:739–44. doi: 10.1046/j.1440-1681.2003.03906.x. 10.1046/j.1440-1681.2003.03906.x [DOI] [PubMed] [Google Scholar]
- 19.Resende MM, Kauser K, Mill JG. Regulation of cardiac and renal mineralocorticoid receptor expression by captopril following myocardial infarction in rats. Life Sci. 2006;78:3066–73. doi: 10.1016/j.lfs.2005.12.011. 10.1016/j.lfs.2005.12.011 [DOI] [PubMed] [Google Scholar]
- 20.Sartório CL, Fraccarollo D, Galuppo P, Leutke M, Ertl G, Stefanon I, Bauersachs J. Mineralocorticoid receptor blockade improves vasomotor dysfunction and vascular oxidative stress early after myocardial infarction. Hypertension. 2007;50:919–925. doi: 10.1161/HYPERTENSIONAHA.107.093450. 10.1161/HYPERTENSIONAHA.107.093450 [DOI] [PubMed] [Google Scholar]
- 21.Mandarim-de-Lacerda CA, Pereira LMM. The effects of spironolactone monotherapy on blood pressure and myocardial remodeling in spontaneously hypertensive rats: a stereological study. J Biomed Sci. 2003;10:50–57. doi: 10.1007/BF02255997. 10.1007/BF02255997 [DOI] [PubMed] [Google Scholar]
- 22.Endemman DH, Touyz MI, Savoia C, Schiffin EL. Eplerenone prevents salt-induced vascular remodeling and cardiac fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2004;43:1252–7. doi: 10.1161/01.HYP.0000128031.31572.a3. 10.1161/01.HYP.0000128031.31572.a3 [DOI] [PubMed] [Google Scholar]
- 23.Lal A, Veinot JP, Leenen FHH. Prevention of high salt diet-induced cardiac hypertrophy and fibrosis by spironolactone. Am J Hypertens. 2003;16:319–23. doi: 10.1016/s0895-7061(02)03268-5. [DOI] [PubMed] [Google Scholar]
- 24.Engelmann GL, Vitullo JC, Gerrity RG. Morphometric analysis of cardiac hypertrophy during development, maturation, and senescence in spontaneously hypertensive rats. Circ Res. 1987;60:487–94. doi: 10.1161/01.res.60.4.487. [DOI] [PubMed] [Google Scholar]
- 25.Rocha WA, Lunz W, Baldo MP, Pimentel EB, Dantas EM, Rodrigues SL, et al. Kinetics of cardiac and vascular remodeling by spontaneously hypertensive rats after discontinuation of long-term captopril treatment. Braz J Med Biol Res. 2010;43:390–6. doi: 10.1590/s0100-879x2010007500023. [DOI] [PubMed] [Google Scholar]
- 26.Zamo FS, Lacchini S, Mostarda C, Chiavegatto S, Silva ICM, Oliveira EM, et al. Hemodynamic, morphometric and autonomic patterns in hypertensive rats – renin-angiotensin system modulation. Clinics. 2010;65:85–92. doi: 10.1590/S1807-59322010000100013. 10.1590/S1807-59322010000100013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veliotes DGA, Woodiwiss AJ, Deftereos DAJ, Gray D, Osadchii O, Norton GR. Aldosterone receptor blockade prevents the transition to cardiac pump dysfunction induced by β-adrenoreceptor activation. Hypertension. 2005;45:914–920. doi: 10.1161/01.HYP.0000164567.62172.c5. 10.1161/01.HYP.0000164567.62172.c5 [DOI] [PubMed] [Google Scholar]
- 28.Fletcher PJ, Pfeffer JM, Pfeffer MA, Braunwald E. Left ventricular diastolic pressure-volume relations in rats with healed myocardial infarction. Effects on systolic function. Circ Res. 1981;49:618–26. doi: 10.1161/01.res.49.3.618. [DOI] [PubMed] [Google Scholar]
- 29.Smith TL, Hutchins PM. Anesthetic effects on hemodynamics of spontaneously hypertensive and Wistar-Kyoto rats. Am J Physiol Heart Circ Physiol. 1980;238:539–44. doi: 10.1152/ajpheart.1980.238.4.H539. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira PM, Alzamora AC, Santos RAS, Campagnole-Santos MJ. Hemodynamic effect produced by microinjection of angiotensin at the caudal ventrolateral medulla of spontaneously hypertensive rats. Neuroscience. 2008;151:1208–16. doi: 10.1016/j.neuroscience.2007.11.042. 10.1016/j.neuroscience.2007.11.042 [DOI] [PubMed] [Google Scholar]
- 31.Resende MM, Mill JG. Effect of high salt intake on local renin-angiotensin system and ventricular dysfunction following myocardial infarction in rats. Clin Exp Pharmacol Physiol. 2007;34:274–9. doi: 10.1111/j.1440-1681.2007.04556.x. 10.1111/j.1440-1681.2007.04556.x [DOI] [PubMed] [Google Scholar]
- 32.Varagic J, Frohlich ED, Susic D, Ahn J, Matavelli L, López B, et al. AT1 receptor antagonism attenuates target organ effects of salt excess in SHRs without affecting pressure. Am J Physiol Heart Circ Physiol. 2008;294:853–8. doi: 10.1152/ajpheart.00737.2007. 10.1152/ajpheart.00737.2007 [DOI] [PubMed] [Google Scholar]
- 33.Cordaillat M, Rugale C, Casellas D, Mimran A, Jover B. Cardiorenal abnormalities associated with high sodium intake: correction by spironolactone in rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:1137–43. doi: 10.1152/ajpregu.00154.2005. [DOI] [PubMed] [Google Scholar]
- 34.Nagata K, Obata K, Xu J, Ichihara S, Noda A, Kimata H, et al. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension. 2006;47:656–64. doi: 10.1161/01.HYP.0000203772.78696.67. 10.1161/01.HYP.0000203772.78696.67 [DOI] [PubMed] [Google Scholar]
- 35.Grandi AM, Imperiale D, Santillo R, Barlocco E, Bertolini A, Guasti L, et al. Aldosterone antagonist improves diastolic function in essential hypertension. Hypertension. 2002;40:647–52. doi: 10.1161/01.hyp.0000036399.80194.d8. 10.1161/01.HYP.0000036399.80194.D8 [DOI] [PubMed] [Google Scholar]
- 36.Duarte DR, Minicucci MF, Azevedo OS, Matsubara BB, Matsubara LS, Novelli EL, et al. The role of oxidative stress and lipid peroxidation in ventricular remodeling induced by tobacco smoke exposure after myocardial infarction. Clinics. 2009;64:691–7. doi: 10.1590/S1807-59322009000700014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferreira Filho C, Abreu LC, Valenti VE, Ferreira M, Meneghini A, Silveira JA, et al. Anti-hypertensive drugs have different effects on ventricular hypertrophy regression. Clinics. 2010;65:723–8. doi: 10.1590/S1807-59322010000700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cingolani OH, Yang XP, Cavasin MA, Carretero OA. Increased systolic performance with diastolic dysfunction in adult spontaneously hypertension rats. Hypertension. 2003;41:249–254. doi: 10.1161/01.hyp.0000052832.96564.0b. 10.1161/01.HYP.0000052832.96564.0B [DOI] [PubMed] [Google Scholar]
- 39.Lijnen P, Petrov V. Induction of cardiac fibrosis by aldosterone. J Mol Cell Cardiol. 2000;32:865–79. doi: 10.1006/jmcc.2000.1129. 10.1006/jmcc.2000.1129 [DOI] [PubMed] [Google Scholar]
- 40.Burlew BS, Weber KT. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002;27:92–8. doi: 10.1007/s00059-002-2354-y. 10.1007/s00059-002-2354-y [DOI] [PubMed] [Google Scholar]
- 41.Nishikawa N, Yamamoto K, Sakata Y, Mano T, Yoshida J, Umekawa S, et al. Long-term effect of spironolactone on cardiac structure as assessed by analysis of ultrasonic radio-frequency signals in patients with ventricular hypertrophy. Circ J. 2005;69:1394–400. doi: 10.1253/circj.69.1394. 10.1253/circj.69.1394 [DOI] [PubMed] [Google Scholar]
- 42.Susic D, Varagic J, Ahn J, Matavelli L, Frohlich ED. Long-term mineralocorticoid receptor blockade reduces fibrosis and improves cardiac performance and coronary hemodynamics in elderly SHR. Am J Physiol Heart Circ Physiol. 2007;292:175–9. doi: 10.1152/ajpheart.00660.2006. 10.1152/ajpheart.00660.2006 [DOI] [PubMed] [Google Scholar]
- 43.Izawa H, Murohara T, Nagata K, Isobe S, Asano H, Amano T, et al. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: A pilot study. Circulation. 2005;112:2940–5. doi: 10.1161/CIRCULATIONAHA.105.571653. [DOI] [PubMed] [Google Scholar]
- 44.Roongsritong C, Sutthiwan P, Bradley J, Simoni J, Power S, Meyerrose GE. Spironolactone improves diastolic function in the elderly. Clin Cardiol. 2005;28:484–7. doi: 10.1002/clc.4960281008. 10.1002/clc.4960281008 [DOI] [PMC free article] [PubMed] [Google Scholar]