Abstract
This study examined whether acute alcohol (EtOH) intoxication before burn injury potentiates postburn intestinal tissue damage and whether neutrophils have any role in the damage under those conditions. Male rats (~250 g) were gavaged with EtOH to achieve a blood EtOH level of approximately 100 mg/dL or with saline and received either approximately 12.5% or approximately 25% total body surface area (TBSA) burn or sham injury. Rats were killed at 4 or 24 h after injury, and various parameters were measured. As compared with sham animals, burn injury alone (regardless of size) resulted in a significant increase in intestinal tissue myeloperoxidase (MPO; an index of neutrophil infiltration) activity and IL-18 levels 4 h after injury. Furthermore, rats receiving 25% TBSA, but not 12.5%, burn exhibited intestine edema. The IL-18 and MPO activity were normalized at 24 h after injury in rats receiving 12.5% TBSA burn, whereas these parameters remained elevated at 24 h in rats with 25% burn. The presence of EtOH in rats at the time of burn injury exacerbated the levels of IL-18, MPO activity, and edema at 4 and 24 h after burn injury. Treatment of rats with anti–IL-18 antibodies or with anti-neutrophil antiserum prevented the increase in the above parameters after EtOH and burn injury, except that the depletion of neutrophils did not prevent the IL-18 increase. In summary, these findings suggest that acute EtOH intoxication exacerbates postburn intestinal tissue damage after burn injury, and that it is, in part, neutrophil mediated.
Keywords: Trauma, ethanol, myeloperoxidase, intestine permeability, cytokines, inflammation
INTRODUCTION
Major burn injury results in impaired host defense and organ tissue damage, which is the leading cause of morbidity and mortality in the injured host (1–5). Furthermore, an association between alcohol (EtOH) intoxication and burn injury has been recognized in many previous studies (6–10). These studies have indicated that burn patients who sustained injury while intoxicated exhibit a higher incidence of infection and require additional care compared with the patients who have not consumed EtOH before burn injury (6–11). The mechanism by which EtOH intoxication potentiates postinjury complications remains largely unknown. In a recent study, we examined the effect of EtOH and burn injury on intestine permeability and immune functions because the intestine is a critical organ that plays a role in the development of organ dysfunction in trauma, burn, and intensive care unit patients (12, 13). Findings from this study showed that EtOH intoxication before burn injury exacerbates the suppression of intestine immunity, deteriorates intestinal barrier functions, and increases intestine bacterial translocation (12, 14–16). Furthermore, a combined insult of EtOH intoxication and burn injury increases intestinal tissue myeloperoxidase (MPO) activity (an index of neutrophil infiltration), IL-18 production, edema formation, and intestine permeability 24 h after injury (12, 16, 17).
Neutrophils, an essential component of the immune system, are the first line of defense against infectious agents, such as bacteria, viruses, and fungi. They quickly migrate to the site of infection, and by producing a variety of factors, such as cytokines, enzymes, and reactive oxygen species, kill pathogens. Although these functions of neutrophils are beneficial to host defense, inappropriate activation of neutrophils resulting in excessive release of proteases and reactive oxygen species are known to cause tissue damage in many inflammatory conditions, including burn, trauma, and sepsis (4, 18–21). Furthermore, both experimental and clinical findings indicate that burn injury size profoundly influences alterations in host immune defense (2, 22, 23). Several lines of evidence indicate that patients with major burn injury (>20%) total body surface area (TBSA) are more likely to develop complications and are transferred to major burn center (24). However, in addition to burn size, other factors such as age, sex, and preclinical manifestation can also influence the outcome of burn patients, especially the patients with small burn injury (25, 26). Similarly, EtOH exposure at the time of burn injury is being increasingly recognized as a factor that further complicates postburn pathogenesis (6, 10–12). Furthermore, a smaller burn, which by itself may not have an adverse effect on host defense but when combined with existing conditions such as EtOH intoxication, may become detrimental. In this study, we used 12.5% and 25% TBSA burn injury and determined whether EtOH intoxication at the time of injury influences postburn intestine tissue damage. Furthermore, we determined the role of neutrophils in intestinal tissue damage after EtOH intoxication and burn injury. Because our previous studies indicate the role of IL-18 in impaired intestinal barrier functions after EtOH and burn injury, we also examined if IL-18 directly or indirectly, via neutrophil recruitment, causes intestinal tissue damage.
MATERIALS AND METHODS
Animals and reagents
Male Sprague-Dawley rats (225–250 g) were obtained from Charles River Laboratories (Wilmington, Mass). Rabbit anti-rat neutrophil antiserum was obtained from Accurate Chemicals and Scientific (Westbury, NY). Anti-rat IL-18 antibody was obtained from R and D Systems Inc. (Minneapolis, Minn).
Rat model of acute EtOH and burn injury
As described previously (14–16), rats (~250 g) were randomly divided into four groups: saline + sham, EtOH + sham, saline + burn, and EtOH + burn. In EtOH-treated groups, the levels of blood EtOH equivalent to 90 to 100 mg/dL were achieved by gavage feeding of 5 mL of 20% EtOH in saline. In saline groups, animals were gavaged with 5 mL of saline. Four hours after the gavage with saline or EtOH, all animals were anesthetized and transferred into a template, which was fabricated to expose approximately 12.5% of the TBSA burn injury. The surface area was calculated using Meeh formula (A = KW^2/3), where k was equal to 10 as described in the article of Walker and Mason (27). For burn injury, animals were immersed in a boiling water bath (95°C–97°C) for approximately 10 to 12 s. Sham-injured rats were subjected to identical anesthesia and immersed in lukewarm water. For producing a 25% TBSA injury, rats received burn or sham injury on both sides of their dorsum. This procedure resulted in third-degree full-thickness burn injury and has been used in our previous studies (15, 28). The animals were dried immediately and given fluid resuscitation (intraperitoneally) with 10 mL of physiological saline. Animals were allowed to recover from anesthesia, returned to their cages, and allowed food and water ad libitum. Separate groups of rats were killed at 4 and 24 h after injury. All the experiments were carried out in adherence to the National Institutes of Health guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Treatment of rats with anti–IL-18 antibodies
A group of EtOH plus burn–injured rats was treated intraperitoneally with anti–IL-18 antibody (50–100 μg/kg body weight) immediately after burn injury.
Depletion of neutrophils
In a separate group, rabbit anti-rat neutrophil antiserum (0.3 mL diluted in 1 mL of physiological saline) was injected intraperitoneally into the rats approximately 16 h before injury (29). On 1 day after injury, blood samples were drawn from rats via cardiac puncture. The depletion of neutrophils was confirmed by counting the neutrophil numbers in the blood by using Hemavet-850 (Scientific Inc, Oxford, Conn) as reported in a previous study (29).
Preparation of intestinal tissue homogenates
Immediately after anesthetizing the rats, intestine was exposed, and a 3-cm-long segment of small intestine was removed, cleaned, and snap-frozen in liquid nitrogen. The samples were stored at −70°C (16). Equal weights of tissues (100 mg) from various groups were suspended in 1 mL of a buffer (containing 0.5% hexadecyl-trimethylammonium bromide in 50 mM phosphate buffer, pH 6.0) and sonicated at 30 cycles twice for 30 s on ice. Homogenates were cleared by centrifuging at 12,000 rpm at 4°C. The supernatants were stored at −70°C. Protein levels in the homogenates were determined using the BioRad assay kit (Hercules, Calif).
Measurement of MPO activity in intestinal tissue homogenates
The MPO activity in the tissue homogenates was measured as described previously (16). Briefly, samples were incubated with a substrate, o-dianisidine hydrochloride. This reaction was carried out in a 96-well plate by adding 290 μL 50 mM phosphate buffer, 3 μL substrate solution (containing 20 mg/mL o-dianisidine hydrochloride), and 3 μL hydrogen peroxide (20 mM). Samples (10 μL) were added to the wells to start the reaction. Standard MPO (Sigma Chemical Co, St Louis, Mo) was used in parallel to determine MPO activity in the sample. The reaction was stopped by adding 3 μL of sodium azide (30%). Plates were read at 460 nm. The MPO activity was determined by using the curve obtained from the standard MPO and normalized with protein.
Measurement of intestinal tissue IL-18 levels
IL-18 levels in intestinal tissue homogenates were measured using enzyme-linked immunosorbent assay (ELISA) kits (BioSource International, Camarillo, Calif) following manufacturer’s instructions. This ELISA kit detects only mature IL-18.
Measurement of intestinal tissue edema
For the measurement of edema, intestinal tissue water content was measured. In brief, small intestines from various experimental groups were removed, weighed, and dried for 48 h at 80°C (16). Water content (%) of intestine tissue was calculated as (wet weight - dry weight)/wet weight × 100.
Intestine permeability
A separate group of animals was used for the measurement of intestine permeability. As described previously (16), portal vein was cannulated under anesthesia using polyethylene-50 tubing filled with heparin saline (10 U/mL) after a midline laparotomy. Renal artery and vein in both kidneys were ligated. A 20-cm segment of small intestine (ileum) was isolated without damaging the intestine and mesenteric structures, and a polyethylene-50 tubing was inserted into the isolated intestine from the proximal end. A 1-mL solution of 25 mg/mL of 4-kd fluorescein isothiocyanate–conjugated Dextran (FITC-Dextran; Sigma Chemicals) was injected into the isolated intestine. Blood samples were collected from the portal vein at 90 min after infusion of FITC-Dextran. Plasma was separated by centrifuging at 4°C, 8000 rpm for 7 min, and was analyzed for FITC-Dextran concentration using a fluorometer (FL500, Bio-Tek Instruments, Inc, Winooski, Vt) at an excitation wavelength of 480 nm and an emission wavelength of 520 nm. The FITC-Dextran concentration in plasma samples was calculated using standard curves prepared from dilutions of FITC-Dextran in phosphate buffer saline.
Statistical analysis
Results are presented as mean ± SEM and were analyzed using an ANOVA statistical program. The significance between the groups was determined using Tukey test (Statistical Package for Social Sciences Software program, Version 2.0, Sigma Stat). A P < 0.05 between the two groups was considered statistically significant.
RESULTS
Intestinal MPO activity
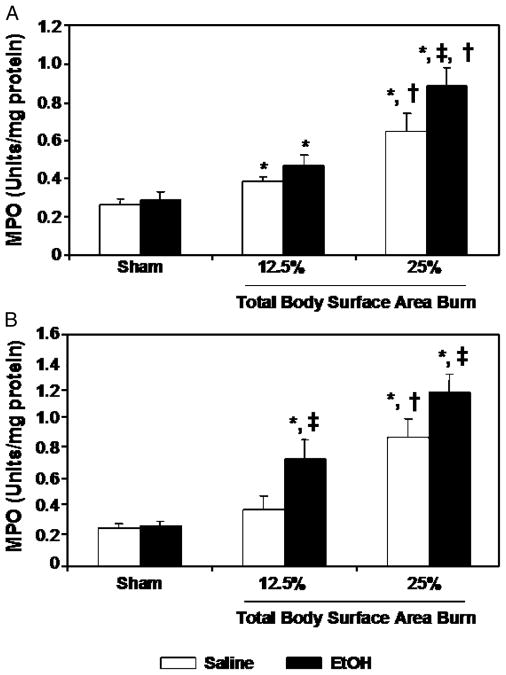
As shown in Figure 1, there was no difference in MPO activity in the intestinal tissue of sham-injured rats gavaged with saline or EtOH either at 4 h or at 24 h after injury. A significant increase in MPO activity in the intestine was observed in rats receiving 12.5% or 25% TBSA burn injury alone in the absence of EtOH intoxication compared with sham-injured rats regardless of EtOH intoxication at 4 h after injury. The MPO activity was normalized at 24 h after injury in rats receiving 12.5% TBSA burn injury. In contrast, intestinal MPO activity remained elevated at 24 h in rats receiving 25% TBSA burn injury in the absence of EtOH exposure. Furthermore, intestinal MPO levels in rats receiving 25% TBSA burn injury were significantly higher compared with the levels observed in rats receiving 12.5% TBSA burn injury at both 4 and 24 h after injury. As indicated in Figures 1A, B, intestinal MPO activity was further increased (P < 0.05) in rats receiving a combined insult of EtOH intoxication and burn injury (regardless of percent area) compared with rats receiving either corresponding similar extent of burn injury in the absence of EtOH intoxication or sham injury regardless of EtOH intoxication at 4 and 24 h after injury.
Fig. 1. Intestinal tissue MPO activity at 4 (A) and 24 (B) h after EtOH intoxication and burn injury.
Intestinal tissues were collected from rats subjected to sham or burn injury with and without EtOH. Equal weights of intestinal tissue from various groups were homogenized. The MPO activity was normalized to the protein contents. Data are means ± SEM from at least six animals in each group. *P < 0.05 vs. Sham; †P < 0.05 vs. 12.5% TBSA burn; ‡P < 0.05 vs. corresponding saline + burn.
Intestinal edema
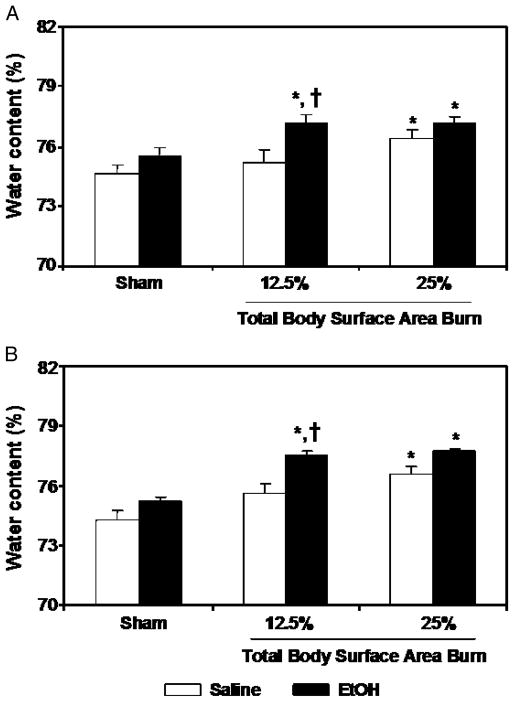
For edema formation, we measured water contents in the intestine. Results from these measurements as shown in Figure 2 indicate no significant difference in intestinal edema in sham rats receiving either EtOH or saline. Furthermore, a difference was also not found in intestinal edema in rats receiving approximately 12.5% TBSA burn injury alone compared with sham rats at 4 and 24 h after injury. However, a significant increase in intestinal edema was observed in rats receiving either approximately 25% TBSA burn injury alone in the absence of EtOH intoxication or a combined insult of EtOH intoxication and burn injury regardless of the percent TBSA burn compared with sham-injured rats at 4 and 24 h after injury (Fig. 2).
Fig. 2. Intestinal tissue edema formation at 4 (A) and 24 (B) h after EtOH intoxication and burn injury.
Intestinal tissues were collected from rats subjected to sham or burn injury with and without EtOH. Intestinal tissue water content was determined by drying the tissue for 48 h at 80°C. Water content (%) of lung tissue was calculated as (wet weight–dry weight)/wet weight × 100. Data are means ± SEM from at least six animals in each group. *P < 0.05 vs. Sham; †P < 0.05 vs. corresponding saline + burn.
IL-18 levels
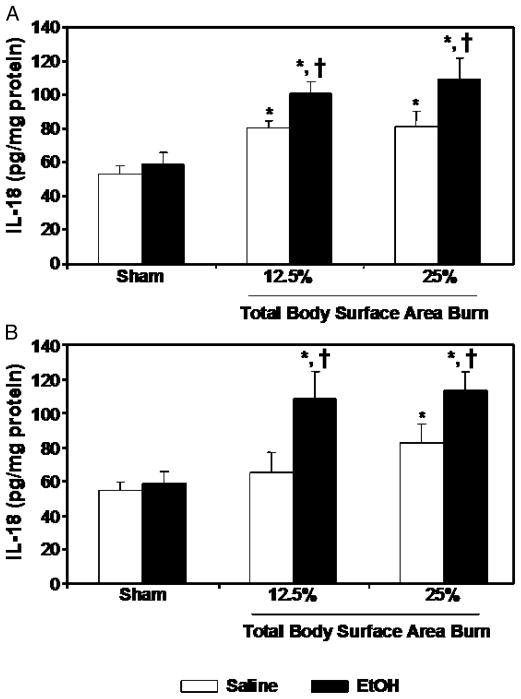
There was no significant difference in IL-18 levels in the intestinal tissue of sham-injured rats gavaged with saline or EtOH at either 4 or 24 h after injury (Fig. 3). However, after a 12.5% or 25% TBSA burn injury in the absence of EtOH intoxication, a significant increase in IL-18 levels in the intestine was observed at 4 h after injury compared with rats receiving sham injury, regardless of EtOH intoxication. At 24 h after injury, IL-18 levels were normalized in rats receiving approximately 12.5% TBSA burn injury; however, IL-18 remained significantly elevated at 24 h in rats receiving 25% TBSA burn injury compared with sham animals. A further significant increase in intestinal IL-18 levels was observed in rats receiving a combined insult of EtOH intoxication and burn injury (regardless of percent TBSA) as compared with rats receiving a similar extent of burn injury in the absence of EtOH intoxication or sham injury, regardless of EtOH intoxication at 4 and 24 h after injury (Fig. 3).
Fig. 3. Intestinal tissue IL-18 levels at 4 (A) and 24 (B) h after EtOH intoxication and burn injury.
Intestinal tissues were collected from all four groups, and equal weights of tissue were homogenized. IL-18 levels in the homogenates were measured using ELISA kits and normalized to the protein. Data are means ± SEM from at least six animals in each group. *P < 0.05 vs. Sham; †P < 0.05 vs. corresponding saline + burn.
Administration of anti–IL-18 antibody prevents the increase in intestinal MPO activity and edema
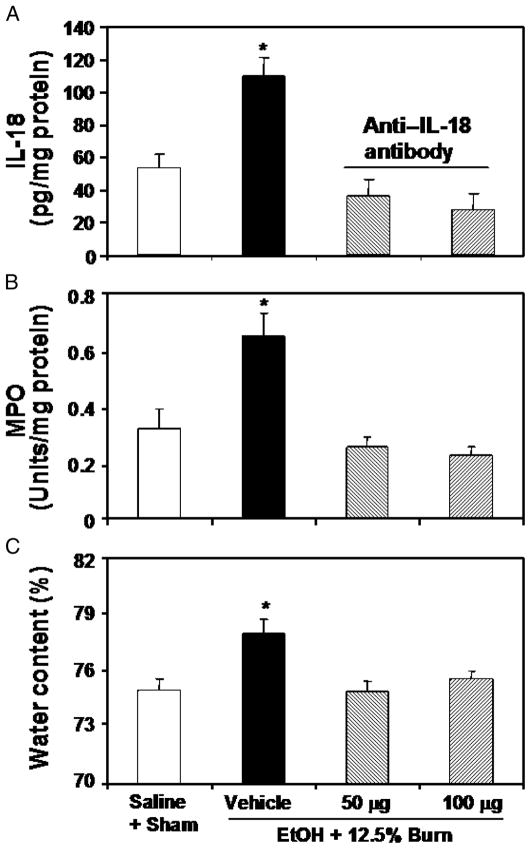
To perform these studies, EtOH plus burn–injured rats were treated with anti–IL-18 antibody (50–100 μg/kg) immediately after burn injury. In these experiments, we used approximately 12.5% TBSA burn injury and did not include all four experimental groups. Rather, we used only the EtOH plus burn group because this was the only group that showed significant differences.
The effect of anti–IL-18 antibodies on intestinal tissue MPO activity and edema was observed at 24 h after burn injury. Results presented in Figure 4 show that treatment of rats with anti–IL-18 antibody significantly attenuated the increase in intestinal MPO activity (Fig. 4B) and the development of edema (Fig. 4C). There was no significant difference between the two doses of anti–IL-18 antibodies that were used to neutralize endogenous IL-18 release. The efficacy of anti–IL-18 antibody was assessed by measuring IL-18 levels, and the results presented in Figure 4A show that treatment of rats with anti–IL-18 antibody prevented the increase in IL-18 levels in the intestinal tissue after a combined insult of EtOH intoxication and burn injury.
Fig. 4. Effect of anti–IL-18 antibody on intestinal tissue IL-18 level (A), MPO activity (B), and edema at 24 h after EtOH intoxication and 12.5% TBSA burn injury.
Animals were treated intraperitoneally with different doses of neutralizing anti–IL-18 antibody immediately after burn injury. Intestinal tissues were collected at 24 h after injury, and equal weights of tissue were homogenized. IL-18 levels and MPO activity in the homogenates were determined and normalized to the protein. Separate segments of intestine were used for measuring intestinal edema. Data are means ± SEM from four to six animals in each group. *P < 0.05 vs. all other groups.
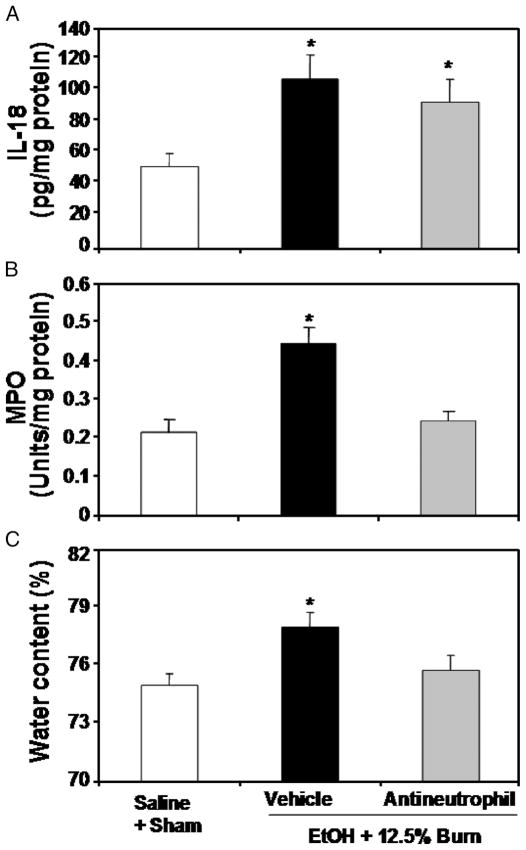
Effect of neutrophil depletion on intestinal tissue IL-18, MPO activity, edema, and permeability
To determine whether neutrophils are critical to the intestinal tissue injury, we treated animals with rabbit anti-rat neutrophil antiserum approximately 16 h before EtOH and burn injury (12.5% TBSA) to deplete neutrophils. As shown previously (29), there was a significant increase in blood neutrophil numbers in rats receiving a combined insult of EtOH intoxication and burn injury (2,371 ± 310/μL) compared with sham animals (360 ± 182/μL) at 24 h after injury. Treatment of rats with anti-neutrophil antiserum prevented the increase in neutrophils (745 ± 96/μL) in circulation 24 h after EtOH intoxication and burn injury. Furthermore, results as presented in Figure 5 suggested that treatment of rats with anti-neutrophil antiserum did not influence intestinal IL-18 levels (Fig. 5A) but significantly reduced the increase in intestinal MPO activity (Fig. 5B) and edema formation after EtOH intoxication and burn injury (Fig. 5C). In subsequent experiments, we further ascertained the role of neutrophil in tissue damage after EtOH and burn injury (12.5% TBSA) by measuring intestinal permeability at 24 h after injury. Results presented in Figure 6 indicate that there was a significant increase in plasma FITC-Dextran levels in portal vein of rats receiving a combined insult of EtOH intoxication and burn injury compared with rats receiving either sham or burn injury alone. Treatment of rats with anti-neutrophil antiserum significantly prevented the increase in FITC-Dextran in portal vein after EtOH and burn injury.
Fig. 5. Effect anti-neutrophil antiserum on intestinal tissue IL-18 levels (A), MPO activity (B), and edema (C) at 24 h after EtOH intoxication and 12.5% TBSA burn injury.
Approximately 16 h before receiving EtOH plus burn injury, rats were treated with rabbit anti-rat neutrophil antiserum (0.3 mL diluted in 1 mL of physiological saline). Intestinal tissues were collected and processed for the measurements of IL-18 levels, MPO activity, and edema. Data are means ± SEM from four to six animals in each group. *P < 0.05 vs. all other groups.
Fig. 6. Effect of anti-neutrophil antiserum on intestinal permeability at 24 h after EtOH intoxication and 12.5% TBSA burn injury.
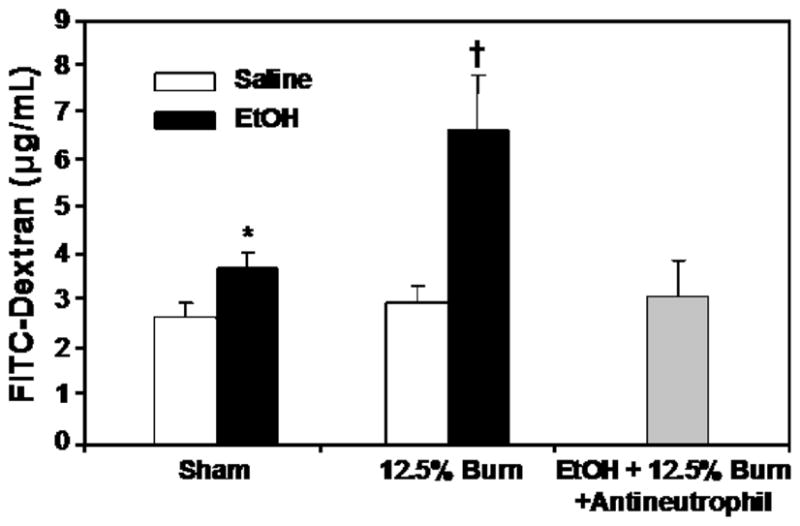
Animals were treated with rabbit anti-rat neutrophil antiserum (0.3 mL diluted in 1 mL of physiological saline) intraperitoneally approximately 16 h before EtOH and burn injury. Intestinal permeability was determined by monitoring the transfer of FITC-Dextran from the isolated intestine segment to blood drawn at 90 min after FITC infusion into isolated intestine segment. Values are mean ± SE from six animals in each group except the anti-neutrophil-treated group that has four animals. *P < 0.05 vs. sham + saline; †P < 0.05 vs. other groups.
DISCUSSION
Alcohol intoxication has been considered a confounding factor in postburn pathogenesis. Although a number of clinical studies have identified an association between EtOH intoxication and burn injury, only a few studies were performed to evaluate the role of EtOH in postburn complications. The findings from many of these studies suggested that burn patients who sustained injury under the influence of EtOH had significantly longer hospital stay; required more surgical procedures, and had more complications compared with patients who sustained a similar extent of burn injury but are not intoxicated at the time of injury (6–11). Moreover, intoxicated patients were more likely to die of smaller burns (11). Additional findings from experimental studies indicate that acute EtOH intoxication before burn injury exacerbates the suppression of host defense, enhances susceptibility to infection, and impairs intestinal immunity (6, 10, 12, 14–16, 28, 30). Using two different burn sizes (12.5% or 25% TBSA), the present study examined the influence of acute EtOH intoxication on postburn intestinal barrier function and the mechanism responsible for altered barrier function under those conditions.
Our findings indicate a significant increase in intestinal IL-18 levels and MPO activity in rats receiving 12.5% or 25% burn injury alone in the absence of EtOH intoxication compared with rats receiving sham injury regardless of EtOH intoxication at 4 h after injury. However, the increase in these parameters was not observed at 24 h after injury in rats subjected to 12.5% burn injury alone. Furthermore, there was no change in water content (edema) in rats receiving 12.5% burn injury. In contrast, there was a significant increase in intestinal edema in rats with 25% burn injury and intestinal IL-18 levels; MPO activity and edema remains elevated in rats with 25% TBSA burn injury at 24 h after injury. Alcohol intoxication at the time of injury exacerbated the increase in intestinal IL-18 levels, MPO activity, and edema in both 12.5% and 25% burn–injured rats. Although similar to our findings, many previous studies have also indicated a surge in neutrophil infiltration into the tissues early after burn injury (31, 32), but some other studies have indicated neutrophil presence in the tissue at 24 or even 72 h after burn injury (21, 33). Several factors ranging from the burn size or species (i.e., mice versus rats) are likely be a factor in the differences in those studies (25, 34, 35). Furthermore, a small burn injury may not be detrimental, but when a small burn injury is combined with other additional insults, such as smoke inhalation, wound infection, other septic complications, and so on, it becomes detrimental (1–3, 12, 22, 36, 37). It is likely that similar to these factors, EtOH intoxication before burn injury serves as an additional insult and thus exacerbates the postburn alterations. These results altogether indicate that burn injury size profoundly impacts the intestinal tissue MPO activity, edema, and IL-18 levels, and that EtOH intoxication at the time of injury further exacerbates these responses.
To delineate the mechanism by which EtOH intoxication potentiates postburn intestinal barrier dysfunction, the present study determined the role of IL-18 and neutrophils in impaired intestinal barrier dysfunction after EtOH and burn injury. Our previous studies had shown that the treatment of animals with Ac-YVAD-CHO, an inhibitor of caspase 1, prevented the increase in neutrophil accumulation in intestinal tissue and the increase in intestinal permeability (16, 17). Caspase 1 is an enzyme that processes IL-1 and IL-18 maturation. Because in the previous study, we found an increase in IL-18, and not in IL-1β, IL-18 was considered to be the cause of neutrophil-dependent intestinal tissue damage (16, 17). Nevertheless, because caspase 1 also processes IL-1 maturation, the effect of caspase 1 inhibitor on IL-18 in that study is somewhat shadowed. We now confirmed those findings by performing an additional study and using a specific antibody that neutralizes endogenous IL-18 levels. The treatment of animals with anti–IL-18 antibody prevented the increase in intestinal MPO activity and edema formation after a combined insult of EtOH intoxication and burn injury. Our findings further indicate that although acute EtOH intoxication before burn injury augments IL-18 release, IL-18 is not directly involved in the intestinal tissue damage, rather it enhances the neutrophil accumulation in the intestine that then contribute to the development of intestinal tissue edema and the increase in intestinal permeability after EtOH and burn injury. Previously we have shown that IL-18 upregulates neutrophil chemokines (i.e., CINC-1 and CINC-3) and the expression of intercellular cell adhesion molecule 1 (16). IL-18, like IL-12, was discovered initially to be a factor that enhances interferon-γ production and promotes cell-mediated immunity, which is essential to host defenses against a variety of infections (38). However, later studies found that like many other proinflammatory cytokines, IL-18 possesses broad immunomodulatory properties including tissue damage. Because neutrophil expresses IL-18 receptor (α and β) (38), IL-18 can activate neutrophil functions including a delay in apoptosis and the release of proteases and O2−. Besides IL-18, many other inflammatory mediators including granulocyte colony-stimulating factor, IL-1, IL-6, and TNF-α are also implicated in the delay of neutrophil apoptosis. Whether these cytokines have any role after EtOH intoxication and burn injury remains to be investigated.
Neutrophils are a major type of leukocytes and have a short life in the circulation. They play a key role in host defense against invading pathogens. They exit blood vessels and migrate to extravascular sites of infection or injury to destroy invading pathogens by releasing proteolytic enzymes and reactive oxygen species. After killing invading pathogens, the neutrophils die by apoptosis and are phagocytosed by macrophages to prevent release of cytotoxic agents by activated neutrophils and thus prevent the subsequent amplification of the inflammatory response. Therefore, neutrophil apoptosis and subsequent clearance are keys to the resolution of acute inflammation. Under infection and many injury conditions, any agent that promotes neutrophil activity and delay neutrophil apoptosis may cause tissue damage. Studies have shown that EtOH intoxication or burn injury results in neutrophil activation and release of proteases and free oxygen radicals (O2−) (20, 39, 40). Furthermore, a role for neutrophils is implicated in organ tissue injury in pathological conditions of acute and chronic EtOH intoxication, acute respiratory distress syndrome, and tissue ischemia (20, 33, 40–43). Our finding that the depletion of neutrophils prevents intestinal tissue damage strongly supports the suggestion that neutrophils play a critical role in intestinal tissue damage in rats that have undergone a combined insult of EtOH intoxication and burn injury.
In summary, our findings indicate a strong correlation between burn size and intestinal tissue damage, with increasing injury size being associated with higher tissue damage. Furthermore, acute EtOH intoxication exacerbates the increase in intestinal tissue MPO activity, IL-18 levels, and edema after burn injury. Treatment of animals with anti–IL-18 antibody prevented the increase in intestinal MPO activity and edema after a combined insult of EtOH and burn injury. Furthermore, the depletion of neutrophils before injury prevented the increase in intestinal MPO activity, edema, and permeability after EtOH and burn injury, but did not influence IL-18 levels. These findings suggest that the presence of neutrophil is critical to intestinal tissue damage after EtOH and burn injury, and that IL-18 seems to play a role in this process.
Acknowledgments
This study was supported by the National Institutes of Health (grant no. R21AA015979-01A1 and 1R01AA015731-01A2).
References
- 1.Murphy TJ, Paterson HM, Mannick JA, Lederer JA. Injury, sepsis, and the regulation of Toll-like receptor responses. J Leukoc Biol. 2004;75:400–407. doi: 10.1189/jlb.0503233. [DOI] [PubMed] [Google Scholar]
- 2.Schwacha MG, Chaudry IH. The cellular basis of post-burn immunosuppression: macrophages and mediators. Int J Mol Med. 2002;10:239–243. [PubMed] [Google Scholar]
- 3.Smith JW, Gamelli RL, Jones SB, Shankar R. Immunologic responses to critical injury and sepsis. J Intensive Care Med. 2006;21:160–172. doi: 10.1177/0885066605284330. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima R, Alexander JW, Gianotti L, Pyles T, Ogle CK. Bacterial translocation-related mortality may be associated with neutrophil-mediated organ damage. Shock. 1995;3:323–328. [PubMed] [Google Scholar]
- 5.Arturson G. Forty years in burns research—the postburn inflammatory response. Burns. 2000;26:599–604. doi: 10.1016/s0305-4179(00)00069-3. [DOI] [PubMed] [Google Scholar]
- 6.Choudhry MA, Chaudry IH. Alcohol intoxication and post-burn complications. Front Biosci. 2006;11:998–1005. doi: 10.2741/1857. [DOI] [PubMed] [Google Scholar]
- 7.Maier RV. Ethanol abuse and the trauma patient. Surg Infect (Larchmt) 2001;2:133–141. doi: 10.1089/109629601750469456. [DOI] [PubMed] [Google Scholar]
- 8.McGill V, Kowal-Vern A, Fisher SG, Kahn S, Gamelli RL. The impact of substance use on mortality and morbidity from thermal injury. J Trauma. 1995;38:931–934. doi: 10.1097/00005373-199506000-00019. [DOI] [PubMed] [Google Scholar]
- 9.McGwin G, Jr, Chapman V, Rousculp M, Robison J, Fine P. The epidemiology of fire-related deaths in Alabama, 1992–1997. J Burn Care Rehabil. 2000;21:75–83. doi: 10.1097/00004630-200021010-00016. [DOI] [PubMed] [Google Scholar]
- 10.Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- 11.Jones JD, Barber B, Engrav L, Heimbach D. Alcohol use and burn injury. J Burn Care Rehabil. 1991;12:148–152. doi: 10.1097/00004630-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Choudhry MA, Rana SN, Kavanaugh MJ, Kovacs EJ, Gamelli RL, Sayeed MM. Impaired intestinal immunity and barrier function: a cause for enhanced bacterial translocation in alcoho intoxication and burn injury. Alcohol. 2004;33:199–208. doi: 10.1016/j.alcohol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Deitch EA. Role of the gut lymphatic system in multiple organ failure. Curr Opin Crit Care. 2001;7:92–98. doi: 10.1097/00075198-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Choudhry MA, Fazal N, Goto M, Gamelli RL, Sayeed MM. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am J Physiol Gastrointest Liver Physiol. 2002;282:G937–G947. doi: 10.1152/ajpgi.00235.2001. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Rana SN, Kovacs EJ, Gamelli RL, Chaudry IH, Choudhry MA. Corticosterone suppresses mesenteric lymph node T cells by inhibiting p38/ERK pathway and promotes bacterial translocation after alcohol and burn injury. Am J Physiol Regul Integr Comp Physiol. 2005;289:R37–R44. doi: 10.1152/ajpregu.00782.2004. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Rana SN, Schwacha MG, Chaudry IH, Choudhry MA. A novel role for IL-18 in corticosterone-mediated intestinal damage in a two-hit rodent model of alcohol intoxication and injury. J Leukoc Biol. 2006;80:367–375. doi: 10.1189/jlb.1205745. [DOI] [PubMed] [Google Scholar]
- 17.Rana SN, Li X, Chaudry IH, Bland KI, Choudhry MA. Inhibition of IL-18 reduces myeloperoxidase activity and prevents edema in intestine following alcohol and burn injury. J Leukoc Biol. 2005;77:719–728. doi: 10.1189/jlb.0704396. [DOI] [PubMed] [Google Scholar]
- 18.Hildebrand F, Hubbard WJ, Choudhry MA, Thobe BM, Pape HC, Chaudry IH. Are the protective effects of 17{beta}-estradiol on splenic macrophages and splenocytes after trauma-hemorrhage mediated via estrogen-receptor (ER)-{alpha} or ER-{beta}? J Leukoc Biol. 2006;79:1173–1180. doi: 10.1189/jlb.0106029. [DOI] [PubMed] [Google Scholar]
- 19.Partrick DA, Moore EE, Offner PJ, Meldrum DR, Tamura DY, Johnson JL, Silliman CC. Maximal human neutrophil priming for superoxide production and elastase release requires p38 mitogen-activated protein kinase activation. Arch Surg. 2000;135:219–225. doi: 10.1001/archsurg.135.2.219. [DOI] [PubMed] [Google Scholar]
- 20.Sayeed MM. Exuberant Ca(2+) signaling in neutrophils: a cause for concern. News Physiol Sci. 2000;15:130–136. [PubMed] [Google Scholar]
- 21.Sir O, Fazal N, Choudhry MA, Goris RJ, Gamelli RL, Sayeed MM. Role of neutrophils in burn-induced microvascular injury in the intestine. Shock. 2000;14:113–117. doi: 10.1097/00024382-200014020-00006. [DOI] [PubMed] [Google Scholar]
- 22.Alexander M, Chaudry IH, Schwacha MG. Relationships between burn size, immunosuppression, and macrophage hyperactivity in a murine model of thermal injury. Cell Immunol. 2002;220:63–69. doi: 10.1016/s0008-8749(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 23.O’Mahony JB, Wood JJ, Rodrick ML, Mannick JA. Changes in T lymphocyte subsets following injury. Assessment of flow cytometry and relationship to sepsis. Ann Surg. 1985;202:580–586. doi: 10.1097/00000658-198511000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mlcak RP, Dimick AR, Mlcak G. Pre-hospital management, transportation and emergency care. In: Herndon DN, editor. Total Burn Care. Philadelphia: W. B. Saunders Company Ltd; 1997. pp. 33–43. [Google Scholar]
- 25.Ananthakrishnan P, Cohen DB, Xu DZ, Lu Q, Feketeova E, Deitch EA. Sex hormones modulate distant organ injury in both a trauma/hemorrhagic shock model and a burn model. Surgery. 2005;137:56–65. doi: 10.1016/j.surg.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 26.McGwin G, Jr, George RL, Cross JM, Reiff DA, Chaudry IH, Rue LW., III Gender differences in mortality following burn injury. Shock. 2002;18:311–315. doi: 10.1097/00024382-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Schwacha MG, Chaudry IH, Choudhry MA. A role of PP1/PP2A in mesenteric lymph node T cell suppression in a two-hit rodent model of alcohol intoxication and injury. J Leukoc Biol. 2006;79:453–462. doi: 10.1189/jlb.0705369. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Kovacs EJ, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication increases interleukin 18-mediated neutrophil infiltration and lung inflammation following burn injury in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1193–L1201. doi: 10.1152/ajplung.00408.2006. [DOI] [PubMed] [Google Scholar]
- 30.Faunce DE, Gregory MS, Kovacs EJ. Acute ethanol exposure prior to thermal injury results in decreased T-cell responses mediated in part by increased production of IL-6. Shock. 1998;10:135–140. doi: 10.1097/00024382-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Toth B, Alexander M, Daniel T, Chaudry IH, Hubbard WJ, Schwacha MG. The role of gammadelta T cells in the regulation of neutrophil-mediated tissue damage after thermal injury. J Leukoc Biol. 2004;76:545–552. doi: 10.1189/jlb.0404219. [DOI] [PubMed] [Google Scholar]
- 32.Patel PJ, Faunce DE, Gregory MS, Duffner LA, Kovacs EJ. Elevation in pulmonary neutrophils and prolonged production of pulmonary macrophage inflammatory protein-2 after burn injury with prior alcohol exposure. Am J Respir Cell Mol Biol. 1999;20:1229–1237. doi: 10.1165/ajrcmb.20.6.3491. [DOI] [PubMed] [Google Scholar]
- 33.Sir O, Fazal N, Choudhry MA, Gamelli RL, Sayeed MM. Neutrophil depletion prevents intestinal mucosal permeability alterations in burn-injured rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1224–R1231. doi: 10.1152/ajpregu.2000.278.5.R1224. [DOI] [PubMed] [Google Scholar]
- 34.Horton JW. Bacterial translocation after burn injury: the contribution of ischemia and permeability changes. Shock. 1994;1:286–290. [PubMed] [Google Scholar]
- 35.Tadros T, Traber DL, Heggers JP, Herndon DN. Effects of interleukin-1alpha administration on intestinal ischemia and reperfusion injury, mucosal permeability, and bacterial translocation in burn and sepsis. Ann Surg. 2003;237:101–109. doi: 10.1097/00000658-200301000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J Immunol. 2005;174:404–410. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 37.Alexander M, Daniel T, Chaudry IH, Schwacha MG. Opiate analgesics contribute to the development of post-injury immunosuppression. J Surg Res. 2005;129:161–168. doi: 10.1016/j.jss.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 39.Fazal N, Al Ghoul WM, Schmidt MJ, Choudhry MA, Sayeed MM. Lyn- and ERK-mediated vs. Ca2+-mediated neutrophil O responses with thermal injury. Am J Physiol Cell Physiol. 2002;283:C1469–C1479. doi: 10.1152/ajpcell.00114.2002. [DOI] [PubMed] [Google Scholar]
- 40.Polikandriotis JA, Rupnow HL, Elms SC, Clempus RE, Campbell DJ, Sutliff RL, Brown LA, Guidot DM, Hart CM. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. Am J Respir Cell Mol Biol. 2006;34:314–319. doi: 10.1165/rcmb.2005-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao XP, Xu N, Sekosan M, Mehta D, Ma SY, Rahman A, Malik AB. Differential role of CD18 integrins in mediating lung neutrophil sequestration and increased microvascular permeability induced by Escherichia coli in mice. J Immunol. 2001;167:2895–2901. doi: 10.4049/jimmunol.167.5.2895. [DOI] [PubMed] [Google Scholar]
- 42.Guo RF, Ward PA. Mediators and regulation of neutrophil accumulation in inflammatory responses in lung: insights from the IgG immune complex model. Free Radic Biol Med. 2002;33:303–310. doi: 10.1016/s0891-5849(02)00823-7. [DOI] [PubMed] [Google Scholar]
- 43.Yu HP, Yang S, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. Maintenance of lung myeloperoxidase activity in proestrus females following trauma-hemorrhage: upregulation of hemeoxygenase-1. Am J Physiol Lung Cell Mol Physiol. 2006;291:L400–L406. doi: 10.1152/ajplung.00537.2005. [DOI] [PubMed] [Google Scholar]