Summary
All organisms have devised strategies to counteract energy depletion in order to promote fitness for survival. We show here that cellular energy depletion puts into play a surprising strategy that leads to absorption of exogenous fuel for energy repletion. We found that the energy depletion sensing kinase AMPK, binds, phosphorylates, and activates the transcriptional coactivator SRC-2, which in a liver-specific manner, promotes absorption of dietary fat from the gut. Hepatocyte-specific deletion of SRC-2 results in intestinal fat malabsorption and attenuated entry of fat into the blood stream. This defect can be attributed to AMPK and SRC-2 mediated transcriptional regulation of hepatic bile-acid secretion into the gut, as it can be completely rescued by replenishing intestinal BA, or by genetically restoring the levels of hepatic Bile Salt Export Pump (BSEP). Our results position the hepatic AMPK-SRC-2 axis as an energy rheostat which upon cellular energy depletion resets whole-body energy by promoting absorption of dietary fuel.
Introduction
In the last three decades the rate of adult obesity in the US has doubled and that of childhood obesity has tripled (Ogden et al., 2006). Obesity associated comorbidities such as diabetes, heart disease, neurodegeneration and certain cancers have seen a proportional increase in incidence along with a rise in the percentage of the gross domestic product spent to treat them (Ogden et al., 2006). The incessant drive of the mammalian body to obtain and store energy is likely the result of a period during evolution when food was scarce (Spiegelman and Flier, 2001). Clearly, one of the more important challenges of the current decade is to enhance our understanding of the basic mechanisms of energy homeostasis in order to gain a deeper insight into the factors that influence body weight. Decoding these factors in mechanistic and molecular terms has great potential for unearthing preventative and therapeutic targets against obesity.
One of the key survival strategies employed by organisms appears to be responding to cellular energy depletion by activating pathways that correct the depletion (Carling, 2004; Hardie and Carling, 1997; Kahn et al., 2005). Two ways to correct cellular energy depletion are deactivation of processes that consume ATP and activation of processes that produce ATP. An ancient energy sensor – AMP activated protein kinase (AMPK) appears to do just that by deactivation of ATP consuming anabolic processes and activation of ATP producing catabolic processes (Carling, 2004; Hardie and Carling, 1997; Kahn et al., 2005; Viollet et al., 2006). Since exogenous fuel is essential for ATP synthesis, it is no surprise that AMPK also drives appetite (Carling, 2004). We show here that once the exogenous fuel is eaten, AMPK, via a previously unlinked systemic process, allows for optimal absorption of the most energy rich fuel – dietary fat. AMPK activates the transcriptional coactivator SRC-2, which, as we demonstrate, promotes absorption of dietary fat by transcriptional modulation of hepatic bile-acid (BA) secretion. The mechanistic cascade we describe, links the cellular energy status with the whole-body energy state. Since, SRC-2 has been described to modulate energy loss via deactivation of brown adipose tissue (Picard et al., 2002), our current results position it as a regulator of energy uptake, and thus as a holistic controller of energy homeostasis.
Results
Whole-body ablation of SRC-2 results in dietary fat malabsorption
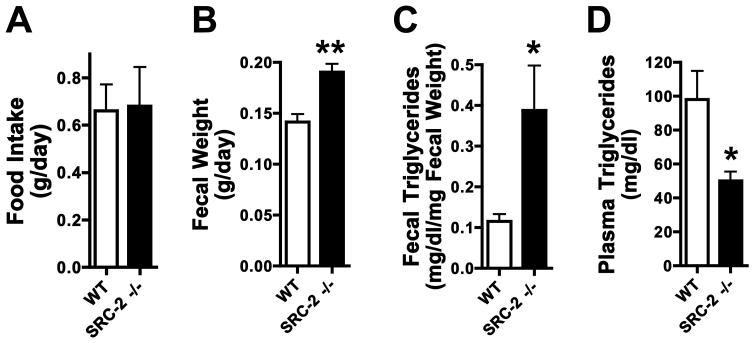
Global SRC-2 (TIF2) null mice are protected from high-fat diet mediated obesity because the coactivator promotes energy conservation by inhibiting brown adipose tissue activation (Picard et al., 2002). We hypothesized that SRC-2 might play an additional role in energy intake, and assessed food intake and fat absorption. Male SRC-2−/− mice fed standard chow ad libitum for 24 hours following an overnight, were compared with WT littermates for food intake, fecal mass, fecal triglyceride content and plasma triglyceride content. Whole body ablation of SRC-2 did not affect food intake, but increased fecal mass and triglyceride content, with a concomitant decrease in plasma triglycerides (Fig. 1A-D), suggesting a reduced capacity to absorb dietary fat from the gut.
Fig. 1. Whole-body ablation of SRC-2 results in dietary fat malabsorption.
A-D) Food intake, fecal output, fecal triglycerides and plasma triglycerides were measured in WT and SRC-2 −/− mice (whole body ablation) fed standard chow ad libitum for 24 hours following an overnight fast (n=5 mice per group).
Data are represented as mean + SEM. Unpaired student's t-test was used for evaluation of statistical significance. One asterisk indicates p < 0.05, two asterisks p < 0.01 and three asterisks p < 0.001.
Hepatocyte-specific ablation of SRC-2 results in intestinal fat malabsorption, which can be rescued by replenishing gut BA levels
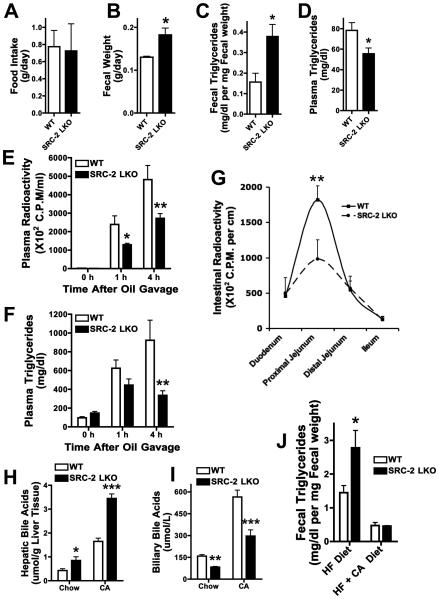
Hepatocyte-specific SRC-2 knockout mice (SRC-2 LKO) (Fig. S1A) consumed the same quantity of standard chow per day as their WT counterparts, but also showed increased fecal mass and triglyceride content, with a plasma triglyceride deficit (Fig. 2A-D), suggesting the liver to be the site of SRC-2 action for intestinal fat absorption. Fecal lipase activity was unaltered in SRC-2 LKO, indicating that pancreatic exocrine function was unaffected by hepatic ablation of SRC-2 (Fig. S1B). Based on the reciprocal effects on fecal and plasma triglycerides in the SRC-2 LKO mice, we assessed the rate of triglyceride entry into the plasma after oral gavage with olive oil labeled with C14 (after injection with Tyloxapol to inhibit plasma lipolysis). Indeed, we found lower triglyceride and radioactivity levels in the plasma of SRC-2 LKO mice (Fig. 2E, F). We also examined intestinal radioactivity after gavaging SRC-2 LKO and WT mice with olive oil labeled with C14, and found a two-fold deficit in proximal jejunal radioactivity (Fig. 2G), suggesting reduced absorption of triglycerides from the gut lumen into the enterocytes. Consistent with this, frozen sections of the proximal jejunal villi stained with Oil-Red-O showed a significantly lower level of triglyceride staining in SRC-2 LKO mice upon exposure to an olive oil gavage (Fig. S1C). We conclude that intestinal fat absorption is defective in SRC-2 LKO mice.
Fig. 2. Hepatic SRC-2 modulates dietary fat absorption in a BA dependant manner.
A-D) Food intake, fecal output, fecal triglycerides and plasma triglycerides were measured in WT and SRC-2 LKO mice (liver specific ablation) fed standard chow ad libitum for 24 hours following an overnight fast (n=5 mice per group).
E-F) Plasma radioactivity and triglyceride levels were measured in chow-fed WT and SRC-2 LKO mice after injection of the lipase inhibitor tyloxapol and gavage with olive oil containing 14C-trioleoylglycerol (n=5 mice per group).
Data are represented as mean + SEM. Two-way ANOVA with Bonferroni posttests to compare replicate means by row was used for evaluation of statistical significance. One asterisk indicates p < 0.05, two asterisks p < 0.01 and three asterisks p < 0.001.
G) Intestinal radioactivity levels were measured in WT and SRC-2 LKO mice 2 hours after gavage with olive oil containing 14C-trioleoylglycerol (n=5 mice per group).
Data are represented as mean + SEM. Two-way ANOVA with Bonferroni posttests to compare replicate means by row was used for evaluation of statistical significance. One asterisk indicates p < 0.05, two asterisks p < 0.01 and three asterisks p < 0.001.
H-I) Hepatic and biliary BA levels were measured in WT and SRC-2 LKO mice on standard-chow and on 1% cholic-acid diet for two weeks. Bile was obtained from the gall bladder and is representative of intestinal BA levels (n=4-5 mice per group).
J) Fecal triglyceride levels were measured in chow-fed WT and SRC-2 LKO mice upon exposure of mice to a high fat diet (60% calories from fat) and a diet containing high fat plus 1% cholic-acid for 48 hours (n=4-5 mice per group).
Data are represented as mean + SEM. Unless otherwise indicated, unpaired student's t-test was used for evaluation of statistical significance. One asterisk indicates p < 0.05, two asterisks p < 0.01 and three asterisks p < 0.001.
See also Fig. S1 and Fig. S2
Since decreased hepatic bile-acid (BA) secretion into the gut could account for this intestinal effect of hepatic SRC-2 deletion, we fed mice either standard-chow or a 1% cholic-acid (CA) diet for 14 days to inhibit hepatic BA production, and assessed BA levels in plasma, liver and bile. Both standard chow-fed and CA diet fed SRC-2 LKO mice displayed increased hepatic BA, but decreased biliary BA levels (Fig. 2H, I), consistent with decreased hepatic BA secretion into the gut. Plasma BA levels were elevated in the CA fed SRC-2 LKO mice (Fig. S1D). Consistent with an elevation in hepatic BA content and a deficit in biliary BA levels, the total BA pool size remained unchanged, along with an unchanged biliary BA composition profile (Fig. S1E and Fig. S2A). Plasma bilirubin levels remained unchanged as well (Fig. S1G). To test whether the failure of BA to reach the gut in sufficient amounts caused the observed fat malabsorption, we assessed fecal triglycerides from SRC-2 LKO mice and WT littermates fed either a high fat diet to stress the fat absorption apparatus, or a high fat diet plus 1% CA to correct the gut BA deficiency. Fecal samples from SRC-2 LKO mice contained two-fold more triglycerides than samples from WT littermates on high fat challenge (Fig. 2J). Remarkably, this fat malabsorption was completely rescued in SRC-2 LKO mice that were fed exogenous BA (Fig. 2J). In order to rule out the possibility that potentially enhanced peripheral energy consumption might account for the low plasma triglyceride levels in SRC-2 LKO mice, we measured oxygen consumption (VO2). The VO2 levels, over a 24 hour period, remained unchanged between WT and SRC-2 LKO mice (Fig. S1F).
SRC-2 regulates the expression of the Bile Salt Export Pump, forced hepatic restoration of which can rescue the fat malabsorption secondary to SRC-2 ablation
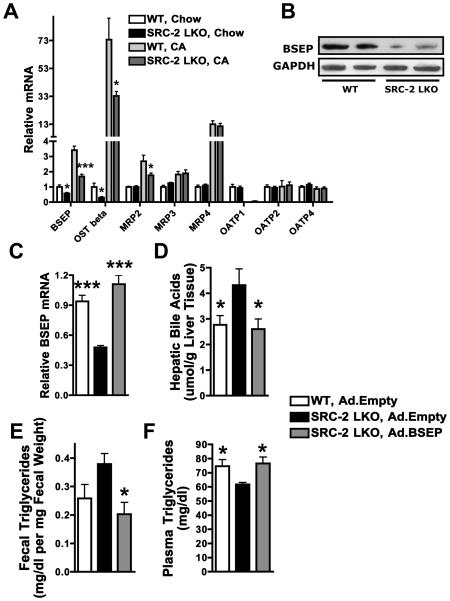
Accumulation of liver and plasma BA and their deficit in bile led us to conclude that SRC-2 positively regulates BA secretion from the liver into the gut. Accordingly, we investigated expression of a series of hepatic transporters, including Bile Salt Export Pump (BSEP), the major BA transporter from hepatocytes to the bile-ducts, in chow fed mice and mice challenged with the CA diet. Consistent with previously published liver microarray results with whole-body SRC-2 null mice (Jeong et al., 2006), BSEP mRNA and protein levels were significantly decreased in SRC-2 LKO mice (Fig. 3A, B). The basolateral transporter OSTbeta was also decreased. The BSEP effect is specific for SRC-2, since its expression was not affected by SRC-1 or SRC-3 deletion (Fig. S3A), demonstrating the specificity of coactivator function in vivo. Particularly as BSEP deficiency in humans is accompanied by severe steatorrhea (Walkowiak et al., 2006), the deficit in BSEP expression likely explains fat malabsorption in SRC-2 LKO mice.
Fig. 3. SRC-2 modulates dietary fat absorption by controlling the expression of the Bile Salt Export Pump (BSEP).
A) Expression of various BA transporter genes was measured via relative quantitation by QPCR in the liver of WT and SRC-2 LKO mice that were exposed to standard-chow or 1% cholic-acid for two weeks (n = 5-7 mice per group).
Data are represented as mean + SEM. Unpaired student's t-test was used for evaluation of statistical significance. One asterisk indicates p < 0.05, two asterisks p < 0.01 and three asterisks p < 0.001.
B) BSEP protein expression was measured via western blot analysis in the liver of WT and SRC-2 LKO mice that were exposed to standard-chow (n=2 mice per group). GAPDH was used as a loading control.
C-F) SRC-2 LKO mice exposed to adenoviral BSEP (Ad. BSEP) were compared with SRC-2 LKO and WT mice exposed to empty adenovirus (Ad. Empty) using tail-vein infusion. 8 days after virus infusion, hepatic BSEP expression, hepatic BA content, fecal triglyceride content and plasma triglyceride content was measured (n=6 mice per group). Statistical comparison was performed between (WT, Ad.Empty) and (SRC-2 LKO, Ad.Empty) and between (SRC-2 LKO, Ad. Empty) and (SRC-2 LKO, Ad.BSEP) groups.
Data are represented as mean + SEM. One-way ANOVA with Tukey's multiple comparison test was used for evaluation of statistical significance. One asterisk indicates p < 0.05, two asterisks p < 0.01 and three asterisks p < 0.001.
See also Fig. S3 and Fig. S4
We assessed the expression of a number of other key genes involved in BA homeostasis, and found a deficit in the expression of BA biosynthesis and import genes (Cyp7a1, Cyp8b1, Cyp7b1, Cyp27a1, NTCP) in SRC-2 LKO mice that suggested a compensatory reduction in gene expression in response to excess hepatic BA levels (Fig. S3B). A decrease in the BA catabolic pathway should increase cholesterol levels, and plasma total cholesterol, HDL, LDL and hepatic total cholesterol showed significant accumulation in SRC-2 LKO mice (Fig. S3D). This was unaccompanied by a change in intestinal absorption of dietary cholesterol (Fig. S3C). While HDL accumulation could suggest involvement of extra-hepatic tissues, this is unlikely as SRC-2 is deleted specifically in the liver. Exploring cholesterol metabolism in more detail, we assessed the expression of several genes important for maintaining cholesterol homeostasis, in the liver and the intestine (Fig. S3E). We found a deficit in liver ABCG5 and ABCG8 in SRC-2 LKO mice, potentially explaining plasma and hepatic cholesterol accumulation, as ABCG5 and ABCG8 are necessary for cholesterol excretion into the bile (Fig. S3E). Expression of LDLR and SR-B1, genes involved in liver cholesterol import, was suppressed in SRC-2 LKO mice (Fig. S3F), possibly to compensate for the increased hepatic cholesterol content, and likely explains accumulation of plasma cholesterol. Assessment of the expression of transcription factors relevant to BA and cholesterol homeostasis showed no significant differences between SRC-2 LKO and WT mice (Fig. S3G). Consistent with an unchanged plasma VLDL profile, the expression of several genes important for hepatic VLDL assembly and export showed no change in SRC-2 LKO mice compared with WT (Fig. S4A).
We tested whether reduced hepatic BSEP expression was responsible for the BA perturbation and fat malabsorption in SRC-2 LKO mice, by restoring hepatic BSEP to physiological levels, using an adenoviral vector. Notably, BSEP restoration completely reversed the hepatic BA accumulation, as well as the fecal triglyceride accumulation and plasma triglyceride deficits displayed by SRC-2 LKO mice (Figs. 3C-F).
SRC-2 modulates BSEP expression in a cell-autonomous manner, by coactivating FXR
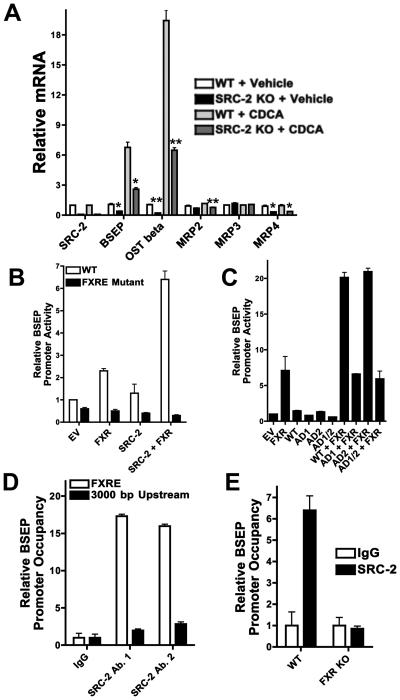
To rule out an indirect, systemic effect of hepatic SRC-2 ablation on the bile secretion program, we investigated primary hepatocytes (PHs) isolated from SRC-2 LKO and WT mice exposed to either vehicle or chenodeoxycholic acid (CDCA) (Lew et al., 2004). We found reductions in mRNA levels of BSEP and OSTbeta in SRC-2 null PHs that mirrored those found in vivo (Fig. 4A) demonstrating a cell-autonomous effect of SRC-2 on the expression of these genes. Repeating this experiment in PHs following acute SRC-2 knock-down demonstrated very similar results, reaffirming the critical nature of SRC-2 for the expression of the hepatic bile secretion program (Fig. S5A).
Fig. 4. SRC-2 modulates BSEP expression in a cell-autonomous manner, by coactivating FXR.
A) Expression of various BA transporter genes was measured via relative quantitation by QPCR in primary hepatocytes exposed to DMSO or to CDCA from WT and SRC-2 LKO mice. Statistical comparison was performed between WT and SRC-2 KO PHs in each treatment group.
B) HepG2 liver hepatoma cells were transfected with a reporter-gene plasmid driven by the wild-type mouse BSEP promoter and the same promoter with a mutated FXRE motif, together with expression plasmids for SRC-2 and FXR and exposed to CDCA. Reporter-gene levels were determined 48 hours after transfection. The empty vector (EV) value was fixed at 1 and the rest of the values are compared relative to that.
C) HepG2 liver hepatoma cells were transfected with a reporter-gene plasmid driven by the wild-type mouse BSEP promoter together with expression plasmids for WT SRC-2, SRC-2 mutant with AD1 deletion, SRC-2 mutant with AD2 deletion and SRC-2 mutant with both AD1 and AD2 deletions, and FXR, and exposed to CDCA. Reporter-gene levels were determined 48 hours after transfection. The empty vector (EV) value was fixed at 1 and the rest of the values are compared relative to that.
D) In vivo ChIP assays were performed using liver tissue from WT mice with 150-200 bp amplicons flanking the region containing the FXRE motif of the BSEP promoter and an irrelevant region 3000 bp upstream of the transcription start site. Sybr-Green QPCR (normalized to input) was used to assess SRC-2 occupancy of the BSEP promoter, using two different antibodies that targeted different regions of SRC-2. Control antibody recognized mouse IgG.
E) In vivo ChIP assays were performed using liver tissue from WT and FXR knockout mice with primers flanking the region containing the FXRE motif of the BSEP promoter. Sybr-Green QPCR (normalized to input) was used to assess SRC-2 occupancy of the BSEP promoter, using an SRC-2 specific antibody. Control antibody recognized mouse IgG.
Data are represented as mean + SEM. For all gene expression data unpaired student's t-test was used for evaluation of statistical significance. One asterisk indicates p < 0.05, two asterisks p < 0.01 and three asterisks p < 0.001.
See also Fig. S5
The BSEP promoter has an evolutionarily conserved recognition-motif for the nuclear receptor FXR (Ananthanarayanan et al., 2001), and we found that SRC-2 synergized with FXR for BSEP promoter activation (Fig. 4B). We examined the role of the two activation domains (AD) of SRC-2 in BSEP transactivation by using deletion mutants for AD1, AD2 and both. Interestingly, we found that AD1 is absolutely essential for SRC-2 mediated transactivation of the BSEP promoter (Fig. 4C). Using in vivo chromatin immunoprecipitation (ChIP, Fig. 4D, E), we confirmed that SRC-2 is recruited to the BSEP promoter in the liver, and this was abolished in the absence of FXR. To unequivocally demonstrate the specificity of SRC-2, we performed in vivo ChIP assays probing SRC-1 and SRC-3 occupancy of the BSEP promoter. In contrast with SRC-2, we found an absence of SRC-1 and SRC-3 at the BSEP prompter (Fig. S5B). Consistent with this, we found unchanged plasma BA levels in SRC-1 and SRC-3 null mice, and unchanged plasma triglyceride levels in SRC-3 null mice (Fig. S5C). We found a significant reduction in plasma triglyceride levels in SRC-1 null mice (Fig. S5C). However, with no change in plasma bile acid content as well as BSEP expression, it is unlikely that the reduction in plasma triglycerides in SRC-1 null mice is due to altered bile acid homeostasis, as is the case in SRC-2 LKO mice.
A hepatocyte-specific screen identifies AMP activated protein kinase (AMPK) as a positive modulator of BSEP expression
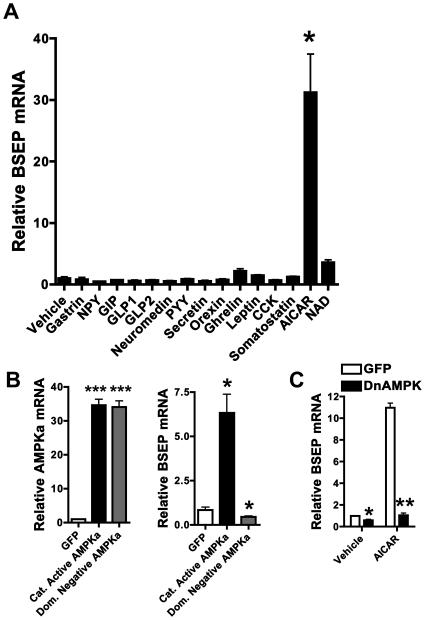
Having substantiated the signaling downstream of SRC-2 that leads to BA secretion and dietary fat absorption, we hypothesized that because SRC-2 promotes energy intake (via dietary fat absorption) and counteracts energy loss (via deactivation of brown adipose tissue) (Picard et al., 2002), it could itself be responsive to changes in energy status. We performed a screen to assess whether a number of hormones and cellular factors known to play a role in energy homeostasis, could affect BSEP expression in primary hepatocytes. We found that, AICAR, the activator of the well known sensor of cellular energy depletion - AMPK (Carling, 2004; Hardie and Carling, 1997; Kahn et al., 2005; Viollet et al., 2006), strongly affected BSEP expression (Fig. 5A). Because of the possibility that AICAR may have promiscuous effects that are independent of AMPK, we over-expressed a constitutively active form of AMPK in primary hepatocytes (with no AICAR present) and found it to be sufficient to transactivate BSEP (Fig. 5B). Conversely, over-expressing a dominant negative form of AMPK (DnAMPK) resulted in downregulation of BSEP expression (Fig. 5B). DnAMPK overexpression also resulted in attenuation of AICAR mediated BSEP transactivation (Fig. 5C).
Fig. 5. A hepatocyte-specific screen identifies AMP activated protein kinase (AMPK) as a putative, positive modulator of BSEP expression.
A) BSEP expression was measured via relative quantitation by QPCR in primary hepatocytes exposed to standard doses of the indicated agents for 24 hours.
B) BSEP expression was measured via relative quantitation by QPCR in primary hepatocytes exposed to adenoviruses containing cDNAs representing either constitutively active AMPK alpha or dominant negative AMPK alpha for 24 hours. Statistical comparison was made between the GFP group and either the constitutively active AMPK alpha or dominant negative AMPK alpha groups.
C) BSEP expression was measured via relative quantitation by QPCR in primary hepatocytes exposed to an adenovirus containing cDNA representing dominant negative AMPK alpha and either vehicle or 1mM AICAR for 18 hours.
All gene expression data are represented as mean + SEM. Unpaired student's t-test was used for evaluation of statistical significance. One asterisk indicates p < 0.05, two asterisks p < 0.01 and three asterisks p < 0.001.
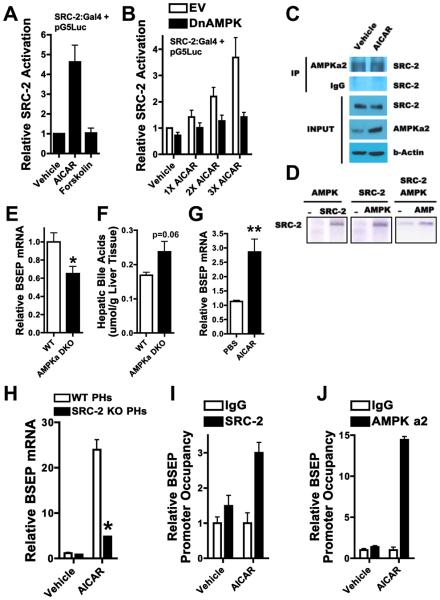
AMPK binds, phosphorylates, and increases SRC-2's intrinsic transcriptional activity, and drives it to the BSEP promoter
AMPK activation in HepG2 cells using AICAR increased the intrinsic transcriptional activity of SRC-2, while cAMP potentiation by forskolin addition had no effect (Fig. 6A, Fig. S6A). Interfering with AMPK activity using a dominant-negative construct abrogated AICAR mediated SRC-2 activation (Fig. 6B), confirming the specificity of AICAR. AICAR had no effect on SRC-2 protein levels (Fig. S6B), suggesting an activation mechanism independent of enhanced SRC-2 expression. Using co-immunoprecipitation assays, we found a physical association between endogenous SRC-2 and AMPK proteins (Fig. 6C). This association was unaffected by AICAR (Fig. 6C), suggesting an activation mechanism independent of increased affinity. Using in vitro kinase assays, with purified full-length SRC-2 and AMPK proteins, we found that AMPK phosphorylated SRC-2 in an AMP dependent manner (Fig. 6D). We next investigated the impact of loss of AMPK in vivo upon BSEP expression. We found a deficit in BSEP mRNA and protein levels in liver tissue from AMPK alpha 1/2 double-knockout mice, along with a modest accumulation of hepatic BA (Fig. 6E, Fig. S6C, Fig. 6F). Consistent with this, AICAR treatment of mice increased hepatic BSEP mRNA and protein expression (Fig. 6G, Fig. S6D), suggesting that AMPK regulates BSEP expression in vivo, presumably through an SRC-2 mediated effect. Indeed, we found SRC-2 to be necessary for AICAR mediated BSEP and OSTbeta transactivation in primary hepatocytes (Fig. 6H, Fig. S6E). We also found FXR to be necessary for AICAR mediated BSEP transactivation (Fig. S6F), suggesting that the SRC-2-FXR complex plays a key role in transducing signals from AMPK to the BSEP promoter. AICAR was also able to activate SRC-1 and SRC-3 in vitro (Fig. S6G), but since SRC-1 and SRC-3 do not impact BSEP transactivation in vivo (Fig. S3A, Fig. S5B, Fig. S5C), this might suggest distinct AMPK mediated functions exerted via the other p160 family members. In cultured liver cells, AICAR treatment resulted in increased SRC-2 recruitment to the BSEP promoter (Fig. 6I). Surprisingly, it also drove AMPK alpha 2 itself to the BSEP promoter (Fig. 6J), suggesting that AMPK, in addition to increasing the intrinsic transcriptional activity of SRC-2, recruits SRC-2 to the BSEP promoter, and also accompanies it there. This is consistent with the predominantly nuclear expression profile of AMPK alpha 2 (Salt et al., 1998), and with a recent report that demonstrates that AMPK alpha 2 plays a direct role in transcription by associating with chromatin, phosphorylating histones and being recruited to promoters of target genes (Bungard et al.). It is quite possible that other AMPK subunits are also present at the BSEP promoter in association with AMPK alpha 2, allowing AMPK to function as a holoenzyme in the transactivation of BSEP.
Fig. 6. Hepatic AMPK increases the intrinsic transcriptional activity of SRC-2, and drives it to the BSEP promoter.
A) HepG2 liver hepatoma cells were transfected with pG5Luc (5 Gal4 binding sites driving luciferase expression) together with pBIND SRC-2 (SRC-2:Gal4 DNA binding domain fusion protein) and exposed to 1 mM AICAR. Luciferase levels were determined 48 hours after transfection. The vehicle value was fixed at 1 and the rest of the values are compared relative to that.
B) HepG2 liver hepatoma cells were transfected with pG5Luc together with pBIND SRC-2 and either empty vector (EV) or dominant negative AMPK alpha 2 (DnAMPK) and exposed to 0.3 mM, 0.6 mM and 1 mM AICAR. Luciferase levels were determined 48 hours after transfection. The vehicle value was fixed at 1 and the rest of the values are compared relative to that.
C) HepG2 cell were treated with 1 mM AICAR for 30 min and subjected to immunoprecipitation with an AMPKa2 specific antibody. Immunoprecipated samples were subjected to immunoblotting along with 10% input as indicated.
D) Purified full length SRC-2 protein was subjected to in vitro phosphorylation with or without purified AMPK holoenzyme and AMP as indicated.
E-F) Hepatic BSEP expression and BA content was measured via relative quantitation by QPCR in the liver of WT and AMPK alpha1/alpha2 double knockout (AMPK alpha DKO) mice that were fasted for 24 hours (n = 7-8 mice per group).
G) Hepatic BSEP expression was measured via relative quantitation by QPCR in the liver of mice that were exposed to either PBS or 0.5 mg/g BW AICAR for 12 hours and fed standard chow (n = 6 mice per group).
H) BSEP expression was measured via relative quantitation by QPCR in primary hepatocytes from WT and SRC-2 LKO mice exposed to 1 mM AICAR for 18 hours.
I-J) ChIP assays were performed using HepG2 cells exposed to either vehicle or 1 mM AICAR for 30 min. with primers flanking the region containing the FXRE motif of the BSEP promoter. Sybr-Green QPCR (normalized to input) was used to assess SRC-2 or AMPK alpha 2 occupancy of the BSEP promoter upon chromatin immunoprecipitation, using an SRC-2 or AMPK a2 specific antibody. Control antibody recognized mouse IgG.
All gene expression data are represented as mean + SEM. Unpaired student's t-test was used for evaluation of statistical significance. One asterisk indicates p < 0.05, two asterisks p < 0.01 and three asterisks p < 0.001.
See also Fig. S6
Discussion
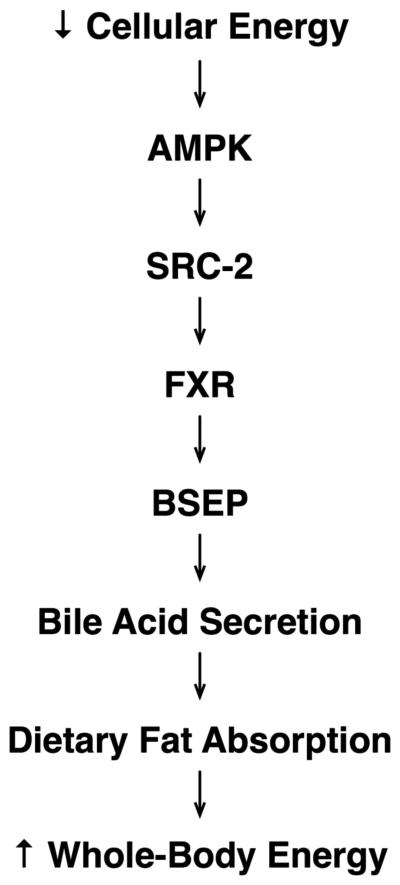
Our results have implicated the SRC-2 coactivator as a newly discovered, essential cog in the AMPK mediated energy regeneration wheel. We have elucidated a pathway that links cellular energy depletion with the act of priming the entry of dietary fuel into the body (Fig. 7), thereby linking the cellular energy state with the whole-animal energy state. We have shown that the cellular energy depletion sensing kinase AMPK activates the transcriptional coactivator SRC-2 which in turn transactivates the BSEP gene by synergizing with the nuclear receptor FXR, resulting in hepatic BA secretion and downstream dietary fat absorption. Interfering with this process by genetic deletion of SRC-2, in a whole-body as well as liver-specific manner, results in abrogation of hepatic BA secretion and results in fat malabsorption in the gut. These effects can be fully rescued by correcting the gut BA deficiency as well as by correcting the hepatic BSEP deficiency genetically.
Fig. 7.
Schematic depicting the cascade that links cellular energy depletion with whole-body energy repletion.
Our results suggest that AMPK promotes absorption of dietary fat in order to provide fuel for its core cellular function of fatty-acid oxidation and ATP regeneration (Minokoshi et al., 2002). The cycle appears to begin with a low cellular ATP state, presumably due to a moderate drop in energy stores between meals, which activates AMPK and SRC-2, thereby allowing the liver to secrete BA into the gall bladder, optimally priming the gut for triglyceride absorption from the next meal, which in turn serves as a fuel for cellular fatty-acid oxidation, thus causing ATP synthesis and deactivation of the above cascade. There is some precedent for ATP levels displaying circadian oscillation (Womac et al., 2009), presumably set by periodic food intake, while AMPK is well known to be under circadian control (Lamia et al., 2009). By that token, we hypothesize that the cascade we have described is activated and deactivated in a circadian manner and is set by the circadian oscillation in ATP levels and AMPK activation. SRC-2 demonstrates exquisite specificity in the modulation of this pathway compared with its family members SRC-1 and SRC-3, much like we have shown in the past for glucose homeostasis (Chopra et al., 2008).
Since SRC-2 has been shown to oppose energy loss (Picard et al., 2002) and our current results have implicated it in energy uptake, it appears to function much like AMPK (Carling, 2004; Hardie and Carling, 1997; Kahn et al., 2005) in preventing energy depletion. Due to its holistic nature in control of energy homeostasis, the AMPK-SRC-2 axis may serve as a potential target to fine tune whole-body energy levels, and combat obesity and associated comorbidities.
Methods
Mice
Whole-body SRC-1, SRC-2 and SRC-3 null mice have been described (Chopra et al., 2008). We used 8-16 week old male, littermate mice for all assays. SRC-2 F/F mice (Mukherjee et al., 2006) were crossed with Albumin-Cre mice (Weisend et al., 2009) to generate liver-specific SRC-2 knockout (SRC-2 LKO) mice. We used 8-16 week old male SRC-2 LKO mice and sex-matched SRC-2 F/F (WT) littermates for all in vivo studies. The results from SRC-2−/− mice (food intake, fecal mass, fecal triglycerides and plasma triglycerides) mirrored those obtained using SRC-2 LKO mice, thereby ruling out any influence by Cre recombinase mediated toxicity in SRC-2 LKO mice. For BSEP mediated genetic rescue experiments, WT and SRC-2 LKO mice were exposed to adenoviral transgenesis (1011 virus particles per mouse), via tail-vein injections as previously described (Chopra et al., 2008). Fecal samples were collected from individual mice using metabolic cages over a 24-hour time period 7 days after virus infusion. Mice were sacrificed and plasma and various organs were isolated 8 days after viral infusion. WT C57 mice were injected subcutaneously with AICAR (0.5 mg/g BW) and livers were isolated 12 hours later. Livers lacking AMPK were obtained from liver-specific knockout mice described previously (Guigas et al., 2006) and were provided by Beniot Viollet. Unless otherwise stated, all experiments were performed in the 24-hour ad libitum fed state, which followed an overnight fast to synchronize the metabolism of all mice. The Baylor College of Medicine Institutional Animal Care and Utilization Committee approved all experiments.
Metabolic Studies
We measured plasma and biliary BA, plasma and biliary phospholipids and plasma triglycerides using colorimetric assays from Diagnostic Chemicals Ltd., Wako Pure Chemicals and Thermo Scientific respectively, using the manufacturer's protocol (bile was obtained from the gall bladders using a 26 gauge needle and syringe). Biliary cholesterol was measured using a colorimetric assay (MBL International), using the manufacturer's protocol. Plasma cholesterol, HDL, LDL, VLDL and bilirubin were measured by the Baylor College of Medicine veterinary pathology core laboratory. Hepatic BAs were extracted by digesting the liver in 70% ethanol followed by a colorimetric assay (Diagnostic chemicals Ltd.) after normalizing to liver weight. BA pool size was determined as previously described (Tiemann et al., 2004). BA composition was determined by HPLC using a reverse phase C18 column with isocratic elution at 0.75 ml/min using a methanol phosphate buffer. Conjugated bile acids were detected by absorbance at 205 nm and identity assignments were made by relative retention times with known standards. To ensure that nothing escaped the HPLC system, one sample from each group of mice was run on a nanoESI-MS system. Intestinal cholesterol absorption was determined as previously described (Temel et al., 2005). Food intake was assessed from individual mice using metabolic cages over a 24-hour time period. Fecal samples were collected from individual mice using metabolic cages over a 24-hour time period. Fecal samples were weighed and subjected to lipid extraction procedures (Kolonin et al., 2004) following which triglyceride measurement was carried out using a colorimetric assay (Thermo Scientific) after normalizing to fecal weight. Fecal lipase activity was measured using a colorimetric kit (Bio Assay Systems) after normalization to fecal weight. VO2 levels were measured using individually housed mice in metabolic cages. Diets containing 1% cholic-acid, high fat (60% calories from fat) and high fat plus 1% cholic-acid were purchased from Harlan-Teklad. Assessment of intestinal triglyceride uptake into the circulation and intestine using C14 labeled olive oil has been described (Yen et al., 2009).
Histological Analyses
We fixed liver samples in 10% formaldehyde for H&E staining. We used frozen proximal jejunal cross sections for oil-red-O staining to evaluate neutral lipid content in intestinal villi.
RNA and Protein Analysis
We used standard RNA extraction procedures (RNeasy Mini Kit from Qiagen). Reverse transcription was carried out using the Superscript III kit (Invitrogen) using the manufacturer's protocol. For gene expression analysis, QPCR was performed using sequence-specific primers and probes from Roche (Universal Probe Library). GAPDH was used as an internal control for all gene-expression assays. All QPCR primers are available upon request. Protein, extracted from frozen livers using tissue lysis buffer (Pierce), was subjected to Western blot analysis using antibodies against BSEP (Abgent), GAPDH (Abcam). HepG2 cell Western blot and co-immunoprecipitation analysis was performed using antibodies against – SRC-2 (Bethyl), phospho-AMPK alpha 2 and AMPK alpha 2 (Cell Signaling) and beta-Actin (Sigma), using standard methodology.
In Vitro Kinase Assays
We used full length purified SRC-2 protein along with purified AMPK holoenzyme (Cell Signaling) for in vitro kinase assays using the manufacturer's protocol (Cell Signaling).
Chromatin Immunoprecipitation
In vivo ChIP was performed by using liver tissue from WT and FXR knockout mice previously infused with collagenase. After percol treatment to exclude dead cells, 1% formaldehyde was added to produce cross linking. The rest of the ChIP procedure was performed using the EZ ChIP kit (Upstate) following the manufacturer's protocol. Standard ChIP procedure was followed for experiments using HepG2 liver hepatoma cells in culture. SRC-2 antibodies are from Bethyl labs. AMPK alpha 2 antibody is from Cell Signaling. QPCR for ChIP was performed using the Sybr-Green technology (Applied Biosystems) using sequence specific primers. Results were normalized to input in each case. Primer sequences are available on request.
Cell Culture
Primary mouse hepatocytes (PHs) were isolated from 8- to 12-week-old WT and SRC-2 LKO mice as described previously (Chopra et al., 2008). Cells were incubated overnight in Williams E media (Invitrogen) containing 10% FBS before transfection with siRNA or treatment with CDCA. Smart-pool siRNA against SRC-2 non-targeting control was purchased from Dharmacon. RNA isolation was carried out 48 hours after treatment. HepG2 cells were transfected with a reporter-gene plasmid driven by the wild-type mouse BSEP promoter (Zhang et al., 2003) and the same promoter with a mutation in the FXRE (Stratagene QuickChange kit), together with expression plasmids for SRC-2, FXR and with WT SRC-2, AD1, AD2 and AD1/2 deletion mutants of SRC-2. Reporter-gene levels were determined 48 hours after transfection and treatment with CDCA. Constitutively active and dominant negative AMPK adenoviruses have been described (Woods et al., 2000). For SRC-2 activation experiments, HepG2 cells were transfected with pG5Luc, bBIND-SRC-2 and pBIND-empty and dominant negative AMPK (DnAMPK). Reporter-gene levels were determined 48 hours after transfection and treatment with 1 mM AICAR.
Statistical Analysis
All results are presented as mean + SEM. P value was calculated by unpaired Student's t test and either one-way or two-way ANOVA where appropriate.
Supplementary Material
Acknowledgements
We would like to thank S. McGuire, S. Settle, V. Yechoor, M. Tsai, A. Beaudet, A. Antebi and H. Taegtmeyer for helpful discussion, R. Lanz for QPCR setup and analysis, J. Xu for SRC-1, SRC-2 and SRC-3 null mice, the Texas Medical Center Digestive Disease Center Molecular Morphology Core (DK 56338) for help with histopathology, L. Hagey for performing bile acid composition analysis. The BSEP promoter reporter construct was kindly provided by P. Edwards. The BSEP adenovirus was a kind gift from Y. Wakabayashi. The AMPK alpha DKO livers were generously provided by B. Viollet. D.M. is supported by the NIH (R01 DK068804). F.D. is supported by the NIDDK (PO1 DK59820). L.C. is supported by the NIH (HL51586). B.W.O. is supported by the NIDDK (PO1 DK59820), NURSA (U19 DK062434) and a grant from the Welch foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. The Journal of biological chemistry. 2001;276:28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- Bungard D, Fuerth BJ, Zeng PY, Faubert B, Maas NL, Viollet B, Carling D, Thompson CB, Jones RG, Berger SL. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science (New York, N.Y. 329:1201–1205. doi: 10.1126/science.1191241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends in biochemical sciences. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, O'Malley BW. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science (New York, N.Y. 2008;322:1395–1399. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigas B, Bertrand L, Taleux N, Foretz M, Wiernsperger N, Vertommen D, Andreelli F, Viollet B, Hue L. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside and metformin inhibit hepatic glucose phosphorylation by an AMP-activated protein kinase-independent effect on glucokinase translocation. Diabetes. 2006;55:865–874. doi: 10.2337/diabetes.55.04.06.db05-1178. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? European journal of biochemistry / FEBS. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Kwak I, Lee KY, White LD, Wang XP, Brunicardi FC, O'Malley BW, DeMayo FJ. The genomic analysis of the impact of steroid receptor coactivators ablation on hepatic metabolism. Molecular endocrinology (Baltimore, Md. 2006;20:1138–1152. doi: 10.1210/me.2005-0407. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell metabolism. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nature medicine. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science (New York, N.Y. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew JL, Zhao A, Yu J, Huang L, De Pedro N, Pelaez F, Wright SD, Cui J. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. The Journal of biological chemistry. 2004;279:8856–8861. doi: 10.1074/jbc.M306422200. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, Lydon JP, O'Malley BW. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Molecular and cellular biology. 2006;26:6571–6583. doi: 10.1128/MCB.00654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. Jama. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Picard F, Gehin M, Annicotte J, Rocchi S, Champy MF, O'Malley BW, Chambon P, Auwerx J. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell. 2002;111:931–941. doi: 10.1016/s0092-8674(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. The Biochemical journal. 1998;334(Pt 1):177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Temel RE, Lee RG, Kelley KL, Davis MA, Shah R, Sawyer JK, Wilson MD, Rudel LL. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. Journal of lipid research. 2005;46:2423–2431. doi: 10.1194/jlr.M500232-JLR200. [DOI] [PubMed] [Google Scholar]
- Tiemann M, Han Z, Soccio R, Bollineni J, Shefer S, Sehayek E, Breslow JL. Cholesterol feeding of mice expressing cholesterol 7alpha-hydroxylase increases bile acid pool size despite decreased enzyme activity. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1846–1851. doi: 10.1073/pnas.0308426100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, Hue L, Andreelli F. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. The Journal of physiology. 2006;574:41–53. doi: 10.1113/jphysiol.2006.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkowiak J, Jankowska I, Pawlowska J, Strautnieks S, Bull L, Thompson R, Herzig KH, Socha J. Exocrine pancreatic function in children with progressive familial intrahepatic cholestasis type 2. Journal of pediatric gastroenterology and nutrition. 2006;42:416–418. doi: 10.1097/01.mpg.0000218154.26792.6a. [DOI] [PubMed] [Google Scholar]
- Weisend CM, Kundert JA, Suvorova ES, Prigge JR, Schmidt EE. Cre activity in fetal albCre mouse hepatocytes: Utility for developmental studies. Genesis. 2009 doi: 10.1002/dvg.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womac AD, Burkeen JF, Neuendorff N, Earnest DJ, Zoran MJ. Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. The European journal of neuroscience. 2009;30:869–876. doi: 10.1111/j.1460-9568.2009.06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Molecular and cellular biology. 2000;20:6704–6711. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CL, Cheong ML, Grueter C, Zhou P, Moriwaki J, Wong JS, Hubbard B, Marmor S, Farese RV., Jr. Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nature medicine. 2009;15:442–446. doi: 10.1038/nm.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kast-Woelbern HR, Edwards PA. Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. The Journal of biological chemistry. 2003;278:104–110. doi: 10.1074/jbc.M209505200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.