Abstract
Purpose
While obesity at time of prostatectomy has been associated with prostate cancer recurrence, it is unknown whether obesity before or after surgery, or weight change from the years prior to surgery to after surgery is associated with recurrence. Thus, we examined the influence of obesity and weight change on recurrence after prostatectomy.
Methods
We conducted a retrospective cohort study of 1,337 men with clinically-localized prostate cancer who underwent prostatectomy performed during 1993-2006 by the same surgeon. Men self-reported weight and physical activity at 5 years before and 1 year after surgery on a survey during follow-up. Mean follow up was 7.3 years. We estimated multivariable-adjusted hazard ratios of prostate cancer recurrence comparing obesity at 5 years before and at 1 year after surgery with normal weight, and a gain of >2.2 kg from 5 years before to 1 year after surgery with stable weight.
Results
During 9,797 person years of follow-up, 102 men recurred. Compared with men who had stable weight, those whose weight increased >2.2 kg had twice the recurrence risk (HR=1.94, 95% CI 1.14-3.32) after taking into account age, pathological stage and grade, and other characteristics. The HR of recurrence was 1.20 (95% CI 0.64-2.23) and 1.72 (95% CI 0.94-3.14) comparing obesity at 5 years before and at 1 year after surgery, respectively, with normal weight. Physical activity (≥5 hrs/wk) did not attenuate risk in men who gained >2.2 kg.
Conclusions
By avoiding weight gain, men with prostate cancer may both prevent recurrence and improve overall well-being.
Keywords: weight change, prostate cancer recurrence, prostatectomy, obesity, physical activity
Introduction
Approximately 20% of men with clinically localized prostate cancer who undergo radical prostatectomy recur within 10 years post-surgery (1). Currently, there are no evidence-based recommendations for men to reduce their risk of recurrence after treatment. Focusing on modifiable factors may provide opportunities for men with prostate cancer to prevent recurrence and improve their overall well-being through behavior change.
Obesity measured at or near time of treatment for early stage prostate cancer has been associated with increased risk of recurrence in most (2-15), but not all studies (16-19). Obesity has also been associated with high grade disease at diagnosis, and the development of advanced or fatal prostate cancer, but not total prostate cancer incidence (20-23). However, the relevant timing of obesity (e.g., before or after diagnosis) to risk of prostate cancer recurrence has not been established. Further, whether weight change influences risk is largely unstudied. Weight gain between young adulthood and time of diagnosis was associated with prostate cancer recurrence in one prospective study; though, the analysis did not account for important clinical and pathological factors that may have confounded the association (8). Determining the relevant timing of obesity and understanding the effect of weight change on prostate cancer recurrence are necessary to both elucidate the mechanism by which adiposity influences risk and to develop strategies to prevent recurrence.
Physical inactivity may exacerbate the obesity-related risk of prostate cancer recurrence, though this relationship has not been explored. While studies of physical activity and prostate cancer incidence have been inconsistent overall (24), several of the recent large studies suggest at least some benefit of increased physical activity on prostate cancer risk (25-27).
We investigated the association of obesity 5 years before and 1 year after prostatectomy, as well as weight change during this time period with prostate cancer recurrence in a retrospective cohort study. We selected the interval of 5 years before surgery to 1 year after surgery to capture the possible influence of weight gain on cancer cells that may have escaped from the primary tumor before surgery. We also assessed both the independent and modifying effects of physical inactivity, an obesity-related behavior.
Methods
Study Population
We conducted a retrospective cohort study of men with clinically-localized prostate cancer who underwent radical retropubic prostatectomy performed by the same surgeon at Johns Hopkins between January 1, 1993 and March 31, 2006 (n=2,498). Men were excluded if they previously had hormone or radiation therapy (1.2%). For all men eligible for this study, electronic or paper medical records were reviewed by one abstractor (AMM) who was blinded to recurrence status. Information on age, race/ethnicity, first degree family history of prostate cancer, preoperative PSA, surgery year, positive surgical margins, pathologic stage and Gleason sum was abstracted from the medical records. Men alive and residing in the United States as of November 2007 were mailed a survey on dietary, lifestyle, and medical factors (n=2,111). This analysis included men who responded to the survey as of August 2009 and had complete information on height, weight, and physical activity (n=1,337). Men were followed for recurrence through August 2009, with mean follow-up time of 7.3 years. The follow-up of this cohort was approved by the Institutional Review Board at the Johns Hopkins School of Medicine. This analysis was additionally approved by the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health.
Exposure Assessment
In the survey, the men self-reported their weight and physical activity level at 5 years before surgery and 1 year after surgery. In this retrospective study, we chose to evaluate 1 year after surgery, as opposed to at the time of surgery, to avoid weight and behavior change specifically related to diagnosis and treatment of the initial prostate cancer.
We calculated body mass index (BMI) using height and weight data, and classified men as normal (<25 kg/m2), overweight (25-29.9 kg/m2), or obese (≥30 kg/m2). Weight change was defined as the difference in self-reported weight at 5 years before surgery and 1 year after surgery. To capture physical activity, men were asked the number of hours per week they spent “doing leisure time physical activity (walking, running, lap swimming, bicycling, and other sports).” Physical activity at 5 years before surgery and at 1 year after surgery were categorized into tertiles for primary analyses (<4 hrs/wk, 4-6 hrs/wk, and ≥7 hrs/wk). For stratified analyses, physical activity at 1 year after surgery was divided at the median value (≥5 hrs/wk as physically active, <5hrs/wk inactive). Men were asked whether they had ever received a diagnosis of diabetes from a doctor and the month and year of the diagnosis. Men were classified as having been diagnosed before or after surgery (but before recurrence).
Outcome Assessment
Men were evaluated by their primary care physicians with PSA tests and digital-rectal examinations every three months for the first year post-operative, semiannually for the second year, and annually thereafter. Recurrence was defined as confirmed PSA re-elevation to ≥0.2 ng/mL above a nadir of non-detectable, local recurrence, metastasis, or death from prostate cancer. Annually, the surgeon contacted the men to request that their physician send their most recent PSA test results. Elevated tests were repeated by the surgeon. PSA information was complete for 99% and 78% of the men at 1 and 5 years after surgery, respectively. Prostate cancer death was obtained through family report or linkage with the National Death Index. Of the 102 men who recurred, 11% had metastases or died from prostate cancer.
Statistical Analysis
We calculated age-adjusted means and proportions for demographic and other factors by BMI 5 years before surgery and weight change using regression modeling. Men began contributing time at risk starting 1 year after surgery. Cases were men who recurred after the first year post surgery. We used Cox proportional hazards regression to estimate the hazard ratio (HR) of recurrence for obesity and physical activity at 5 years before surgery and at 1 year after surgery, and weight change. Weight change was modeled in two ways. First, we used indicator variables for weight loss and gain with maintenance as the reference, defined as a weight change ≤ 2.2 kg, to allow for minor weight fluctuation. Second, to avoid subjective cutpoints and assess the shape of the association, continuous weight change was modeled using restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles of the distribution. The top and bottom 1% of the distribution of weight change were excluded from the restricted quadratic splines model to avoid the influence of extreme values. To test whether the association between weight gain and recurrence was consistent with a linear dose-response, we used the likelihood ratio test to compare the linear and spline models. We confirmed the proportional hazards assumption for all models by including an interaction term between the main effect and follow-up time in the model and testing the coefficient using the Wald test. All models were compatible with the proportional hazards assumption. To test for trend across levels of BMI, physical activity, and weight change, we assigned each man the median value within his category of weight. The median values were modeled and the coefficient was tested with the Wald test. To explore whether physical activity level use modified the association of obesity and weight gain and with recurrence risk, we stratified the analysis by physical activity level.
Three primary analyses were performed for obesity and physical activity: (1) adjusted for age; (2) adjusted for age and non-modifiable risk factors including race/ethnicity, family history, year of prostatectomy, pathological stage and grade; and (3) adjusted for all covariates in the previous models plus modifiable risk factors including cigarette smoking status, and mutual adjustment for physical activity and BMI in the same time period. Because weight and weight change are correlated, to determine the independent association for weight change and recurrence, we adjusted for weight at 5 years before surgery and height along with the non-modifiable factors (listed above), cigarette smoking status and physical activity. In the primary analyses, diabetes did not appear to be a confounder and thus was not included in the models. In sub-analyses, we further adjusted for preoperative PSA, excluded men with positive surgical margins, and excluded men with diabetes. All analyses were conducted using SAS version 9.1 (Cary, NC). All tests were two-sided and results were considered statistically significant if p<0.05.
Results
At 5 years before prostatectomy, approximately 53% of men were overweight and 9% were obese. The men who were obese were also less active 5 years before surgery, more likely to be former smokers and to report a diagnosis of diabetes (Table 1). After pathologic review, obese men were less likely to have organ-confined disease and more likely to have positive surgical margins. Between 5 years before surgery and 1 year after surgery, 13.9% of men gained >2.2 kg and 12.7% of men lost >2.2 kg. Men who gained weight were younger and more likely to be current smokers (Table 1). Men who lost weight were more likely to be diabetic. Compared with men who maintained their weight, men who lost weight had a higher BMI and were less active at 5 years before prostatectomy. Pathologic characteristics did not differ among categories of weight change.
Table 1.
*Age-adjusted demographic and pathologic tumor characteristics by body mass index 5 years before prostatectomy and weight change.
| Body Mass Index (kg/m2) | Weight Change (>2.2 kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| <25.0 (n=503) |
25.0-29.9 (n=716) |
≥30.0 (n=118) |
p-value | Loss (n=170) |
Maintenance (n=981) |
Gain (n=186) |
p-value | |
| Mean age (SD) | 56.4(6.8) | 56.4(6.5) | 57.0(6.3) | 0.66 | 57.0(6.1) | 56.8(6.5) | 53.9(6.9) | <.001 |
| Race/ethnicity (%) | ||||||||
| White | 95.6 | 94.4 | 95.7 | 94.7 | 94.9 | 95.9 | ||
| Black | 0.6 | 2.5 | 2.6 | 0.14 | 0.0 | 2.0 | 2.5 | 0.91 |
| Other/missing | 3.8 | 3.1 | 1.7 | 5.3 | 3.2 | 1.6 | ||
| Family history of prostate cancer (%) | ||||||||
| No | 66.1 | 67.9 | 66.5 | 70.1 | 66.2 | 69.3 | ||
| Yes | 27.3 | 26.1 | 27.6 | 0.97 | 21.1 | 27.9 | 25.4 | 0.27 |
| Missing | 6.6 | 6.0 | 5.8 | 8.7 | 5.9 | 5.3 | ||
| Smoking status (%) | ||||||||
| Never | 57.8 | 50.4 | 46.8 | 42.6 | 55.9 | 46.0 | ||
| Former | 38.6 | 45.8 | 53.1 | 0.04 | 54.9 | 40.7 | 49.5 | <0.01 |
| Current | 2.0 | 2.5 | 0 | 1.2 | 2.1 | 2.9 | ||
| Missing | 1.6 | 1.3 | 0 | 1.2 | 1.2 | 1.6 | ||
| Mean pre-operative PSA (SE) | 6.4(0.2) | 6.7(0.2) | 6.6(0.4) | 0.52 | 6.8(0.3) | 6.5(0.1) | 6.9(0.3) | 0.36 |
| Median surgery year | 1999.2 | 1999.1 | 1999.4 | 0.77 | 1999.5 | 1999.1 | 1999.7 | 0.07 |
| Mean pathologic Gleason sum (SE) | 6.3(0.03) | 6.3(0.02) | 6.4(0.06) | 0.17 | 6.3(0.05) | 6.3(0.02) | 6.3(0.05) | 0.72 |
| Pathologic stage (%) | ||||||||
| Organ-confined | 78.7 | 73.7 | 59.6 | 71.6 | 74.9 | 73.9 | ||
| Focal or established capsular penetration | 17.7 | 22.5 | 35.3 | 0.001 | 23.8 | 21.8 | 20.4 | 0.60 |
| Seminal vesicle or lymph node positive | 0.03 | 0.04 | 0.04 | 4.6 | 3.2 | 5.1 | ||
| Positive surgical margins (%) | 4.4 | 6.4 | 11.8 | 0.01 | 7.6 | 6.2 | 4.7 | 0.54 |
| Diabetes mellitus diagnosis (%) | ||||||||
| Never | 98.6 | 96.8 | 84.8 | 90.6 | 97.4 | 96.6 | ||
| Before prostatectomy | 0.6 | 1.5 | 9.3 | 0.001 | 4.7 | 1.4 | 1.7 | <0.01 |
| After prostatectomy | 0.8 | 1.7 | 5.9 | 4.7 | 1.2 | 1.7 | ||
| Body mass index, kg/m2 | ||||||||
| Mean 5 years before prostatectomy (SE) | 23.2(0.06) | 26.8(0.05) | 32.4(0.1) | <0.001 | 28.8(0.2) | 25.4(0.09) | 26.3(0.2) | <0.001 |
| Mean 1 year after prostatectomy (SE) | 23.4(0.08) | 27.0(0.06) | 31.2(0.2) | <0.001 | 26.7(0.2) | 25.5(0.09) | 28.1(0.2) | <0.001 |
| Mean weight change, kg (SE) | 0.6(0.2) | 0.4(0.1) | -3.6(0.3) | <0.001 | -6.6(0.2) | 0.3(0.07) | 5.7(0.2) | <0.001 |
| Physical activity, hrs/wk | ||||||||
| Mean 5 years before prostatectomy (SE) | 7.1(0.3) | 6.7(0.2) | 5.3(0.6) | 0.01 | 5.6(0.5) | 6.8(0.2) | 7.5(0.5) | 0.01 |
| Mean 1 year after prostatectomy (SE) | 7.0(0.3) | 6.6(0.2) | 6.3(0.6) | 0.32 | 6.6(0.5) | 6.8(0.2) | 6.3(0.5) | 0.50 |
Characteristics of 1,337 men undergoing radical prostatectomy at Johns Hopkins Hospital 1993-2006. Standard deviation (SD). Standard error (SE). Prostate-specific antigen (PSA).
Men who were obese at 5 years before prostatectomy appeared to have an increased age-adjusted risk of prostate cancer recurrence compared to normal weight men; though this association was not statistically significant and was attenuated in the multivariable models (Table 2). Overweight men did not have an increased risk of recurrence as compared to normal weight men. Likewise, physical inactivity at 5 years before prostatectomy was not associated with risk of prostate cancer recurrence.
Table 2.
Association of body mass index and physical activity 5 years before and 1 year after prostatectomy with prostate cancer recurrence.
| 5 Years Before Prostatectomy* | 1 Year After Prostatectomy* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases/Total | Person Years | Age Adjusted HR (95%CI) |
Multivariable Adjusted1 HR (95% CI) |
Multivariable Adjusted1,2 HR (95% CI) |
Cases/Total | Person Years | Age Adjusted HR (95%CI) |
Multivariable Adjusted† HR (95% CI) |
Multivariable Adjusted†,‡ HR (95% CI) |
|
| Body mass index (kg/m2) | ||||||||||
| <25.0 | 40/503 | 3,686 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 35/497 | 3,690 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 25.0-29.9 | 47/716 | 5,289 | 0.82 (0.54,1.25) | 0.76 (0.50,1.16) | 0.73 (0.47,1.13) | 50/721 | 5,266 | 1.00 (0.65,1.54) | 0.92 (0.60,1.43) | 0.89 (0.57,1.38) |
| ≥30.0 | 15/118 | 822 | 1.64 (0.91,2.98) | 1.27 (0.69,2.33) | 1.20 (0.64,2.23) | 17/119 | 841 | 2.17 (1.22,3.88) | 1.78 (0.98,3.21) | 1.72 (0.94,3.14) |
| Test for trend§ | 0.27 | 0.71 | 0.84 | 0.02 | 0.10 | 0.13 | ||||
| Physical activity (hrs/wk) | ||||||||||
| <4 | 28/405 | 2,966 | 0.95 (0.59,1.55) | 0.94 (0.57,1.53) | 0.95 (0.58,1.56) | 25/384 | 2,811 | 0.99 (0.59,1.65) | 0.99 (0.59-1.66) | 0.96 (0.57,1.63) |
| 4-6 | 35/415 | 2,948 | 1.20 (0.76,1.90) | 1.24 (0.78,1.98) | 1.27 (0.80,2.02) | 42/439 | 3,198 | 1.43 (0.91,2.24) | 1.34 (0.86,2.10) | 1.32 (0.84,2.07) |
| ≥7 | 39/517 | 3,883 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 35/514 | 3,788 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Test for trend§ | 0.99 | 0.98 | 0.97 | 0.71 | 0.76 | 0.83 | ||||
1337 men undergoing radical prostatectomy at Johns Hopkins Hospital 1993-2006. Follow-up began 1 year after prostatectomy.
Hazard ratio (HR) and 95% confidence interval (CI) adjusted for age, race/ethnicity, family history, year of prostatectomy, stage, and grade.
HR (95%CI) adjusted for smoking status, and mutually adjusted for body mass index and physical activity.
Median values of each category modeled in test for trend.
Obesity at 1 year after surgery was associated with a two-fold increase in risk of prostate cancer recurrence as compared to normal weight men after adjustment for age. After multivariable adjustment, the association was modestly attenuated, and no longer statistically significant. In sub-analyses, non-statistically significant positive associations between obesity at 1 year after surgery and recurrence were observed with additional adjustment for pre-surgery PSA (multivariable adjusted HR: 1.64; 95%CI: 0.89,3.03), after exclusion of men with positive surgical margins (HR: 1.59; 95%CI: 0.81,3.13), and after exclusion of men with diabetes (HR: 1.50; 95%CI: 0.78,2.87). Physical inactivity at 1 year after prostatectomy was not associated with an increased risk of prostate cancer recurrence; this association did not change in sub-analyses further adjusting for pre-surgery PSA, excluding men with positive surgical margins, or excluding men with diabetes (data not shown).
Men who gained weight were at a nearly two-fold increased risk of prostate cancer recurrence when compared to men who maintained their weight; this result was statistically significant (Table 3). Men who lost weight appeared to have a lower risk of prostate cancer recurrence, though the association was not statistically significant. When the association between weight change and recurrence was modeled using restricted quadratic splines, the risk of prostate cancer recurrence increased with increasing weight gain, whereas risk appeared to decrease with weight loss (Figure 1). This association was statistically consistent with a linear dose-response. In sub-analyses, there was a similar association between weight gain and recurrence with additional adjustment for pre-surgery PSA (HR: 1.85; 95%CI: 1.07,3.20) and when men with diabetes were excluded (HR: 1.90; 95%CI: 1.10,3.29); although the association was attenuated when men with positive surgical margins were excluded (HR: 1.47; 95%CI: 0.80,2.69). The association between weight change and recurrence did not differ by BMI (<25, ≥25 kg/m2) at 5 years before surgery (data not shown).
Table 3.
Association of weight change from 5 years before to 1 year after prostatectomy with prostate cancer recurrence.
| Weight Change >2.2kg | ||||
|---|---|---|---|---|
| Median Weight Change (kg) |
Cases/Total | Person Years | HR* (95% CI) |
|
| Decrease | -5.22 | 11/170 | 1,186 | 0.77 (0.38,1.53) |
| Maintenance | 0 | 71/981 | 7,391 | 1.00(ref) |
| Increase | 4.54 | 20/186 | 1,220 | 1.94 (1.14,3.32) |
| Test for trend† | 0.02 | |||
1,337 men at Johns Hopkins Hospital 1993-2006. Hazard ratio (HR) and 95% confidence interval (CI) adjusted for weight 5 years before prostatectomy, height, physical activity 1 year after prostatectomy, age, race/ethnicity, family history, year of prostatectomy, stage, grade, and smoking status.
Median values of each category modeled in test for trend.
Figure 1.
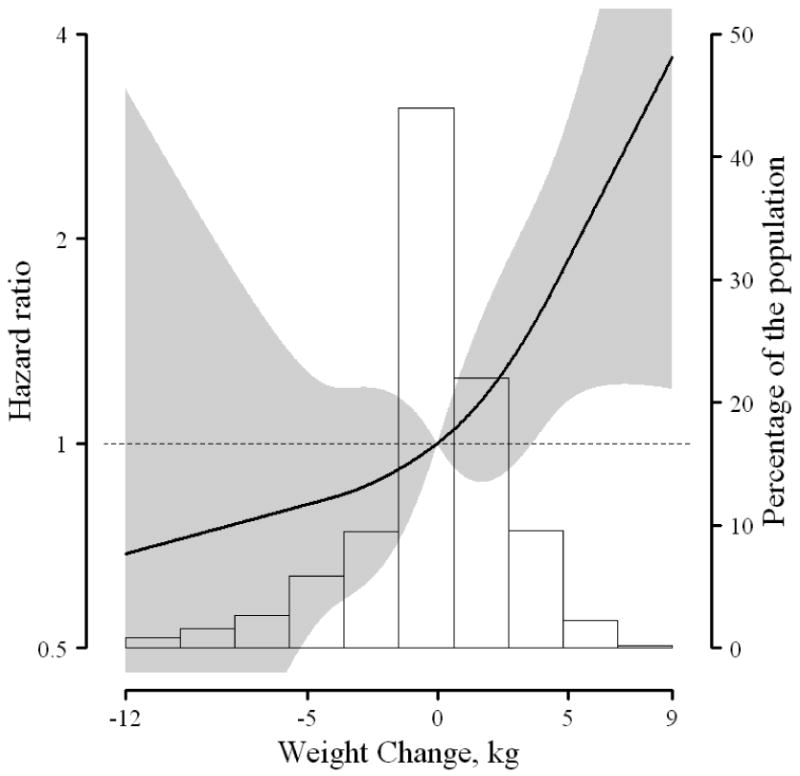
Multivariable-adjusted hazard ratio of prostate cancer recurrence associated with weight change using restricted quadratic splines. Adjusted for weight 5 years before prostatectomy, height, physical activity 1 year after prostatectomy, age, race/ethnicity, family history, year of prostatectomy, stage, grade, and smoking status. Gray shading: Represents confidence intervals. Background: Distribution of weight change in study population.
Among inactive men, obesity at 1 year after surgery was associated with a nearly two-fold increased risk of prostate cancer recurrence, though not statistically significant (Table 4), whereas the association for obesity 1 year after surgery was attenuated among active men. Physical inactivity did not modify the elevated risk of recurrence in men who gained weight; the risk of recurrence was higher in men who gained weight irrespective of physical activity level (Table 4).
Table 4.
Association between body mass index 1 year after prostatectomy and prostate cancer recurrence by physical activity level.
| Physical Activity <5 hrs/wk | |||||||
|---|---|---|---|---|---|---|---|
| Body Mass Index (kg/m2) 1 year after |
Cases/Total | Person Years | HR* (95% CI) |
Weight Change > 2kg |
Cases/Total | Person Years | HR† (95% CI) |
| <25.0 | 12/176 | 1,286 | 1.00 (ref) | Decrease | 6/69 | 482 | 0.76 (0.27,2.11) |
| 25.0-29.9 | 17/270 | 1,969 | 0.76 (0.35,1.65) | Maintenance | 26/370 | 2,758 | 1.00 (ref) |
| ≥30.0 | 10/65 | 437 | 1.94 (0.76,4.93) | Increase | 7/72 | 452 | 1.52 (0.57,4.06) |
| Test for trend‡ | 0.12 | 0.42 | |||||
| Physical Activity ≥5 hrs/wk | |||||||
| <25.0 | 23/321 | 2,404 | 1.00 (ref) | Decrease | 5/101 | 704 | 0.68 (0.25,1.85) |
| 25.0-29.9 | 33/451 | 3,297 | 0.85 (0.49,1.47) | Maintenance | 45/611 | 4,633 | 1.00 (ref) |
| ≥30.0 | 7/54 | 404 | 1.11 (0.45,2.73) | Increase | 13/114 | 768 | 2.10 (1.06,4.18) |
| Test for trend‡ | 0.94 | 0.02 | |||||
1,337 men undergoing radical prostatectomy at Johns Hopkins Hospital 1993-2006. Hazard ratio (HR) and 95% confidence interval (CI) adjusted for age, race/ethnicity, family history, year of prostatectomy, stage, grade, smoking status, and physical activity 1 year after prostatectomy.
Further adjusted for weight at 5 years before prostatectomy and height.
Median values of each category modeled in test for trend.
Discussion
In this retrospective cohort study, weight gain from 5 years before prostatectomy to 1 year after was associated with a nearly two-fold increased risk of prostate cancer recurrence. This association remained significant even among physically active men. Further, there appeared to be a linear association between weight change and recurrence, suggesting that risk of recurrence increased with increasing weight gain, and decreased with increasing weight loss.
Obesity and weight gain may influence risk of prostate cancer recurrence through several mechanisms, including metabolic, hormonal, and inflammatory pathways. Obese men tend to have higher insulin and leptin levels and lower androgen levels (23). These metabolic and hormonal changes may differ throughout the natural history of weight change. The consequences of these adipose-mediated metabolic and hormonal changes may influence prostate cancer differently depending on when they occur during the natural history of prostate cancer carcinogenesis: before disease is present, early in the natural history, or after detection or treatment. We focused on the time frame when the tumor is present, but not yet diagnosed, and cancer cells are escaping from the primary tumor before the prostatectomy. We thus addressed the influence of weight change on the ability of those cancer cells to escape, survive and proliferate. Future studies should address whether weight change in the years following prostatectomy influences recurrence independent of weight change circa diagnosis and surgery.
While weight gain from young adulthood to middle age is not associated with or inversely associated with prostate cancer incidence in most (28-37) but not all studies (38), only one previous study has addressed weight gain and prostate cancer recurrence. Strom and colleagues evaluated annual weight gain from age 25 to time of diagnosis; they reported an unadjusted two-fold increased risk of recurrence among men with an annualized average weight gain >1.5 kg/yr (8). Because only an unadjusted analysis was reported, it is unclear whether weight gain was a risk factor for recurrence independent of obesity and/or pathologic characteristics. In our study the positive association between weight gain and recurrence was independent of pathological tumor characteristics as well as cigarette smoking status, physical activity level, weight and height.
In our study, obesity 1 year after surgery appeared to be associated with an increased risk of recurrence; an association that was attenuated among physically active men. Obesity in middle age has been extensively studied for prostate cancer incidence and mortality, and obesity at time of surgery for risk of prostate cancer recurrence. Specifically, obesity in middle age has been associated with a higher incidence of advanced stage and higher grade prostate cancer, a higher risk of death from prostate cancer, and obesity at diagnosis has been associated with higher case fatality in men with prostate cancer (22, 23). In contrast, obesity has been observed to be either not associated or inversely associated with risk of early stage and low grade disease (22, 23). Our findings for obesity at 1 year after surgery, while not statistically significant, are consistent with those from studies reporting a positive association between obesity at the time of surgery and recurrence (2-4, 7, 8, 12, 13, 15), including for a prior study conducted in prostate cancer patients of the one surgeon (5) and of multiple surgeons at Johns Hopkins Hospital (6).
We also evaluated whether the associations for weight change and obesity were modified by physical inactivity. We assessed these interactions because this potential modifier s may also influence metabolic, hormonal and inflammatory pathways (23, 39). While we found suggestions that physical activity differentially influenced the associations of obesity and weight change with recurrence, these findings were not statistically significantly different.
We evaluated obesity, physical activity, and weight change in a cohort of men with prostate cancer who underwent radical prostatectomy performed by the same surgeon. Obesity is associated with worse stage/grade at time of diagnosis (22, 23), which is, in turn, a strong predictor of recurrence. Thus in our analysis, we adjusted for pathologic stage and grade, so that the independent effects of obesity and of weight gain could be detected. Because all men had to be healthy enough to undergo surgery, the prevalence of obesity was much lower in our sample than in the US population. Despite this select group of patients of a single surgeon, we expect that our findings are generalizable across the range of BMI and weight change present in our study. Because these men had clinically-localized disease, we had low power to perform sub-group analyses by stage and grade. Although obesity is associated with PSA in men without a diagnosis of prostate cancer, previous work found no difference in the performance of PSA as a predictor of biochemical recurrence by weight status (40). Adjustment for pre-surgery PSA level did not change our inferences. We asked the men to report their leisure time physical activity using a single question; this measure was not validated and may not capture the full range of activity in all men. Our measures were retrospective, so may be subject to recall bias.
Obesity and weight gain in adulthood have been associated with numerous adverse health outcomes. Our study suggests that weight gain may also increase the risk of prostate cancer recurrence among men who have undergone radical prostatectomy to treat clinically-localized prostate cancer. Thus, by avoiding weight gain, men with prostate cancer may both prevent recurrence and improve overall well-being.
Acknowledgments
Funding/Support: This research was supported by Nation Cancer Institute grants T32 CA009314 (Dr. Joshu and Dr. Mondul) and P50 CA58236.
Footnotes
Publisher's Disclaimer: Disclaimers: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Han M, Partin AW, Piantadosi S, Epstein JI, Walsh PC. Era specific biochemical recurrence-free survival following radical prostatectomy for clinically localized prostate cancer. J Urol. 2001;166:416–9. [PubMed] [Google Scholar]
- 2.Amling CL, Riffenburgh RH, Sun L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439–45. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 3.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–53. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 4.Bassett WW, Cooperberg MR, Sadetsky N, et al. Impact of obesity on prostate cancer recurrence after radical prostatectomy: data from CaPSURE. Urology. 2005;66:1060–5. doi: 10.1016/j.urology.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 5.Freedland SJ, Isaacs WB, Mangold LA, et al. Stronger association between obesity and biochemical progression after radical prostatectomy among men treated in the last 10 years. Clin Cancer Res. 2005;11:2883–8. doi: 10.1158/1078-0432.CCR-04-2257. [DOI] [PubMed] [Google Scholar]
- 6.Freedland SJ, Grubb KA, Yiu SK, et al. Obesity and risk of biochemical progression following radical prostatectomy at a tertiary care referral center. J Urol. 2005;174:919–22. doi: 10.1097/01.ju.0000169459.78982.d7. [DOI] [PubMed] [Google Scholar]
- 7.Mallah KN, DiBlasio CJ, Rhee AC, Scardino PT, Kattan MW. Body mass index is weakly associated with, and not a helpful predictor of, disease progression in men with clinically localized prostate carcinoma treated with radical prostatectomy. Cancer. 2005;103:2030–4. doi: 10.1002/cncr.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strom SS, Wang X, Pettaway CA, et al. Obesity, weight gain, and risk of biochemical failure among prostate cancer patients following prostatectomy. Clin Cancer Res. 2005;11:6889–94. doi: 10.1158/1078-0432.CCR-04-1977. [DOI] [PubMed] [Google Scholar]
- 9.Strom SS, Kamat AM, Gruschkus SK, et al. Influence of obesity on biochemical and clinical failure after external-beam radiotherapy for localized prostate cancer. Cancer. 2006;107:631–9. doi: 10.1002/cncr.22025. [DOI] [PubMed] [Google Scholar]
- 10.Palma D, Pickles T, Tyldesley S. Obesity as a predictor of biochemical recurrence and survival after radiation therapy for prostate cancer. BJU Int. 2007;100:315–9. doi: 10.1111/j.1464-410X.2007.06897.x. [DOI] [PubMed] [Google Scholar]
- 11.Stroup SP, Cullen J, Auge BK, L'Esperance JO, Kang SK. Effect of obesity on prostate-specific antigen recurrence after radiation therapy for localized prostate cancer as measured by the 2006 Radiation Therapy Oncology Group-American Society for Therapeutic Radiation and Oncology (RTOG-ASTRO) Phoenix consensus definition. Cancer. 2007;110:1003–9. doi: 10.1002/cncr.22873. [DOI] [PubMed] [Google Scholar]
- 12.Hisasue S, Yanase M, Shindo T, et al. Influence of body mass index and total testosterone level on biochemical recurrence following radical prostatectomy. Jpn J Clin Oncol. 2008;38:129–33. doi: 10.1093/jjco/hym162. [DOI] [PubMed] [Google Scholar]
- 13.Magheli A, Rais-Bahrami S, Trock BJ, et al. Impact of body mass index on biochemical recurrence rates after radical prostatectomy: an analysis utilizing propensity score matching. Urology. 2008;72:1246–51. doi: 10.1016/j.urology.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King CR, Spiotto MT, Kapp DS. Obesity and risk of biochemical failure for patients receiving salvage radiotherapy after prostatectomy. Int J Radiat Oncol Biol Phys. 2009;73:1017–22. doi: 10.1016/j.ijrobp.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 15.Komaru A, Kamiya N, Suzuki H, et al. Implications of body mass index in Japanese patients with prostate cancer who had undergone radical prostatectomy. Jpn J Clin Oncol. 40:353–9. doi: 10.1093/jjco/hyp164. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui SA, Inman BA, Sengupta S, et al. Obesity and survival after radical prostatectomy: A 10-year prospective cohort study. Cancer. 2006;107:521–9. doi: 10.1002/cncr.22030. [DOI] [PubMed] [Google Scholar]
- 17.Motamedinia P, Korets R, Spencer BA, Benson MC, McKiernan JM. Body mass index trends and role of obesity in predicting outcome after radical prostatectomy. Urology. 2008;72:1106–10. doi: 10.1016/j.urology.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 18.van Roermund JG, Kok DE, Wildhagen MF, et al. Body mass index as a prognostic marker for biochemical recurrence in Dutch men treated with radical prostatectomy. BJU Int. 2009;104:321–5. doi: 10.1111/j.1464-410X.2009.08404.x. [DOI] [PubMed] [Google Scholar]
- 19.van Roermund JG, Hinnen KA, Battermann JJ, et al. Body mass index is not a prognostic marker for prostate-specific antigen failure and survival in Dutch men treated with brachytherapy. BJU Int. 105:42–8. doi: 10.1111/j.1464-410X.2009.08687.x. [DOI] [PubMed] [Google Scholar]
- 20.Amling CL, Kane CJ, Riffenburgh RH, et al. Relationship between obesity and race in predicting adverse pathologic variables in patients undergoing radical prostatectomy. Urology. 2001;58:723–8. doi: 10.1016/s0090-4295(01)01373-5. [DOI] [PubMed] [Google Scholar]
- 21.Rohrmann S, Roberts WW, Walsh PC, Platz EA. Family history of prostate cancer and obesity in relation to high-grade disease and extraprostatic extension in young men with prostate cancer. Prostate. 2003;55:140–6. doi: 10.1002/pros.10211. [DOI] [PubMed] [Google Scholar]
- 22.Freedland SJ, Platz EA. Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev. 2007;29:88–97. doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 23.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–25. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 24.World Cancer Research Fund / American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 25.Johnsen NF, Tjonneland A, Thomsen BL, et al. Physical activity and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer. 2009;125:902–8. doi: 10.1002/ijc.24326. [DOI] [PubMed] [Google Scholar]
- 26.Moore SC, Peters TM, Ahn J, et al. Age-specific physical activity and prostate cancer risk among white men and black men. Cancer. 2009;115:5060–70. doi: 10.1002/cncr.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orsini N, Bellocco R, Bottai M, et al. A prospective study of lifetime physical activity and prostate cancer incidence and mortality. Br J Cancer. 2009;101:1932–8. doi: 10.1038/sj.bjc.6605404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nomura A, Heilbrun LK, Stemmermann GN. Body mass index as a predictor of cancer in men. J Natl Cancer Inst. 1985;74:319–23. [PubMed] [Google Scholar]
- 29.Cerhan JR, Torner JC, Lynch CF, et al. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States) Cancer Causes Control. 1997;8:229–38. doi: 10.1023/a:1018428531619. [DOI] [PubMed] [Google Scholar]
- 30.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:557–63. [PubMed] [Google Scholar]
- 31.Putnam SD, Cerhan JR, Parker AS, et al. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann Epidemiol. 2000;10:361–9. doi: 10.1016/s1047-2797(00)00057-0. [DOI] [PubMed] [Google Scholar]
- 32.Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol. 2000;151:541–9. doi: 10.1093/oxfordjournals.aje.a010241. [DOI] [PubMed] [Google Scholar]
- 33.Spitz MR, Strom SS, Yamamura Y, et al. Epidemiologic determinants of clinically relevant prostate cancer. Int J Cancer. 2000;89:259–64. doi: 10.1002/1097-0215(20000520)89:3<259::aid-ijc8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson F, Wolk A, Pedersen NL, et al. Obesity and hormone-dependent tumors: cohort and co-twin control studies based on the Swedish Twin Registry. Int J Cancer. 2003;106:594–9. doi: 10.1002/ijc.11266. [DOI] [PubMed] [Google Scholar]
- 35.Friedenreich CM, McGregor SE, Courneya KS, Angyalfi SJ, Elliott FG. Case-control study of anthropometric measures and prostate cancer risk. Int J Cancer. 2004;110:278–83. doi: 10.1002/ijc.20110. [DOI] [PubMed] [Google Scholar]
- 36.Littman AJ, White E, Kristal AR. Anthropometrics and prostate cancer risk. Am J Epidemiol. 2007;165:1271–9. doi: 10.1093/aje/kwm013. [DOI] [PubMed] [Google Scholar]
- 37.Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109:675–84. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez BY, Park SY, Wilkens LR, Henderson BE, Kolonel LN. Relationship of body mass, height, and weight gain to prostate cancer risk in the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:2413–21. doi: 10.1158/1055-9965.EPI-09-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banez LL, Sun L, Trock BJ, et al. Body mass index and prostate specific antigen as predictors of adverse pathology and biochemical recurrence after prostatectomy. J Urol. 2009;182:491–6. doi: 10.1016/j.juro.2009.04.007. discussion 6-8. [DOI] [PubMed] [Google Scholar]


