Abstract
The Six1 homeodomain protein is a developmental transcription factor that has been implicated in tumor onset and progression. Our recent work demonstrates that Six1 overexpression in human breast cancer cell lines is sufficient to induce epithelial-to-mesenchymal transition (EMT) and metastasis. Importantly, Six1-induced EMT and metastasis is dependent on TGF–β signaling. The TGF-β pathway plays a dual role in cancer, acting as a tumor suppressor in early lesions, but enhancing metastatic spread in more advanced tumors. Our previous work indicated that Six1 may be a critical mediator of the switch in TGF-β signaling from tumor suppressive to tumor promotional. However, the mechanism by which Six1 impinges on the TGF-β pathway was, until now, unclear. In this work, we identify the TGF-β type I receptor (TβRI) as a target of Six1 and a critical effector of Six1-induced TGF-β signaling and EMT. We demonstrate that Six1-induced upregulation of TβRI is both necessary and sufficient to activate TGF-β signaling and induce properties of EMT. Interestingly, increased TβRI expression is not sufficient to induce experimental metastasis, providing in vivo evidence that Six1 overexpression is required to switch TGF-β signaling to the pro-metastatic phenotype, and demonstrating that induction of EMT is not sufficient to induce experimental metastasis. Together, these results demonstrate a novel mechanism for the activation of TGF-β signaling, identify TβRI as a new target of Six1, and implicate Six1 as a determinant of TGF-β function in breast cancer.
Keywords: Six1, TGF-β signaling, breast cancer metastasis, type I TGF-β Receptor, Epithelial-Mesenchymal Transition
Introduction
Six1 is a member of the Six family of homeodomain transcription factors and is a critical developmental regulator in numerous organs, including the brain (1), muscle (2), and kidney (3). In mammalian development, Six1 is required for progenitor cell proliferation and survival (4), and contributes to the epithelial plasticity in muscle and potentially renal development (3, 5). Six1 is a component of a genetic network that is conserved from Drosophila to humans (6), in which it interacts with other developmental regulators, including Eya and Dach (4), to regulate gene expression.
Previous studies have discovered that the misexpression of embryonic proteins in cancer, particularly homeodomain transcription factors, can induce developmental programs out of context that contribute to tumor onset and progression (7). In particular, inappropriate activation of developmental programs that regulate epithelial plasticity has been identified in cancer and is hypothesized to contribute to local spread and metastasis of tumor cells through the induction of an oncogenic epithelial-mesenchymal transition (EMT) (8). Consistent with other developmental regulators, Six1 is misexpressed in numerous cancers including breast (9), ovarian (10), rhabdomyosarcoma (11) and hepatocellular carcinoma (12). In human breast cancer, Six1 correlates with advanced disease and adverse patient outcomes (9). Similar to the observed functions of Six1 in development, Six1 increases cancer cell proliferation and survival (10, 13) and regulates epithelial plasticity through the induction of EMT (14, 15). Importantly, forced Six1 expression in mouse models of breast cancer initiation and metastasis demonstrate that Six1 induces tumor formation and increases metastatic spread (14-16). The mechanisms underlying the pro-tumorigenic and pro-metastatic properties of Six1 have begun to be elucidated, and include the activation of multiple pathways. The proliferative effects of Six1 in breast cancer are in part mediated by upregulation of Cyclin A1 (13). In contrast, the Six1-induced EMT is dependent on activation of the TGF–β pathway, which results in concomitant activation of the Wnt signaling pathway (14, 15). Notably, Six1-induced activation of TGF–β signaling is necessary for its pro-metastatic activity, suggesting that TGF–β signaling is a critical downstream mediator of Six1-induced breast cancer progression.
TGF–β signaling is an important pathway in numerous homeostatic and pathologic processes and plays a significant role in cancer (17). Similar to the other developmental pathways implicated in cancer, the normal function of TGF-β during organogenesis can parallel its effect in cancer. For example, during development TGF-β induces the EMT required for cardiac valve formation and palatal fusion, and similarly, treatment of numerous cancer cell lines with TGF-β also induces an EMT (18-21). Importantly, in cancer, the consequence of activated TGF-β signaling is highly context dependent and TGF-β can be classified as both tumor suppressive and tumor promotional (22). In breast cancer, early lesions typically are growth inhibited by TGF–β, highlighting its tumor suppressive activity (23). However, in later stages of breast cancer, the cells become resistant to the growth inhibitory activity of TGF–β (24) and instead TGF-β promotes metastatic progression through multiple mechanisms, likely including its ability to induce EMT (20, 25). Interestingly, the mechanism underlying the switch in TGF-β signaling from tumor suppressive to pro-metastatic is not well understood.
Recent work has established the TGF–β receptors as a critical point of regulation for both the magnitude and the specificity of TGF–β signaling. For instance, in breast cancer cells, responsiveness to TGF–β is enhanced after treatment with an HDAC inhibitor, which increases the TGF-β type I receptor (TβRI) expression (26). Additionally, the level of the type II receptor (TβRII) expression in a colon cancer line determines the level of activation of the TGF–β pathway, as well as its biological effects, suggesting that receptor expression is not simply a passive requirement of signaling, but instead that levels of the receptors can actively modulate TGF–β responses (27). The significance of the precise regulation of TGF–β receptor expression is consistent with clinical data suggesting that the level and potentially the ratio of the type I and II receptors regulate TGF–β responses in systemic sclerosis (28), and the observations from cervical cancer, glial blastoma multiforme and pulmonary adenocarcinoma that show a correlation between TβRI levels and advanced disease (29-31).
In this work, we identify TβRI as a relevant downstream target of Six1 in MCF7 breast cancer cells. We further show that TβRI is necessary and sufficient to induce TGF-β signaling and EMT. However, even though we show that TβRI upregulation alone induces TGF-β signaling and EMT, it is not sufficient to induce metastasis. Instead, TβRI upregulation in the absence of Six1 overexpression actually inhibits metastatic spread in vivo in an experimental metastasis model, suggesting that activation of TGF-β signaling must cooperate with Six1 expression to induce metastasis. These data implicate Six1 specifically in the switch of TGF-β signaling from tumor suppressive to tumor promotional. Together, these results elucidate a novel mechanism by which Six1 regulates TGF–β signaling, and further demonstrate that Six1 promotes the pro-metastatic functions of TGF-β signaling.
Materials and Methods
Plasmids
pBabe-Flag-TβRI was provided as a gift from the laboratory of Boris Pasche. The Flag-TβRI was subcloned into the retroviral MSCV-GFP plasmid. The pGL2-TbRI promoter-luciferase was provided as a gift from the laboratory of Sudhakar Ammanamanchi. Truncations of the TβRI promoter were made using PCR and cloned into pGL3. The truncations were verified by sequencing.
Western blots
For western blots, E-cadherin and β-catenin antibodies were obtained from BD Biosciences Transduction Laboratories. TβRI, TβRII and TβRIII antibodies were obtained from Santa Cruz Biotechnology. β-actin and β-tubulin antibodies were obtained from Sigma-Aldrich and p-Smad3 antibody from Cell Signaling. Fractionation of the indicated cell lines was performed as previously described (32). The chemiluminescent signal was quantitated and the ratio of soluble to insoluble protein was calculated.
Cell Culture
MCF7 and MCF12A cells were cultured as recommended by ATCC at 37°C in 5% CO2. MCF7-Ctrl and MCF7-Six1 stable expressing clones were previously described (33) and MCF12A-Ctrl and MCF12A-Six1 stable expressing clones were previously described (16). For TβRI overexpressing cell lines, the MCF7 parental line was transduced with either retrovirus encoding MSCV-GFP or MSCV-TβRI-GFP. The cells were then sorted by flow cytometry to isolate GFP-expressing cells or selected with puromycin. TβRI and Six1 siRNA knockdown was performed using an siGENOME Smartpool (Thermo Scientific). 50nM of the siRNA oligonucleotides were transfected into MCF7-Ctrl or MCF-Six1 cells using Lipofectamine 2000 (Invitrogen). For antibody treatments, MCF7 cell lines were treated with 5 ug/ml of either Control (GST Santa Cruz Biotechnology) or TGF-β Inactivating antibodies (1D11 – R&D Systems) under low serum conditions (1% fetal bovine serum) for 24 hours prior to luciferase analysis. The particular lines used within the paper were fingerprinted (the MCF7s and all clonal isolates) in November 2007 (while the work was ongoing) to ensure that all clonal isolates were derived from MCF7 and that they matched the ATCC MCF7 lines. In addition, the MCF12A cell lines used in the supplemental data were karyotyped and confirmed to be MCF12A cells in June of 2006.
Luciferase Experiments
The indicated cell lines were transfected with p3TP-luciferase or pTopflash-luciferase and a Renilla luciferase (10 fold lower concentration) using Fugene (Roche). For the promoter assays, pGL3-TβRI promoter-luciferase constructs either full length or truncations were cotransfected with pcDNA3.1-Six1 with pcDNA-Eya2 (or empty vectors) and a Renilla luciferase (10 fold lower concentration) using Fugene (Roche). After 48 hours, the cells were harvested in passive lysis buffer (Promega). The luciferase activity was determined using Dual Luciferase Kit (Promega) on a Modulus Luminometer (Turner BioSystems). The calculation used to determine luciferase activity was as follows: (Six1+Eya2 on the TβRI promoter) − (pcDNA3.1 empty vectors on the TβRI-promoter) divided by (Six1+Eya2 on the promoterless pGL3 vector) − (pcDNA3.1 empty vectors on the promoterless pGL3 vector).
Quantitative PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) as described in the product insert. The RNA was reverse transcribed using iScript kit (BioRad). TβRI and cyclophilin B primer and probesets (Applied Biosystems) were used. Detection of the amplified products was performed on a CFX96 Realtime PCR machine (Biorad). Values represent the ratio of the relative quantity of TβRI to the relative quantity of Cyclophilin B.
Northern Blot
Northern blot analysis was performed as previously described (33). The TβRI template was obtained by PCR amplification of TβRI cDNA and radiolabelled using the Megaprime DNA Labeling System (Amersham). The β-actin probe was made by radiolabeling a plasmid-derived β-actin insert.
In vivo Experiments
For intracardiac injections, 3-4 week old female athymic nude mice were obtained (Taconic) and anesthetized using avertin. 1 × 105 MSCV-GFP or MSCV-TβRI MCF7 cells were injected into the left ventricle of these mice as previously described (14). An estrogen pellet containing 2mg of estrogen in 8mg of α-cellulose was implanted into the flank of the mice. The mice were measured for their bioluminescent signal approximately every 7 days. Values represent the average bioluminescent signal per mouse in photons/second. For orthotopic injections, 3-4 week old female NOD/Scid mice were obtained (Taconic) and anesthetized using avertin. 1 × 106 MCF7-Ctrl or MCF7-Six1 expressing a control or TβRIIDN were injected into the mammary fat pad in 100μl of growth factor reduced matrigel (BD Biosciences). An estrogen pellet containing 2mg of estrogen in 8mg of α-cellulose was implanted into the flank of the mice. The mice were monitored until the tumors reached 2cm3 (volume = 0.5 × width2 × length).
Results
Six1 expression in mammary carcinoma cells increases TβRI expression
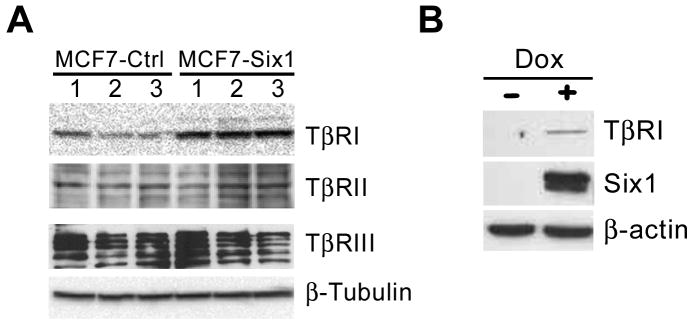
Based on our previous work demonstrating that Six1 increases TGF–β signaling in MCF7 mammary carcinoma cells and that TGF-β signaling mediates the pro-metastatic properties of Six1 (14), we set out to determine the mechanism by which Six1 increases TGF–β signaling. Previously, we showed that Six1-overexpressing MCF7 cells (MCF7-Six1) have increased activation of TGF–β signaling in response to TGF–β treatment compared to control MCF7 cells, suggesting that Six1 sensitizes mammary carcinoma cells to TGF–β. MCF7 cells in culture have a variable response to TGF–β signaling, which has been attributed to decreased expression of the TGF–β receptors (34, 35). In fact, de-repression of TβRI expression with an HDAC inhibitor restores responsiveness of MCF7 cells to TGF–β (26). Thus, we determined whether MCF7-Six1 cells display differential expression of the TGF–β receptors, including TβRI, TβRII and TβRIII. Interestingly, we observed an increase in expression of the TβRI protein in MCF7-Six1 cells with no consistent change in TβRII or TβRIII protein levels (Figure 1A).
Figure 1. Six1 expression in a mammary carcinoma cell line increases expression of the type I TGF-β receptor (TβRI).
(A) Western blot analysis was performed on lysates from clonal isolates of control or Six1-expressing MCF7 cells. Antibodies were used against TGF-β receptors including TβRI, TβRII or TβRIII. (B) Western blot analysis using antibodies that recognize TβRI and Six1 was performed on lysates from MCF7 cells with doxycycline-inducible Six1 expression either in the absence (-) or presence (+) of 5mg/ml doxycycline.
To confirm the results from the stable Six1 expressing clones and to test the regulation of TβRI by Six1 under more immediate conditions, we utilized a doxycycline inducible expression system. Short term induction of Six1 expression also led to increases in TβRI protein (Figure 1B), suggesting that Six1 regulates TβRI protein expression during both long and short time courses. Finally, to determine whether upregulation of TβRI remains dependent on Six1 expression in our stable clones, we knocked down Six1 in MCF7-Six1 clones (Supplementary Figure 1A) and demonstrate that TβRI upregulation is indeed dependent on continued expression of Six1 (Supplementary Figure 1B).
Six1 regulates the transcript levels of TβRI and activates its promoter
TβRI protein levels are regulated at multiple steps, from the level of the TβRI transcript to post-translational ubiquitination and degradation of the TβRI protein (36). However, based on the role of Six1 as a transcription factor, we investigated whether its overexpression can activate TβRI transcription in MCF7 cells. First, we tested whether TβRI transcript levels were altered in response to Six1 overexpression, and discovered an increase in the levels of TβRI transcript using Northern blot analysis (Figure 2A).
Figure 2. Six1 expression in MCF7 cells increases the expression of the TβRI transcript and activates the TβRI promoter.
(A) Northern blot analysis of TβRI transcript in MCF7-control or -Six1 clones. β-actin is used as a loading control. (B) Analysis of TβRI-promoter activity after transfection of a TβRI-luciferase plasmid, Eya2 and increasing amounts of Six1 plasmid. Values represent the mean ratio of Firefly luciferase to Renilla luciferase +/- the standard deviation and are representative of three independent experiments. (C) Analysis of the transcriptional activity of TβRI promoter truncations after transfection of Six1 and Eya2. Values represent the mean ratio of Firefly luciferase to Renilla luciferase and are normalized to the promoterless pGL3 construct +/- the standard deviation.
As a transcription factor, Six1 regulates the expression of multiple target genes including Cyclin A1, Cyclin D1, c-Myc (13, 37). Thus, we hypothesized that Six1 regulates TβRI expression at the level of the promoter. Using a promoter luciferase assay, we show that Six1 co-expression with its required cofactor Eya2 results in a dose dependent activation (up to 20-fold) of TβRI promoter activity (Figure 2B). Thus, Six1 overexpression activates TβRI expression at the promoter level. It should be noted that while Eya2 can be found in MCF7 cells, its levels are low and thus transient transfection of Six1 requires co-transfection of Eya2 due to the high levels of Six1 and the TβRI promoter introduced into the cell. In contrast, stable overexpression of Six1 in MCF7 cells leads to lower levels of Six1 expression, and thus endogenous Eya2 is sufficient to serve as the co-activator of Six1 in this context (data not shown).
To identify the region of the TβRI promoter through which Six1 acts, we generated truncations of the promoter and tested their ability to be activated by Six1 (Figure 2C). The region of the TβRI promoter from -175 to +25 was determined to be necessary for maximal activation by Six1. Recently, multiple Six1 consensus binding sites have been identified (38-40). Interestingly, an inspection of the TβRI promoter within the defined region did not reveal a known consensus Six1 binding site suggesting that: 1) Either Six1 is binding to a novel sequence or, 2) Six1 is not binding directly to the TβRI promoter, but instead is indirectly activating the TβRI promoter through as yet unidentified intermediate factors.
Previously, we have shown that Six1 overexpression in the immortalized, but non-transformed mammary epithelial cell line, MCF12A, also induces EMT and activates TGF-β signaling (14). To determine if Six1 upregulates TβRI in this system, we analyzed the protein level of TβRI and observed that TβRI is in fact upregulated in the MCF12A-Six1 cells (Supplementary Figure 2A). Next we tested whether Six1 and Eya2 coexpression activates the TβRI promoter in the MCF12A cells and found that Six1 and Eya2 co-expression can also increase activity of the TβRI promoter, albeit to a lesser degree, in this cell line (Supplementary Figure 2B). Together, these results are consistent with the hypothesis that TβRI is a target of Six1 in the MCF7 and MCF12A mammary cell lines and suggests that regulation of TβRI may be an underlying mechanism of Six1-dependent activation of TGF-β signaling and induction of EMT.
TβRI upregulation is necessary for Six1-induced TGF–β signaling and EMT
Although we previously demonstrated that TGF-β signaling is necessary for Six1-induced EMT (14), it is unclear whether TβRI upregulation is required for both Six1-induced activation of TGF-β signaling and the induction of EMT. Thus, TβRI levels were decreased in MCF7-Six1 cells by transiently transfecting an siRNA pool of 4 different TβRI-targeting siRNA constructs into the cells. Quantitative PCR analysis and western blot analysis confirmed the downregulation of TβRI compared to cells transfected with an siRNA non-targeting control pool (Figure 3A, Supplementary Figure 3). Analysis of the TGF–β responsive 3TP luciferase reporter demonstrated a significant reversal of the Six1-induced increase in TGF–β signaling to levels observed in the MCF7 control line (Figure 3B). In addition, TβRI knockdown also decreased Six1-induced levels of phospho-Smad (Supplementary Figure 3). These data strongly suggest that upregulation of TβRI is critical to Six1-induced TGF–β signaling.
Figure 3. TβRI is necessary for Six1-induced TGF-β signaling and properties of EMT.
(A) Realtime PCR analysis of TβRI in MCF7-Control or MCF7-Six1 clones after TβRI siRNA knockdown. Values represent the level of the TβRI transcript normalized to Cyclophilin B, and are the mean of three independent experiments. (B) Analysis of the TGF-β-responsive promoter, 3TP-luciferase, in MCF7-Control versus -Six1 cells after TβRI siRNA knockdown. Values represent the ratio of Firefly luciferase to the Renilla luciferase and are the mean values of three independent experiments. (C) Quantification of western blot analysis of E-cadherin or β-catenin in the soluble versus insoluble fraction of MCF7-Control versus MCF7-Six1 cells after TβRI siRNA knockdown. Values represent the ratio of soluble to insoluble fractions and are the mean of three independent experiments. (D) Analysis of a β-catenin responsive promoter, Topflash-luciferase, in MCF7-Control versus -Six1 cells after TβRI siRNA knockdown. Values represent the ratio of Firefly luciferase to the Renilla luciferase and are the mean values of three independent experiments. In all panels, error bars represent the standard error of the mean and the p values were calculated using the Student's t test.
We then asked whether upregulation of TβRI is necessary for Six1-induced EMT. We previously determined that Six1 expression induces properties of EMT, and that a subset of those properties are dependent on TGF-β signaling (14), including relocalization of E-cadherin and β-catenin from the adherens junctions to the cytoplasm, and activation of β-catenin-dependent transcription (14). Consistent with our results that TβRI is necessary for Six1-induced TGF-β signaling, and that TGF-β signaling is necessary for the above mentioned properties of Six1-induced EMT (14), knockdown of TβRI also significantly reversed the E-cadherin and β-catenin relocalization (Figure 3C) and decreased the Six1-induced β-catenin-dependent transcriptional activity (Figure 3D). Thus, the reversal of Six1-induced properties of EMT after TβRI knockdown confirms that TβRI upregulation is necessary both for Six1-induced TGF–β signaling and properties of EMT including E-cadherin and β-catenin relocalization.
TβRI overexpression is sufficient to activate TGF–β signaling and to induce properties of EMT
Although TβRI is a necessary component of the TGF–β pathway, it is not clear whether increased expression of TβRI alone would activate TGF–β signaling and induce EMT, or whether TβRI upregulation cooperates with other Six1-induced targets to activate these pathways. To test whether TβRI is sufficient to activate TGF–β signaling in MCF7 cells, we expressed a FLAG-tagged TβRI (MCF7-TβRI) or as a control, GFP (MCF7-GFP) in MCF7 cells (Figure 4A). Importantly, MCF7-TβRI cells showed increased 3TP-luciferase activity and phosphorylation of Smad3, suggesting that TβRI overexpression is sufficient to increase TGF–β signaling (Figure 4B, Supplementary Figure 4). Interestingly, the MCF7-TβRI cells displayed an approximately 1.5-fold induction of 3TP activity compared to MCF7-GFP cells, whereas MCF7-Six1 cells displayed anywhere between 3-5 fold induction over the MCF7-Control cells. These results suggest that in addition to the upregulation of TβRI protein levels, Six1 may act through other mechanisms to further enhance TGF–β signaling.
Figure 4. Overexpression of TβRI alone is sufficient to activate TGF-β signaling and induce properties of EMT.
(A) Western blot analysis on lysates from MCF7-GFP and MCF7-Flag-tagged TβRI using an antibody for the Flag-epitope. β-actin is used as a loading control. (B) Analysis of a TGF-β responsive promoter, 3TP-luciferase, in MCF7-GFP versus -TβRI cells. Values represent the ratio of Firefly luciferase to the Renilla luciferase and are the mean values of three independent experiments. (C) Quantification of western blot of analysis of E-cadherin or β-catenin in the soluble versus of insoluble fraction of MCF7-GFP or -TβRI cells. Values represent the ratio of soluble to insoluble fractions and are the mean of three independent experiments. (D) Analysis of a β-catenin responsive promoter, Top-flash-luciferase, in MCF7-GFP and -TβRI cells. Values represent the ratio of Firefly luciferase to the Renilla luciferase and are the mean values of three independent experiments. In all panels, error bars represent the standard error of the mean and the p values were calculated using the Student's t test.
Because TβRI upregulation alone is sufficient to activate TGF-β signaling in the MCF7 model, we investigated whether this induction was dependent on the presence of the TGF-β ligand. Other receptor signaling pathways, including EGF (41, 42) and TNF (43), have been shown to function independent of their ligand, particularly when the receptors are overexpressed. In addition, current TGF-β inhibitors including TGF-β inactivating antibodies (44), TGF-β siRNA constructs (45) and small molecule receptor kinase inhibitors (46), target different levels of the TGF-β pathway. As a result, depending on the mechanism of activation of the TGF-β pathway, tumor cells may have variable responsiveness to each inhibitor. While we had previously shown that Six1-induced TGF-β signaling can be inhibited by a TβRI kinase inhibitor (14), it was not clear whether this Six1 induction depended on the TGF-β ligand. However, the increased TGF-β signaling in MCF7-Six1 clones was significantly decreased after treatment with a TGF-β inactivating antibody (Supplementary Figure 5A). In addition, treatment of MCF7-TβRI cells with the TGF-β inactivating antibody completely abolished the increase in TGF-β signaling (Supplementary Figure 5B). These data confirm that both Six1- and TβRI-induced TGF-β signaling is dependent on the TGF-β ligand. Additionally, these results predict that Six1 overexpressing cells will be sensitive to TGF-β inhibitors targeting the TGF-β ligand.
Based on the observation that TβRI upregulation is sufficient to increase TGF-β signaling, we next tested whether TβRI upregulation alone is sufficient to induce properties of EMT or whether it cooperates with other Six1-induced targets. Consistent with our hypothesis that TβRI is a relevant target for Six1-induced EMT, MCF7-TβRI cells displayed a relocalization of E-cadherin and β-catenin from the insoluble to soluble fraction (Figure 4C). In addition, the relocalization of β-catenin corresponded to an increase in the β-catenin responsive promoter Top-flash (Figure 4D), properties previously demonstrated in MCF7-Six1 cells to be dependent on TGF–β signaling (14). Taken together, these results establish the TβRI level in MCF7 cells as a modulator of TGF–β signaling and EMT. Additionally, the finding that TβRI upregulation is sufficient to increase TGF-β signaling dependent on the TGF-β ligand and to induce EMT, highlights TβRI as a relevant target of Six1 in breast cancer cells.
Overexpression of TβRI in a mammary carcinoma cell line attenuates its metastatic spread in an experimental metastasis model
TGF-β signaling plays a complex role in tumor progression, suppressing tumor formation in normal tissue and early lesions while promoting invasion and metastatic dissemination in later stages of tumor development. We previously demonstrated, using an intracardiac injection experimental metastasis model, that Six1 expression led to an increase in metastatic burden and a decrease in survival, and that this Six1-induced increase in metastatic burden and decrease in survival was dependent on TGF-β signaling (14). Surprisingly, we also observed that while loss of TGF-β signaling in Six1 overexpressing cells decreased the metastatic burden in the experimental metastasis model, loss of TGF-β signaling in MCF7 control cells actually increased the metastatic burden (14). This observation led us to postulate that TGF-β signaling is acting as a tumor suppressor in MCF7 control cells, however in the context of Six1 overexpression, increased TGF-β signaling instead promotes metastasis in the same cell type. These data suggest that Six1 mediates a switch in TGF-β signaling from tumor suppressive to tumor promotional. To further investigate the in vivo activity of TGF-β signaling in MCF7 cells, we performed intracardiac injections of the MCF7-TβRI and control cell lines on athymic nude mice. Interestingly, the mice injected with the MCF7-TβRI cells exhibited a dramatic inhibition of experimental metastasis and a significant decrease in the metastatic burden as determined by total body luminescence compared to the control MCF7-GFP cells (Figure 5A-B). Additionally, unlike MCF7-Six1 cells which substantially decreased the overall survival of these mice, injection of MCF7-TβRI cells trended towards improving their overall survival compared to MCF7-GFP cells (Supplementary Figure 6). Thus, although increased levels of TβRI in MCF7 cells are sufficient to induce TGF-β signaling and EMT, they are not sufficient to induce experimental metastasis. In summary, TGF-β signaling is necessary for Six1-induced metastasis (14), yet in the absence of Six1 overexpression, increased TGF-β signaling is unable to increase metastastic dissemination or decrease overall survival, implicating Six1 as a mediator of the switch in TGF-β response in MCF7 cells.
Figure 5. Overexpression of TβRI alone in MCF7 cells suppresses metastatic dissemination.
(A) Bioluminescent imaging of athymic nude mice on day 48 after intracardiac injection of either MCF7-GFP or -TβRI cells. False color logarithmic scale represents the intensity of the bioluminescent signal in photons. (B) Quantification of the total flux of the bioluminescent signal per mouse in photons per second over the course of the experiment. Values represent the average of the total flux for all mice on the indicated day. p-values were calculated using the Mann-Whitney t-test. Error bars represent the standard error of the mean.
As outlined above, TGF-β signaling plays a dual role in tumorigenesis and depending on the context can be tumor suppressive or tumor promotional (22). One mechanism by which TGF-β signaling is thought to be tumor suppressive is through growth inhibition (23). To test the role of Six1 in modulating the effect of TGF-β signaling on tumor growth rate in vivo, we measured the growth of orthotopic tumors derived from MCF7-Ctrl and MCF7-Six1 cells expressing either a control or a dominant negative TβRII (TβRIIDN) (Supplementary Figure 7). Inhibition of TGF-β signaling in the MCF7-Ctrl tumors significantly decreased the time required for the tumors to reach 2cm3, which is consistent with the growth inhibitory effect of TGF-β signaling. Importantly, in the context of Six1 overexpression (MCF7-Six1), inhibition of TGF-β signaling had no significant effect on the time required to reach 2cm3 suggesting that Six1 antagonizes the growth inhibitory effect of TGF-β signaling in vivo. Together, these results highlight the context dependence role of TGF-β signaling in cancer, establishing Six1 as a significant determinant of TGF-β activity and emphasizing the striking consequences of Six1 misexpression on cancer progression.
Discussion
Homeodomain proteins comprise a large transcription factor family with an enormous diversity of functions. From the earliest stages of development, homeodomain proteins are critical to normal development and physiology. However, it comes as little surprise that homeodomain protein function also underlies various pathologies, most notably cancer (7). Indeed, homeodomain proteins, including Six1, have been recognized as significant contributors to tumor initiation and progression (14, 47-49). However, potentially due to the promiscuity of DNA binding or the complexity of genetic regulation by homeodomain proteins, few mechanisms of homeodomain protein action have been identified.
The Six1 homeoprotein has been implicated in both tumor initiation and tumor progression in many human cancers (11, 12, 14, 16), but the molecular mechanisms of Six1 activity still are not fully understood. However, we do know that Six1 contributes to tumor formation and metastatic progression by regulating multiple activities of the cancer cell, including genome stability (16), responsiveness to apoptotic stimuli (10), cell proliferation (13) and epithelial differentiation (14). While a limited number of Six1 targets have been identified, the mechanisms of the majority of Six1-induced activities are unclear. Previously we have shown that in breast cancer, Six1 induces TGF-β signaling and EMT. Importantly, Six1 also increases the metastatic potential of breast cancer cells in a TGF-β dependent manner, thereby establishing the TGF-β pathway as a critical target of Six1 overexpression. Furthermore, in samples of human invasive ductal carcinoma, Six1 expression significantly correlates with activated TGF-β signaling linking Six1 with the TGF-β pathway in human breast cancer (14). However, the exact mechanism by which Six1 overexpression increases TGF-β signaling was previously unknown. In this work, we identify TβRI as a novel target of Six1 activity and link the upregulation of this receptor with Six1-induced TGF-β signaling and the induction of EMT.
However our work also reveals a complex relationship between TGF-β signaling, EMT and metastasis. Although upregulation of TβRI in MCF7 cells is sufficient to induce TGF-β signaling and EMT, it is not sufficient to increase metastasis in a tumorigenic context. In fact, activation of the TGF-β signaling pathway alone inhibits the metastatic burden of mice in an experimental metastasis model, whereas Six1 overexpression and the resulting activation of TGF-β signaling dramatically increases metastasis dependent on TGF-β signaling. These results suggest that Six1 overexpression is necessary for the induction of the pro-metastatic effects of TGF-β signaling and EMT. Without Six1 overexpression, TGF-β signaling alone in MCF7 cells is tumor suppressive. Therefore, not only can Six1 expression induce TGF-β signaling, but it can also directly determine the effect of activated TGF-β signaling. Together, the ability of Six1 to induce TGF-β signaling and act as a regulator of TGF-β function provides a double hit in favor of metastatic spread. Consequently, Six1 expression in breast cancer potentially is an indicator not only of TGF-β activity, but specifically of its pro-metastatic effects. In addition, we propose that Six1 modulates the effect of TGF-β signaling by antagonizing its growth inhibitory activity as we demonstrate that tumor growth in vivo is accelerated when TGF-β signaling is inhibited in control tumors. However in the context of Six1 expression, inhibition of TGF-β signaling has no effect on tumor growth, suggesting that Six1 counteracts the growth inhibitory effect of TGF-β signaling. Together these results suggest a model whereby TGF-β signaling inhibits tumor progression in normal tissue and early lesions. However, Six1 misexpression in these tumors has the potential to not only increase TGF-β signaling through upregulation of TβRI, but to also antagonize the tumor suppressive effects of TGF-β signaling, selectively enhancing its pro-metastatic effects (Figure 6).
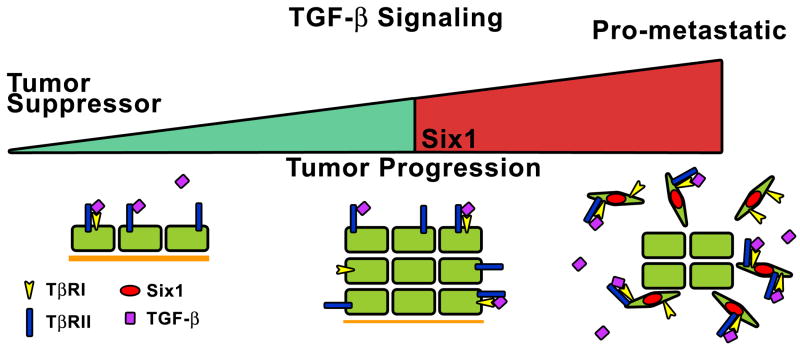
Figure 6. Six1 mediates the switch in TGF-β signaling from tumor suppressive to tumor promotional.
In early stage lesions, TGF-β signaling acts as a tumor suppressor blocking tumor development and suppression. However as the tumor progresses, Six1 overexpression leads to increased TGF-β signaling through upregulation of TβRI. In addition, Six1 switches the function of TGF-β from tumor suppressive to pro-metastastic, facilitating the dissemination of the tumor cells from the primary tumor to secondary sites.
Not only do these results have implications for understanding the natural progression and development of cancer, but they also hold potential therapeutic implications. As TGF-β targeted therapies are developed and tested (44-46, 50), understanding the mechanisms underlying the switch between the tumor suppressive and pro-metastatic functions of TGF-β signaling will be essential in selecting the patients most likely to respond to the therapies and avoiding the possibility of promoting the aggressiveness of the disease. Based on this work, we propose that Six1 should be investigated more thoroughly as a potential marker of TGF-β mediated pro-metastatic activities.
Supplementary Material
Acknowledgments
Financial Support: This work was funded by grants from the National Cancer Institute (2RO1-CA095277), The American Cancer Society (RSG-07-183-01-DDC) and The Susan G. Komen Foundation (BCTR0707562) to H.L.F. D.S.M, C.W., and S.M.F were funded by predoctoral fellowships from the Department of Defense Breast Cancer Research Program (W81XWH-06-1-0757 and W81ZWH-10-1-0162 respectively).
References
- 1.Brugmann SA, Pandur PD, Kenyon KL, Pignoni F, Moody SA. Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development. 2004;131:5871–81. doi: 10.1242/dev.01516. [DOI] [PubMed] [Google Scholar]
- 2.Laclef C, Hamard G, Demignon J, Souil E, Houbron C, Maire P. Altered myogenesis in Six1-deficient mice. Development. 2003;130:2239–52. doi: 10.1242/dev.00440. [DOI] [PubMed] [Google Scholar]
- 3.Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development. 2003;130:3085–94. doi: 10.1242/dev.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Oghi KA, Zhang J, et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–54. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- 5.Grifone R, Demignon J, Houbron C, et al. Six1 and Six4 homeoproteins are required for Pax3 and Mrf expression during myogenesis in the mouse embryo. Development. 2005;132:2235–49. doi: 10.1242/dev.01773. [DOI] [PubMed] [Google Scholar]
- 6.Heanue TA, Reshef R, Davis RJ, et al. Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev. 1999;13:3231–43. doi: 10.1101/gad.13.24.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2:777–85. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 8.Micalizzi DS, Ford HL. Epithelial-mesenchymal transition in development and cancer. Future Oncol. 2009;5:1129–43. doi: 10.2217/fon.09.94. [DOI] [PubMed] [Google Scholar]
- 9.Reichenberger KJ, Coletta RD, Schulte AP, Varella-Garcia M, Ford HL. Gene amplification is a mechanism of Six1 overexpression in breast cancer. Cancer Res. 2005;65:2668–75. doi: 10.1158/0008-5472.CAN-04-4286. [DOI] [PubMed] [Google Scholar]
- 10.Behbakht K, Qamar L, Aldridge CS, et al. Six1 overexpression in ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and is associated with poor survival. Cancer Res. 2007;67:3036–42. doi: 10.1158/0008-5472.CAN-06-3755. [DOI] [PubMed] [Google Scholar]
- 11.Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10:175–81. doi: 10.1038/nm966. [DOI] [PubMed] [Google Scholar]
- 12.Ng KT, Man K, Sun CK, et al. Clinicopathological significance of homeoprotein Six1 in hepatocellular carcinoma. Br J Cancer. 2006;95:1050–5. doi: 10.1038/sj.bjc.6603399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coletta RD, Christensen K, Reichenberger KJ, et al. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci U S A. 2004;101:6478–83. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micalizzi DS, Christensen KL, Jedlicka P, et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. J Clin Invest. 2009;119:2678–90. doi: 10.1172/JCI37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCoy EL, Iwanaga R, Jedlicka P, et al. Six1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transition. J Clin Invest. 2009;119:2663–77. doi: 10.1172/JCI37691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coletta RD, Christensen KL, Micalizzi DS, Jedlicka P, Varella-Garcia M, Ford HL. Six1 overexpression in mammary cells induces genomic instability and is sufficient for malignant transformation. Cancer Res. 2008;68:2204–13. doi: 10.1158/0008-5472.CAN-07-3141. [DOI] [PubMed] [Google Scholar]
- 17.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 18.Azhar M, Runyan RB, Gard C, et al. Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev Dyn. 2009;238:431–42. doi: 10.1002/dvdy.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nawshad A, LaGamba D, Hay ED. Transforming growth factor beta (TGFbeta) signalling in palatal growth, apoptosis and epithelial mesenchymal transformation (EMT) Arch Oral Biol. 2004;49:675–89. doi: 10.1016/j.archoralbio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Rees JR, Onwuegbusi BA, Save VE, Alderson D, Fitzgerald RC. In vivo and in vitro evidence for transforming growth factor-beta1-mediated epithelial to mesenchymal transition in esophageal adenocarcinoma. Cancer Res. 2006;66:9583–90. doi: 10.1158/0008-5472.CAN-06-1842. [DOI] [PubMed] [Google Scholar]
- 21.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 22.Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006;25:435–57. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- 23.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–21. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 24.Chen CR, Kang Y, Massague J. Defective repression of c-myc in breast cancer cells: A loss at the core of the transforming growth factor beta growth arrest program. Proc Natl Acad Sci U S A. 2001;98:992–9. doi: 10.1073/pnas.98.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 26.Ammanamanchi S, Brattain MG. Restoration of transforming growth factor-beta signaling through receptor RI induction by histone deacetylase activity inhibition in breast cancer cells. J Biol Chem. 2004;279:32620–5. doi: 10.1074/jbc.M402691200. [DOI] [PubMed] [Google Scholar]
- 27.Rojas A, Padidam M, Cress D, Grady WM. TGF-beta receptor levels regulate the specificity of signaling pathway activation and biological effects of TGF-beta. Biochim Biophys Acta. 2009;1793:1165–73. doi: 10.1016/j.bbamcr.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pannu J, Gore-Hyer E, Yamanaka M, et al. An increased transforming growth factor beta receptor type I:type II ratio contributes to elevated collagen protein synthesis that is resistant to inhibition via a kinase-deficient transforming growth factor beta receptor type II in scleroderma. Arthritis Rheum. 2004;50:1566–77. doi: 10.1002/art.20225. [DOI] [PubMed] [Google Scholar]
- 29.Soufla G, Sifakis S, Baritaki S, Zafiropoulos A, Koumantakis E, Spandidos DA. VEGF, FGF2, TGFB1 and TGFBR1 mRNA expression levels correlate with the malignant transformation of the uterine cervix. Cancer Lett. 2005;221:105–18. doi: 10.1016/j.canlet.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Kjellman C, Olofsson SP, Hansson O, et al. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int J Cancer. 2000;89:251–8. doi: 10.1002/1097-0215(20000520)89:3<251::aid-ijc7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Takanami I, Tanaka F, Hashizume T, Kodaira S. Roles of the transforming growth factor beta 1 and its type I and II receptors in the development of a pulmonary adenocarcinoma: results of an immunohistochemical study. J Surg Oncol. 1997;64:262–7. doi: 10.1002/(sici)1096-9098(199704)64:4<262::aid-jso3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Shtutman M, Levina E, Ohouo P, Baig M, Roninson IB. Cell adhesion molecule L1 disrupts E-cadherin-containing adherens junctions and increases scattering and motility of MCF7 breast carcinoma cells. Cancer Res. 2006;66:11370–80. doi: 10.1158/0008-5472.CAN-06-2106. [DOI] [PubMed] [Google Scholar]
- 33.Ford HL, Kabingu EN, Bump EA, Mutter GL, Pardee AB. Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: a possible mechanism of breast carcinogenesis. Proc Natl Acad Sci U S A. 1998;95:12608–13. doi: 10.1073/pnas.95.21.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalkhoven E, Roelen BA, de Winter JP, et al. Resistance to transforming growth factor beta and activin due to reduced receptor expression in human breast tumor cell lines. Cell Growth Differ. 1995;6:1151–61. [PubMed] [Google Scholar]
- 35.Ko Y, Banerji SS, Liu Y, et al. Expression of transforming growth factor-beta receptor type II and tumorigenicity in human breast adenocarcinoma MCF-7 cells. J Cell Physiol. 1998;176:424–34. doi: 10.1002/(SICI)1097-4652(199808)176:2<424::AID-JCP21>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Lonn P, Moren A, Raja E, Dahl M, Moustakas A. Regulating the stability of TGFbeta receptors and Smads. Cell Res. 2009;19:21–35. doi: 10.1038/cr.2008.308. [DOI] [PubMed] [Google Scholar]
- 37.Yu Y, Davicioni E, Triche TJ, Merlino G. The homeoprotein six1 transcriptionally activates multiple protumorigenic genes but requires ezrin to promote metastasis. Cancer Res. 2006;66:1982–9. doi: 10.1158/0008-5472.CAN-05-2360. [DOI] [PubMed] [Google Scholar]
- 38.Noyes MB, Christensen RG, Wakabayashi A, Stormo GD, Brodsky MH, Wolfe SA. Analysis of homeodomain specificities allows the family-wide prediction of preferred recognition sites. Cell. 2008;133:1277–89. doi: 10.1016/j.cell.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spitz F, Demignon J, Porteu A, et al. Expression of myogenin during embryogenesis is controlled by Six/sine oculis homeoproteins through a conserved MEF3 binding site. Proc Natl Acad Sci U S A. 1998;95:14220–5. doi: 10.1073/pnas.95.24.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger MF, Badis G, Gehrke AR, et al. Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell. 2008;133:1266–76. doi: 10.1016/j.cell.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donepudi M, Resh MD. c-Src trafficking and co-localization with the EGF receptor promotes EGF ligand-independent EGF receptor activation and signaling. Cell Signal. 2008;20:1359–67. doi: 10.1016/j.cellsig.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Fiore PP, Pierce JH, Kraus MH, Segatto O, King CR, Aaronson SA. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237:178–82. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 43.Boldin MP, Mett IL, Varfolomeev EE, et al. Self-association of the “death domains” of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. J Biol Chem. 1995;270:387–91. doi: 10.1074/jbc.270.1.387. [DOI] [PubMed] [Google Scholar]
- 44.Nam JS, Terabe M, Mamura M, et al. An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008;68:3835–43. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlingensiepen KH, Schlingensiepen R, Steinbrecher A, et al. Targeted tumor therapy with the TGF-beta 2 antisense compound AP 12009. Cytokine Growth Factor Rev. 2006;17:129–39. doi: 10.1016/j.cytogfr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Halder SK, Beauchamp RD, Datta PK. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005;7:509–21. doi: 10.1593/neo.04640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamada J, Omatsu T, Okada F, et al. Overexpression of homeobox gene HOXD3 induces coordinate expression of metastasis-related genes in human lung cancer cells. Int J Cancer. 2001;93:516–25. doi: 10.1002/ijc.1357. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Chen H, Parker B, et al. HOXB7, a Homeodomain Protein, Is Overexpressed in Breast Cancer and Confers Epithelial-Mesenchymal Transition. Cancer Res. 2006;66:9527–34. doi: 10.1158/0008-5472.CAN-05-4470. [DOI] [PubMed] [Google Scholar]
- 49.Yu M, Smolen GA, Zhang J, et al. A developmentally regulated inducer of EMT, LBX1, contributes to breast cancer progression. Genes Dev. 2009;23:1737–42. doi: 10.1101/gad.1809309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hau P, Jachimczak P, Schlingensiepen R, et al. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides. 2007;17:201–12. doi: 10.1089/oli.2006.0053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.