Abstract
Bioactive components of many foods added during cooking have potential antioxidant, anti-inflammatory, antimicrobial, antibacterial and chemopreventive properties. However, epidemiologic studies generally do not collect detailed information on these items which include spices, chilies, coconuts, garlic, onions, and oils. Since India has some of the highest spice consumption in the world, we developed a computer-based food preparer questionnaire to estimate per capita consumption of 19 spices, chilies, coconuts, garlic, onions, and 13 cooking oils among 3,625 participants in the India Health Study, a multicenter pilot study in three regions of India. We observed notable regional differences in consumption of spices, chilies, coconut, garlic, and onions. In Trivandrum, over 95 percent of the participants consumed 12 different spices, while in New Delhi and Mumbai, 95 percent of participants consumed only four and five spices, respectively. Cooking oil use also varied, as ghee was most common in New Delhi (96.8%) followed by mustard seed oil (78.0%), while in Trivandrum the primary oil was coconut (88.5%) and in Mumbai it was peanut (68.5%). There was some variation in consumption by education, income, and religion. Using a novel method for assessing food items primarily added during cooking, we successfully estimated per capita consumption within an epidemiologic study. Based on basic science research and suggestive ecologic level data on cancer incidence and spice consumption, improving epidemiologic assessment of these potentially chemopreventive food items may enhance our understanding of diet and cancer risk.
Keywords: India, diet, spices, cooking oils, cancer prevention
Introduction
Foods that are primarily added during cooking for flavor or seasoning, such as spices, allium vegetables (garlic and onions), chilies, coconuts, and oils contain bioactive components with potential antioxidant, antimutagenic, anti-inflammatory, and antimicrobial/antibacterial properties (Pehowich et al., 2000; Bianchini and Vainio, 2001; Surh, 2002; Lampe, 2003; Larsson et al., 2004; Sengupta et al., 2004; Psota et al., 2006; Slimestad et al., 2007; Kaefer and Milner, 2008; Krishnaswamy, 2008; Powolny and Singh, 2008; Aggarwal and Sung, 2009; Butt et al., 2009; Iciek et al., 2009; Russo, 2009; Ganguly, 2010; Prasad et al., 2010). Spices have been of particular interest in basic science research in relation to chronic disease risk as they contain many phytochemicals, including flavonoids, tannins, phenolic acids, and terpenes, that may be relevant to chronic disease. Onions and garlic may also be relevant to disease etiology as they contain organosulfur compounds and flavonoids (Shelef, 1984; Bianchini and Vainio, 2001; Sengupta et al., 2004; Slimestad et al., 2007; Kaefer and Milner, 2008; Krishnaswamy, 2008; Powolny and Singh, 2008; Butt et al., 2009; Iciek et al., 2009; Prasad et al., 2010). Coconut oil and whole coconuts, which contain primarily medium chain fatty acids, are important sources of dietary fat in certain populations (Elson, 1992) and may be healthier than other foods high in saturated fat (Pehowich et al., 2000), but have not been investigated to a great extent. Fatty acids from cooking oil may also be related to cancer risk (WCRF, 2007) via immunity, inflammation, and cell signaling (Larsson et al., 2004). Omega-3 and omega-6 fatty acids from some nut and seed oils, may have health implications for cardiovascular outcomes (Rastogi et al., 2004; Psota et al., 2006; Russo, 2009).
Even though research suggests a role for some of these seasonings and other items added during cooking in cancer and other chronic diseases, epidemiologic studies of diet generally do not capture information on these foods. India is an ideal setting to evaluate consumption of these food items, as they are key components of the traditional diets. Moreover, in developing countries with limited access to diverse foods, certain spices may also be important dietary sources of micronutrients, such as iron and calcium (Ramasastri, 1983), which may themselves also be related to disease risk. The general format of current dietary assessment instruments that collect frequency and portion size may not be appropriate for ascertaining foods added during cooking, as they are generally consumed in small amounts within larger mixed dishes (Kaefer and Milner, 2008). A few small studies in India have assessed these foods, but have generally been limited by querying a small number of food items or utilizing methods that may not be practical in large population-based studies (Thimmayamma et al., 1983; Uma Pradeep et al., 1993; Beegom and Singh, 1997; Gupta and Prakash, 1997; Kumar, 1997; Mathew et al., 2000; Phukan et al., 2001; Nayak et al., 2009). If an individual does not cook most of their own food, they may not know all the food items contained in mixed dishes, therefore the food preparer may be best suited to provide information on items that added during cooking.
Although there is a multitude of laboratory and animal studies suggesting a potential role for spices and seasonings in cancer prevention, there are very little epidemiologic data in this area due in part to the difficult in assessing consumption of these foods. The ability to assess all aspects of the diet may be particularly relevant for understanding the complex role of diet in chronic disease, especially cancer, when conducting epidemiologic investigations worldwide. Therefore, we developed a food preparer questionnaire as part of a multicenter epidemiologic pilot study of diet in India to estimate per capita consumption of spices, chilies, coconuts, garlic, onions, and cooking oils. As a supplement to this investigation, we also evaluated ecologic data on global spice consumption and all cancer incidence.
Materials and Methods
Data sources for global spice consumption and all cancer incidence
We used cross sectional food availability data from the Food and Agriculture Organization of the United Nation’s Statistical Databases (FAOSTAT) (http://faostat.fao.org/), to look at spice consumption trends overtime and by region (Food and Agriculture Organization of the United Nations, 2010). Food availability or consumption, as measured in “crops primary equivalent” values, estimates the total amount of the commodity available for human consumption taking into account exports, and other waste from farm to household). In general, spices can be defined as parts (bark, buds, fruit or flower bulbs, roots, seeds, stems) of tropical plants that have been dried, while herbs are the leafy parts (fresh or dried) of temperate zone plants. Total spice consumption from FAOSTAT included the following spices: vanilla, cinnamon, nutmeg, mace, cardamom, anise, badian, fennel, coriander, ginger, and an other spices category which included bay leaves, dill seed, fenugreek seed, saffron, thyme, turmeric, as well as curry power and other spice mixtures. All cancer (excluding non-melanoma skin cancer) age-standardized incidence rates (per 100,000) for 2008 were obtained from GLOBOCAN (http://globocan.iarc.fr/) (Ferlay et al., 2010).
India Health Study
The India Health Study (IHS) was a multi-center pilot study undertaken to assess the feasibility of establishing a cohort study of diet and cancer in India. The IHS enrolled individuals from December 2006 through July 2008 in three regions of India; New Delhi in the north (All India Institute of Medical Sciences), Mumbai in the west (Healis-Sekhsaria Institute for Public Health), and Trivandrum in the south (Regional Cancer Center). The centers were selected to capture established cancer registries, as well as a range of economic, ethnic, and urbanization patterns. Human ethics committees from each study center and the Special Studies Institutional Review Board of the United States National Cancer Institute reviewed and approved the study protocol prior to study commencement. Due to the nature of the international collaboration, the Indian Health Ministry Screening Committee, which is part of the Indian Council of Medical Research reporting to the Government of India, also reviewed and approved the study.
Participants were recruited at the household level from each region. Households (excluding slums and temporary housing) were identified from census data and/or voter registration lists in New Delhi and Trivandrum. In Mumbai, an existing cohort database (Gupta, 1996) was used to identify households or neighboring households. In addition, recruitment of households at each center was stratified by religion (Hindu/Muslim/Christian), and for Trivandrum only, residence status (urban/rural). Field interviewers verified household eligibility during the first in-home recruitment visit.
Participants were eligible if they were between 35-69 years old, resided in study area for a minimum period of one year (to minimize the number of frequent movers), had no prior history of cancer or cardiovascular event, could speak English or the primary regional language, had no physical ailments that would prevent them from fully participating in the study, were willing to provide biological samples, and were not pregnant, if female. We recruited approximately equal numbers of subjects for each five-year age category and one male and one female per household for equal gender distribution and cost efficiency.
Trained field personnel administered questionnaires and diet assessments in the participants’ homes. Socio-demographic, household, diet (described in detail below), and lifestyle information were collected upon enrollment. From the 6,355 persons identified from 3,033 screened households, 4,671 (74%) met all eligibility criteria. The final response rate was 89% (n=4,177), as 11% were unwilling to participate. Of the 4,177 enrolled participants, 4,144 provided demographic information and/or diet history information.
Assessment of spices, chilies, coconut, garlic, onions, and cooking oils
Field personnel administered a detailed computer-based diet questionnaire using the New Interactive Nutrition Assistant– Diet in India Study of Health (NINA-DISH) software, which was adapted from software originally developed by Novo Nordisk Pharma India (Bangalore, India) (Kapur et al., 1997). The diet questionnaire consisted of three sections: (1) defined questions on frequency and portion size, similar to a food frequency questionnaire; (2) an openended section for each mealtime; and (3) a food-preparer questionnaire.
The food preparer questionnaire was completed by either (1) a study participant identified as the household’s primary food preparer; or (2) a study participant with the help of the primary food preparer for their household. The questionnaire elicited detailed information on 19 spices (including salt), chilies (dried, green), coconuts, garlic, onions (small, red), and 13 cooking oils. The food preparers were asked how much (g, kg, or number) of each of these items they purchased for the household during a specific time span (week, month).
This information was then linked to data on the number and ages of people living in each household. To account for the varying amounts of food consumed by different age groups, individuals less than five years of age were counted as 0.70 of a person-unit, individuals five to 12 years of age were counted as 0.90 of a person-unit, and individuals greater than 12 years of age contributed 1.0 person-units. For example, a household with 2 adults and 2 children under age five would have 3.4 total person-units. This approach was adapted from consumer surveys conducted by the government of India (National Sample Survey Organization, 1996). We then divided the total amount of each food item (standardized to g/month or n/month) by the total person units in the household to arrive at a per capita estimate of consumption.
Statistical Analysis
For the ecologic level data, spice consumption over time by region was graphed with smoothed lines. We also calculated the Pearson correlation coefficient to evaluate the relationship between spice consumption from 2007 from FAOSTAT (most recently available data) and all cancer incidence from 2008 (most current estimates). For the IHS data, we calculated simple descriptive statistics to assess the range of consumption of each food item by study region. If a food item was missing the measurement unit (g or kg) or the time unit (week, month), we imputed the mean per capita consumption for the household’s region (New Delhi, Trivandrum, Mumbai). We also calculated the percentage of participants that had ever used each individual food item, with never defined as 0 g/month or 0 n/month. In addition, we assessed the association between mean consumption and selected demographic characteristics controlling for region (New Delhi, Trivandrum, Mumbai), using a F-test and then tested for pairwise differences with correction for multiple comparisons based on the Tukey method. Reported p-values are all two-sided and analyses were conducted using SAS software (SAS Institute, Cary, NC, Version 9).
Individuals were eligible for this analysis if they or another individual in their household had completed the food preparer questionnaire. Food preparer data were missing for 519 participants for various reasons (no food preparer identified for the household, inaccurate information on the number people in household, or missing household identification or linking information), leaving us with an analytic population of 3,625 (New Delhi, n=835; Trivandrum, n=2,044; Mumbai, n=746). For analyses involving demographic characteristics, we further excluded 6 individuals who were missing this information.
Results
Global spice consumption and all cancer incidence
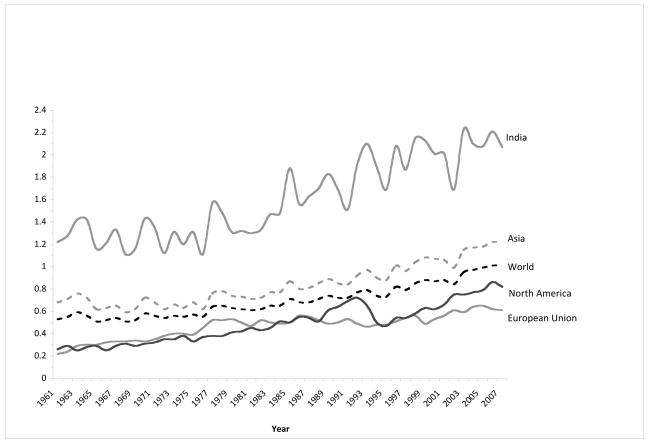
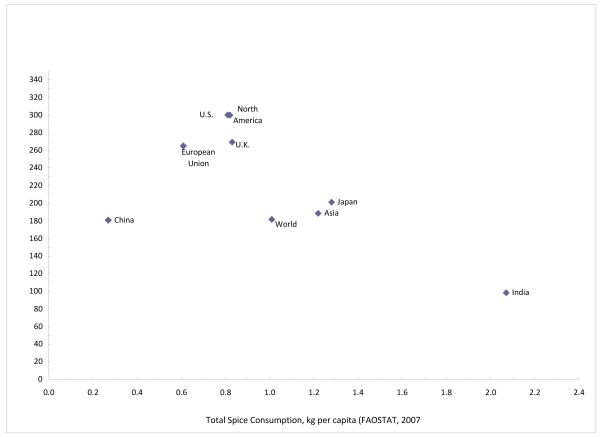
Based on food availability data from FAOSTAT, since 1961 per capita spice consumption globally and within various regions have been increasing (Figure 1). With the most current data for 2007, even within Asia, which had higher consumption (1.22 kg/capita) than the global average (1.01 kg/capita), India’s consumption (2.07 kg/capita) was exceptionally high (Figure 1). At an ecologic level, there was a suggestive inverse correlation between spice consumption and all cancer (excluding non-melanoma skin cancer) age standardized incidence rates (Pearson correlation coefficient=-0.621, p-value=0.074) (Figure 2).
Figure 1.
Trends in per capita total spice consumptiona by region based on FAOSTAT (http://faostat.fao.org/), 1961-2007.
aTotal spice consumption includes the following spices: vanilla, cinnamon, cutmeg, mace, cardamom, anise, badian, fennel, coriander, ginger, and an other spices category which included bay leaves, dill seed, fenugreek seed, saffron, thyme, turmeric, as well as curry power and other spice mixtures.
Figure 2.
Correlation between per capita total spice consumption for 2007 (FAOSTAT, http://faostat.fao.org/) and all cancer age-stardardized incidence rates for 2008 (GLOBOCAN, http://globocan.iarc.fr/) (Pearson correlation coefficient = −0.621, p-value = 0.074).
aTotal spice consumption includes the following spices: vanilla, cinnamon, cutmeg, mace, cardamom, anise, badian, fennel, coriander, ginger, and an other spices category which included bay leaves, dill seed, fenugreek seed, saffron, thyme, turmeric, as well as curry power and other spice mixtures.
bAll cancer (excluding non-melanome skin cancer) age-standarded incidence rates (per 100,000).
Consumption of spices and seasonings in the IHS
We observed notable regional differences in per capita spice consumption among the 3,625 participants with food preparer information (Table 1). In Trivandrum, over 95 percent of the participants reported consuming 12 different spices. However, in New Delhi and Mumbai, 95 percent reported consuming only four and five spices, respectively. Only ginger, salt, and turmeric were consumed by more than 95% of the population in each of the three regions. Median per capita consumption of ginger was highest in Mumbai, while turmeric consumption was highest in New Delhi. Reported median consumption of salt was highest in New Delhi (285.7 g/month/person) and slightly lower in Trivandrum and Mumbai (250.0 g/month/person). Excluding salt, in New Delhi, the median per capita consumption (g/month) of ginger (41.7) was highest, followed by chili powder (35.7), coriander (33.3), garam masala (33.3), and turmeric (28.6). Trivandrum was characterized by high consumption (g/month/person) of chili powder (166.7), tamarind (135.1), coriander (102.0), curry leaves (43.3), and ginger (37.3). In Mumbai, both ginger (58.3) and chili powder (58.3) had the highest median per capita consumption (g/month/person), followed by curry leaves (27.1), turmeric (21.7), and cumin (20.0).
Table 1.
Per capita spice consumption (grams/month/person) in New Delhi (n=835), Trivandrum (n=2044), and Mumbai (n=746)
| New Delhi | Trivandrum | Mumbai | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Spice | Median |
10th-90th percentile |
% Ever used |
Median |
10th-90th percentile |
% Ever used |
Median |
10th-90th percentile |
% Ever used |
| Asafoetida | 0.0 | (0.0-3.8) | 30.2 | 12.5 | (5.6-25.0) | 97.1 | 3.6 | (0.0-14.7) | 98.8 |
| Basil | 0.0 | (0.0-0.0) | 5.5 | 0.0 | (0.0-0.0) | 2.3 | 0.0 | (0.0-0.0) | 8.2 |
| Bay leaves | 0.0 | (0.0-0.0) | 5.6 | 0.0 | (0.0-0.0) | 3.0 | 2.0 | (0.0-8.8) | 68.5 |
| Black mustard seeds | 0.0 | (0.0-20.0) | 45.0 | 25.0 | (13.2-50.0) | 98.7 | 16.7 | (4.4-34.5) | 92.8 |
| Black pepper | 8.8 | (0.0-17.2) | 69.9 | 18.5 | (6.6-41.7) | 98.0 | 3.0 | (0.0-16.7) | 65.2 |
| Cardamom | 0.0 | (0.0-12.5) | 38.9 | 1.7 | (0.0-14.9) | 58.7 | 4.6 | (0.0-17.5) | 72.1 |
| Chili powder | 35.7 | (16.7-62.5) | 95.2 | 166.7 | (89.3-274.0) | 99.6 | 58.3 | (0.0-125.0) | 85.7 |
| Cloves | 0.0 | (0.0-25.0) | 48.0 | 0.0 | (0.0-37.5) | 25.7 | 3.1 | (0.0-12.5) | 69.6 |
| Coriander | 33.3 | (0.0-52.6) | 89.5 | 102.0 | (50.0-197.4) | 99.0 | 12.5 | (0.0-37.3) | 79.1 |
| Cumin | 25.0 | (0.0-52.6) | 86.8 | 25.0 | (12.5-37.5) | 97.9 | 20.0 | (7.8-50.0) | 97.2 |
| Curry leaves | 0.0 | (0.0-0.0) | 6.4 | 43.3 | (6.3-149.4) | 99.5 | 27.1 | (7.5-100.0) | 96.1 |
| Curry powder | 0.0 | (0.0-20.0) | 19.4 | 0.0 | (0.0-53.2) | 49.0 | 0.0 | (0.0-33.3) | 49.2 |
| Fenugreek | 0.0 | (0.0-15.0) | 49.3 | 25.0 | (14.9-50.0) | 99.0 | 0.0 | (0.0-25.0) | 45.0 |
| Garam masala | 33.3 | (16.7-50.0) | 96.7 | 17.0 | (5.7-38.5) | 97.5 | 12.5 | (0.0-50.0) | 67.4 |
| Ginger | 41.7 | (16.7-108.3) | 96.9 | 37.3 | (10.0-100.0) | 95.8 | 58.3 | (10.0-216.7) | 96.9 |
| Saffron | 0.0 | (0.0-5.0) | 58.6 | 0.0 | (0.0-0.0) | 0.8 | 0.0 | (0.0-0.0) | 7.9 |
| Salt | 285.7 | (166.7-500.0) | 99.3 | 250.0 | (125.0-500.0) | 99.6 | 250.0 | (125.0-408.2) | 98.8 |
| Tamarind | 0.0 | (0.0-6.3) | 30.1 | 135.1 | (75.0-250.0) | 99.6 | 2.2 | (0.0-37.5) | 52.4 |
| Turmeric | 28.6 | (10.0-52.1) | 98.1 | 25.0 | (15.0-59.4) | 99.5 | 21.7 | (10.0-50.0) | 99.3 |
We also observed differences in consumption of chilies (dried and green), coconuts, garlic, and onions (small and red) (Table 2). Coconut consumption was common in both Trivandrum and Mumbai, with 100% and 90.5% of individuals, respectively, reporting this food, but was nearly absent from New Delhi cuisine (3.7%). Garlic was common to all three regions, but consumption in Mumbai (7.2 heads/month/person) was more than double that in New Delhi (3.4) and Trivandrum (3.3) Green chilies and red onions were also common across the three regions, while small white onions and dried chilies were predominantly used only in Trivandrum.
Table 2.
Per capita consumption (unit/month/person) of coconuts, chilies, onions, and garlic in New Delhi (n=835), Trivandrum (n=2044), and Mumbai (n=746)
| Region | Food item | 10th Percentile | Median | 90th Percentile | % Ever used |
|---|---|---|---|---|---|
| New Delhi | Coconuts (n) | 0.00 | 0.0 | 0.0 | 3.7 |
| Garlic heads (n) | 1.4 | 3.4 | 36.1 | 92.7 | |
| Green chilies (g) | 61.9 | 130.0 | 433.3 | 98.2 | |
| Red onions (g) | 153.5 | 1444.4 | 2766.0 | 95.2 | |
| Small onions (g) | 0.0 | 0.0 | 0.0 | 2.3 | |
| Dried chilies (g) | 0.0 | 0.0 | 111.1 | 37.1 | |
| Trivandrum | Coconuts (n) | 5.3 | 8.7 | 15.2 | 100.0 |
| Garlic heads (n) | 1.4 | 3.3 | 7.4 | 99.9 | |
| Green chilies (g) | 48.2 | 108.3 | 221.1 | 99.9 | |
| Red onions (g) | 380.1 | 1083.3 | 2166.7 | 99.6 | |
| Small onions (g) | 188.4 | 541.7 | 1083.3 | 99.2 | |
| Dried chilies (g) | 0.0 | 27.1 | 108.3 | 75.4 | |
| Mumbai | Coconuts (n) | 0.4 | 2.2 | 5.4 | 90.5 |
| Garlic heads (n) | 2.9 | 7.2 | 91.1 | 99.2 | |
| Green chilies (g) | 36.1 | 108.3 | 252.8 | 99.2 | |
| Red onions (g) | 541.7 | 1444.4 | 3033.3 | 96.1 | |
| Small onions (g) | 0.0 | 0.0 | 0.0 | 5.5 | |
| Dried chilies (g) | 0.0 | 0.0 | 57.0 | 28.2 |
There was also diversity in the type of cooking oils used, as ghee was most common in New Delhi (96.8%), followed by mustard seed oil (78.0%), while in Trivandrum the primary oils were coconut (88.5%) and ghee (40.1%), and in Mumbai they were peanut (68.5%) and ghee substitute (47.7%) (Table 3). Other oils that were less common across all three regions were cottonseed oil, olive oil, safflower oil, and vegetable oil. Only ghee and sunflower oil appeared in the top 5 oils across all three regions.
Table 3.
Top five most commonly used cooking oils in New Delhi (n=835), Trivandrum (n=2044), and Mumbai (n=746) based on ever versus never use and per capita consumption (grams/person/month)
| Region | Food item | 10th Percentile | Median | 90th Percentile | % Ever used |
|---|---|---|---|---|---|
| New Delhi | Ghee | 200.0 | 400.0 | 750.0 | 96.8 |
| Mustard oil | 0 | 400.0 | 800.0 | 78.0 | |
| Soybean oil | 0.0 | 0.0 | 500.0 | 38.4 | |
| Sunflower oil | 0.0 | 0.0 | 500.0 | 20.7 | |
| Ghee substitute | 0.0 | 0.0 | 166.7 | 12.9 | |
| Trivandrum | Coconut oil | 0.0 | 384.6 | 760.4 | 88.5 |
| Ghee | 0.0 | 0.0 | 26.7 | 40.1 | |
| Palm oil | 0.0 | 0.0 | 270.3 | 28.3 | |
| Sunflower oil | 0.0 | 0.0 | 125.0 | 11.4 | |
| Sesame seed oil | 0.0 | 0.0 | 0.0 | 8.5 | |
| Mumbai | Peanut oil | 0.0 | 746.0 | 1333.3 | 68.5 |
| Ghee substitute | 0.0 | 0.0 | 147.1 | 47.7 | |
| Ghee | 0.0 | 0.0 | 125.0 | 26.7 | |
| Sunflower oil | 0.0 | 0.0 | 1000.0 | 24.7 | |
| Palm oil | 0.0 | 0.0 | 0.0 | 5.6 |
Finally, we evaluated associations between mean consumption of selected food items by demographic characteristics and controlled for region (Table 4). There were some consumption differences by education level, as consumption of red onions increased with higher education levels, while consumption of garlic decreased with higher education levels. Similarly, higher income was associated with higher red onion and lower garlic consumption. Higher income was also associated with higher consumption of ghee. Consumption of red onions, chili powder, and ginger also varied by religion, but these differences were not statistically significant for all pair-wise comparisons
Table 4.
Mean* per capita consumption (unit/month/person) of selected dietary variables by demographic characteristics (N=3,619)
| Characteristic | n | Ghee (g) (mean ± SE) |
Red onions (g) (mean ± SE) |
Garlic heads (n) (mean ± SE) |
Chili powder (g) (mean ± SE) |
Turmeric (g) (mean ± SE) |
Ginger (g) (mean ± SE) |
Saffron (g) (mean ± SE) |
|---|---|---|---|---|---|---|---|---|
| Education | ||||||||
| 1. Middle school or less | 1464 | 154.1 ± 3.4 | 1309.8 ± 25.6 | 19.9 ± 0.8 | 97.2 ± 2.2 | 31.4 ± 0.7 | 60.2 ± 1.7 | 0.52 ± 0.05 |
| 2. Secondary education | 1588 | 157.8 ± 3.3 | 1503.9 ± 24.6 | 18.4 ± 0.7 | 98.3 ± 2.1 | 31.0 ± 0.6 | 67.5 ± 1.6 | 0.58 ± 0.05 |
| 3. University or post-graduate | 567 | 160.0 ± 5.4 | 1549.2 ± 40.6 | 12.7 ± 1.2 | 91.1 ± 8/5 | 30.9 ± 1.1 | 66.0 ± 2.7 | 0.51 ± 0.08 |
| p-valuea | <0.001 | <0.001 | <0.001 | <0.001 | 0.673 | <0.001 | <0.001 | |
| Pairwise p-valuesb | ||||||||
| 1 vs 2 | 0.68 | <0.001 | 0.29 | 0.92 | <0.001 | 0.56 | ||
| 1 vs 3 | 0.63 | <0.001 | <0.001 | 0.31 | 0.16 | 0.99 | ||
| 2 vs 3 | 0.94 | 0.61 | <0.001 | 0.20 | 0.90 | 0.75 | ||
| Household monthly income | ||||||||
| 1. < 5,000 | 1730 | 144.6 ± 4.0 | 1337.1 ± 30.2 | 24.3 ± 0.9 | 101.8 ± 2.6 | 30.8 ± 0.8 | 63.7 ±2.0 | 0.54 ± 0.06 |
| 2. 5,000-10,000 | 1023 | 154.5 ± 4.1 | 1429.3 ± 30.7 | 22.9 ± 0.9 | 94.7 ± 2.6 | 31.6 ± 0.8 | 67.7 ± 2.0 | 0.47 ± 0.06 |
| 3. >10,000 | 866 | 172.3 ± 5.0 | 1554.8 ± 37.6 | 6.6 ± 1.1 | 92.3 ± 3.2 | 31.1 ± 1.0 | 62.2 ± 2.5 | 0.61 ± 0.07 |
| p-valuea | <0.001 | <0.001 | <0.001 | <0.001 | 0.754 | <0.001 | <0.001 | |
| Pairwise p-valuesb | ||||||||
| 1 vs 2 | <0.001 | 0.04 | <0.001 | 0.08 | 0.24 | 0.58 | ||
| 1 vs 3 | 0.12 | <0.001 | 0.41 | 0.11 | 0.91 | 0.75 | ||
| 2 vs 3 | 0.03 | 0.04 | <0.001 | 0.86 | 0.25 | 0.31 | ||
| Religion | ||||||||
| 1. Hindu | 1790 | 154.1 ± 2.9 | 1384.7 ± 22.1 | 19.0 ± 0.7 | 100.1 ± 1.9 | 31.7 ± 0.6 | 62.3 ± 1.5 | 0.48 ± 0.04 |
| 2. Muslim | 792 | 154.6 ± 5.7 | 1680.7 ± 43.0 | 16.1 ± 1.3 | 78.0 ± 3.7 | 30.6 ± 1.1 | 74.9 ± 2.8 | 0.48 ± 0.04 |
| 3. Christian | 667 | 145.6 ± 5.8 | 1619.2 ± 43.4 | 18.1 ± 1.3 | 96.1 ± 3.7 | 31.9 ± 1.1 | 79.5 ± 2.9 | 0.58 ± 0.08 |
| 4. Other | 430 | 174.0 ± 6.3 | 1311.5 ± 46.8 | 16.0 ± 1.4 | 98.6 ± 4.0 | 29.3 ±1.2 | 54.1 ± 3.1 | 0.74 ± 0.09 |
| p-valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Pairwise p-valuesb | ||||||||
| 1 vs 2 | 0.99 | <0.001 | 0.17 | <0.001 | 0.81 | <0.001 | 1.00 | |
| 1 vs 3 | 0.53 | <0.001 | 0.93 | 0.76 | 0.99 | <0.001 | 0.74 | |
| 1 vs 4 | 0.02 | 0.51 | 0.22 | 0.99 | 0.32 | 0.09 | 0.04 | |
| 2 vs 3 | 0.52 | 0.60 | 0.51 | <0.001 | 0.72 | 0.48 | 0.75 | |
| 2 vs 4 | 0.15 | <0.001 | 0.99 | <0.001 | 0.90 | <0.001 | 0.18 | |
| 3 vs 4 | 0.01 | <0.001 | 0.73 | 0.98 | 0.47 | <0.001 | 0.56 | |
Adjusted for region (New Delhi, Trivandrum, Mumbai).
For F-test
If F-test was statistically significant, pairwise p-values were adjusted for multiple comparisons based on Tukey Method.
Discussion
Based on global estimates of spice consumption and all cancer incidence, we observed a suggestive inverse correlation. In an effort to move toward epidemiologic investigations of spices and other potentially bioactive food items used during cooking, we assessed consumption of spices, chilies, coconuts, garlic, onions, and cooking oils using a computer-based food preparer questionnaire in a pilot study in India. Our novel assessment method was successful in capturing consumption across three diverse regions and our results demonstrated a wide range and variation in the food items added during cooking, as well as some differences by education, income, and religion. Since most epidemiologic dietary assessment methods are unlikely to capture intake of these potentially preventive agents with adequate detail or accuracy, our methodology could be used in future studies to more fully capture the whole diet and enhance the study of diet and chronic diseases. More detailed epidemiologic investigation of these foods is necessary to understand if the hypotheses raised by laboratory and animal experiments as well as ecological level data presented here, are supported in human populations.
The computer-based questionnaire format was easy to administer in our population. Other computer-based dietary-assessments have been developed and found to be valid and reliable for diverse purposes and target groups (Ngo et al., 2009). The format of the food preparer questionnaire was different from other epidemiologic dietary assessment tools, as it did not query an individual’s dietary habits. However, since food costs in India constitute a large percent of a household’s total income, querying amounts of particular foods the food preparer had purchased for the household was an ideal way to elicit information on those foods that cannot be easily captured through standard dietary assessments. Furthermore, people are unlikely to recall the relative proportions of these somewhat discreet items eaten in combination with other main foods, particularly if they were not involved in the food preparation process.
Some cross-sectional and case-control studies of these food items have been conducted in India, but in general they have had small sample sizes or only assessed a limited number of food items in broad terms, such as (high, low, none) (Thimmayamma et al., 1983; Uma Pradeep et al., 1993; Beegom and Singh, 1997; Gupta and Prakash, 1997; Kumar, 1997; Mathew et al., 2000; Phukan et al., 2001; Nayak et al., 2009). In addition, many of the current studies either did not describe the full methodology for assessing intake (Uma Pradeep et al., 1993; Gupta and Prakash, 1997; Kumar, 1997; Nayak et al., 2009) or used methods, such as food records and weighing or observing all meals (Thimmayamma et al., 1983; Beegom and Singh, 1997), which would not be feasible in a large-scale study. A cross-sectional survey of 100 housewives most closely resembled our approach, as these women were likely to be food preparers (Uma Pradeep et al., 1993).
Large-scale epidemiologic investigations of these exposures are generally limited to items queried in standard diet questionnaires. Thus, they focus on onions and garlic, as a category of vegetables or to estimate flavonoid consumption (Dorant et al., 1996a; Dorant et al., 1996b; WCRF, 2007; Galeone et al., 2009), or the most commonly used cooking oils for estimating fatty acid intake (Kabagambe et al., 2005; WCRF, 2007), but the precision with which these cooking additives can be recalled or estimated may be low (Daniel et al., 2009). There are some ecologic level investigations of spices globally, including a review of recipes to estimate the types, but not amounts, of spices used in different countries (Billing and Sherman, 1998), government surveys in India that assessed overall nutritional status including spices and condiments grouped with many other food items (National Sample Survey Organization, 1996), and evaluations of U.S. Department of Agriculture spice availability data (Kaefer and Milner, 2008). Even in other populations in Asia, where there is likely to be high consumption of these spices and condiments, very little epidemiologic research on these items has been conducted (Lindeberg and Lundh, 1993; Lipoeto et al., 2004).
We observed some variation in consumption of food preparatory items by demographic characteristics. Income and education likely reflect the ability to purchase a range of food items (red onions, ghee) without regard to cost, but may also reflect a switch to a more non-traditional diet, as we noted a decrease in the consumption of garlic. A survey of Indian households, found that rural and low-income urban individuals consumed less garlic and ginger combined, than high-income urban participants (Thimmayamma et al., 1983). A cross-sectional study in Costa Rica found that higher socioeconomic status and levels of health awareness predicted use of unsaturated cooking oil over saturated cooking oil (Colon-Ramos et al., 2007). In contrast to this, we observed an increased use of ghee, a type of clarified butter very high in saturated fat, with higher income. This association could be explained by the high cost of ghee in India. Some of the differences by religion may by indicative of variation in traditional cuisine among these groups. Since little epidemiologic research on these foods exists, these associations need to be evaluated in other studies.
The IHS study was strengthened by a specially developed easy to administer computer-based questionnaire for food items added during cooking. Since the IHS was constructed to include three regions of India, we were able to examine consumption of under-studied foods among a diverse group of people with a wide range of intake. However, rather than individual intake, we only have an estimate of per capita consumption based on household data. Yet, although, like other aspects of the diet, these food items may be measured or recalled with some error, our methodology was sufficient to capture variability in consumption. A potential limitation of the IHS restricting our generalizability is the lack of a pure population-based random sample. Finally, our study did not capture information on cooking processes or the foods with which these spices are often consumed, so we cannot assess potential interactions with other foods, what happens to bioactive components during cooking, or the solubility/absorption of these food components.
Public Health Implications
Spices have been of particular interest with regard to diseases in both cell and animals studies, but there is little known about the impact of these foods at the human or epidemiologic level. Curcumin for example, a polyphenol in turmeric responsible for its yellow color, has been found to have anti-inflammatory, anti-mutagenic, anti-atherosclerotic, and anti-carcinogenic effects (Surh, 2002; Aggarwal et al., 2008; Kaefer and Milner, 2008; Krishnaswamy, 2008; Aggarwal and Sung, 2009; Epstein et al., 2010; Huang et al., 2010).Yet, research does not indicate that all spices have solely beneficial properties in relation to disease outcomes. This is true for capsaicin from chilies, which exhibits anti-mutagenic and anti-carcinogenic properties in some studies, but also pro-carcinogenic properties in others (Surh and Lee, 1996; Surh et al., 1998; Surh, 2002; Verschoyle et al., 2007).
Studies have reported many disease fighting properties of garlic and onions including anti-bacterial, anti-carcinogenic, anti-inflammatory, and anti-thrombotic activity (Shelef, 1984; Bianchini and Vainio, 2001; Sengupta et al., 2004; Kaefer and Milner, 2008; Krishnaswamy, 2008; Butt et al., 2009; Iciek et al., 2009). Although there are epidemiologic data on garlic and onions from studies assessing vegetable intake (Rahman and Lowe, 2006; WCRF, 2007; Kim and Kwon, 2009), current dietary assessment methods may not adequately capture total consumption of these foods, especially when a large amount are added during cooking.
There is evidence that polyunsaturated (alpha-linolenic and linoleic acid) and monounsaturated fatty acids, derived from nut, vegetable, and seed oils may reduce the risk of chronic diseases (Rastogi et al., 2004; Psota et al., 2006; Russo, 2009) via modulation of inflammatory (Lopez-Garcia et al., 2004), insulin (Tsitouras et al., 2008), and cholesterol pathways (Lecerf, 2009). Fatty acids from cooking oil may also be related to cancer risk (WCRF, 2007), through effects on immunity, inflammation, and cell signaling (Larsson et al., 2004). Certain tropical oils, like palm oil may also play a protective role in carcinogenesis due to a high content of carotenoids and tocotrienols (Elson, 1992); and coconut oil and whole coconuts that contain primarily medium chain fatty acids, may be healthier than other foods high in saturated fat (Pehowich et al., 2000).
Conclusions
Using a specially developed computer-based food-preparer questionnaire, we successfully identified and described regional variability in consumption of spices, chilies, coconut, garlic, onions, and cooking oils in India, with some additional consumption differences by education, income, and religion. Our software could be easily adapted for use in many study designs and populations; however, in the short-term, it may be directly applied to Indian diets in other parts of the world with a substantial South Asian population. Research on the complexity of diet and its impact on chronic disease is continually evolving and this study has shown a novel and effective way for collecting dietary exposures that have a multitude of potentially beneficial disease-related properties. With increasing spice consumption globally and the ecologic level correlation between spice consumption and cancer incidence, future nutritional epidemiology studies should consider measuring these foods to better assess the currently hypothesized mechanisms involving spices and seasonings in relation to carcinogenesis.
Acknowledgements
We are indebted to the participants in the IHS for their patience and outstanding cooperation even during the busy times of their day. The authors would like to thank all the field, laboratory, data-entry, and office staff at the three centers for their dedicated work to make this study possible. Our special thanks go to Sriram Hariharan of Atribs for developing the NINA-DISH software and Mangesh Pednekar and Binukumar for their continued support. The network support provided by the team at the Capital Technology Information Services, Inc., Raj Shah, Anil Srivastava, Chirag Shah, Sachin Parikh, and Keyur Shah, was invaluable for the success of the IHS. In addition, we would like to express our gratitude to Mary McAdams and Eric Berger of Information Management Systems, Inc. and Judy Walsh, Sujata Dixit-Joshi and Viji Narayana of Westat for data management.
Funding: This research was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
References
- Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 2008;74(13):1560–9. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30(2):85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Beegom R, Singh RB. Association of higher saturated fat intake with higher risk of hypertension in an urban population of Trivandrum in south India. Int J Cardiol. 1997;58(1):63–70. doi: 10.1016/s0167-5273(96)02842-2. [DOI] [PubMed] [Google Scholar]
- Bianchini F, Vainio H. Allium vegetables and organosulfur compounds: do they help prevent cancer? Environ Health Perspect. 2001;109(9):893–902. doi: 10.1289/ehp.01109893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing J, Sherman PW. Antimicrobial functions of spices: why some like it hot. Q Rev Biol. 1998;73(1):3–49. doi: 10.1086/420058. [DOI] [PubMed] [Google Scholar]
- Butt MS, Sultan MT, Butt MS, Iqbal J. Garlic: nature’s protection against physiological threats. Crit Rev Food Sci Nutr. 2009;49(6):538–51. doi: 10.1080/10408390802145344. [DOI] [PubMed] [Google Scholar]
- Colon-Ramos U, Kabagambe EK, Baylin A, et al. Socio-economic status and health awareness are associated with choice of cooking oil in Costa Rica. Public Health Nutr. 2007;10(11):1214–22. doi: 10.1017/S1368980007699571. [DOI] [PubMed] [Google Scholar]
- Daniel CR, McCullough ML, Patel RC, et al. Dietary intake of omega-6 and omega-3 fatty acids and risk of colorectal cancer in a prospective cohort of U.S. men and women. Cancer Epidemiol Biomarkers Prev. 2009;18(2):516–25. doi: 10.1158/1055-9965.EPI-08-0750. [DOI] [PubMed] [Google Scholar]
- Dorant E, van den Brandt PA, Goldbohm RA. A prospective cohort study on the relationship between onion and leek consumption, garlic supplement use and the risk of colorectal carcinoma in The Netherlands. Carcinogenesis. 1996a;17(3):477–84. doi: 10.1093/carcin/17.3.477. [DOI] [PubMed] [Google Scholar]
- Dorant E, van den Brandt PA, Goldbohm RA, Sturmans F. Consumption of onions and a reduced risk of stomach carcinoma. Gastroenterology. 1996b;110(1):12–20. doi: 10.1053/gast.1996.v110.pm8536847. [DOI] [PubMed] [Google Scholar]
- Elson CE. Tropical oils: nutritional and scientific issues. Crit Rev Food Sci Nutr. 1992;31(1-2):79–102. doi: 10.1080/10408399209527562. [DOI] [PubMed] [Google Scholar]
- Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010;103(11):1545–57. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] 2010 Retrieved June 17, 2010, from http://globocan.iarc.fr.
- Food and Agriculture Organization of the United Nations FAO Statistical Databases (FAOSTAT): Food Supply and Consumption Data. 2010 Retrieved June 17, 2010, from http://faostat.fao.org.
- Galeone C, Pelucchi C, Dal Maso L, et al. Allium vegetables intake and endometrial cancer risk. Public Health Nutr. 2009;12(9):1576–9. doi: 10.1017/S1368980008003820. [DOI] [PubMed] [Google Scholar]
- Ganguly C. Flavoring agents used in Indian cooking and their anticarcinogenic properties. Asian Pac J Cancer Prev. 2010;11(1):25–8. [PubMed] [Google Scholar]
- Gupta PC. Survey of sociodemographic characteristics of tobacco use among 99,598 individuals in Bombay, India using handheld computers. Tob Control. 1996;5(2):114–20. doi: 10.1136/tc.5.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Prakash H. Association of dietary ghee intake with coronary heart disease and risk factor prevalence in rural males. J Indian Med Assoc. 1997;95(3):67–9. 83. [PubMed] [Google Scholar]
- Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer. 2010;62(1):1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- Iciek M, Kwiecien I, Wlodek L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen. 2009;50(3):247–65. doi: 10.1002/em.20474. [DOI] [PubMed] [Google Scholar]
- Kabagambe EK, Baylin A, Ascherio A, Campos H. The type of oil used for cooking is associated with the risk of nonfatal acute myocardial infarction in costa rica. J Nutr. 2005;135(11):2674–9. doi: 10.1093/jn/135.11.2674. [DOI] [PubMed] [Google Scholar]
- Kaefer CM, Milner JA. The role of herbs and spices in cancer prevention. J Nutr Biochem. 2008;19(6):347–61. doi: 10.1016/j.jnutbio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A, Kapur K, Prakash W. NovoCare Interactive Nutrition Assistant - A Computer Aided Interactive Diabetes Nutrition Management Program. NNDU; 1997. [Google Scholar]
- Kim JY, Kwon O. Garlic intake and cancer risk: an analysis using the Food and Drug Administration’s evidence-based review system for the scientific evaluation of health claims. Am J Clin Nutr. 2009;89(1):257–64. doi: 10.3945/ajcn.2008.26142. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy K. Traditional Indian spices and their health significance. Asia Pac J Clin Nutr. 2008;17(Suppl 1):265–8. [PubMed] [Google Scholar]
- Kumar PD. The role of coconut and coconut oil in coronary heart disease in Kerala, south India. Trop Doct. 1997;27(4):215–7. doi: 10.1177/004947559702700409. [DOI] [PubMed] [Google Scholar]
- Lampe JW. Spicing up a vegetarian diet: chemopreventive effects of phytochemicals. Am J Clin Nutr. 2003;78(3 Suppl):579S–83S. doi: 10.1093/ajcn/78.3.579S. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79(6):935–45. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- Lecerf JM. Fatty acids and cardiovascular disease. Nutr Rev. 2009;67(5):273–83. doi: 10.1111/j.1753-4887.2009.00194.x. [DOI] [PubMed] [Google Scholar]
- Lindeberg S, Lundh B. Apparent absence of stroke and ischaemic heart disease in a traditional Melanesian island: a clinical study in Kitava. J Intern Med. 1993;233(3):269–75. doi: 10.1111/j.1365-2796.1993.tb00986.x. [DOI] [PubMed] [Google Scholar]
- Lipoeto NI, Agus Z, Oenzil F, Wahlqvist M, Wattanapenpaiboon N. Dietary intake and the risk of coronary heart disease among the coconut-consuming Minangkabau in West Sumatra, Indonesia. Asia Pac J Clin Nutr. 2004;13(4):377–84. [PubMed] [Google Scholar]
- Lopez-Garcia E, Schulze MB, Manson JE, et al. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004;134(7):1806–11. doi: 10.1093/jn/134.7.1806. [DOI] [PubMed] [Google Scholar]
- Mathew A, Gangadharan P, Varghese C, Nair MK. Diet and stomach cancer: a case-control study in South India. Eur J Cancer Prev. 2000;9(2):89–97. doi: 10.1097/00008469-200004000-00004. [DOI] [PubMed] [Google Scholar]
- National Sample Survey Organization DoS, Government of India . Nutritional Intake in India: Fifth Quinquennial Survey on Consumer Expenditure. Government of India; Kolkata, India: 1996. [Google Scholar]
- Nayak SP, Sasi MP, Sreejayan MP, Mandal S. A case-control study of roles of diet in colorectal carcinoma in a South Indian Population. Asian Pac J Cancer Prev. 2009;10(4):565–8. [PubMed] [Google Scholar]
- Ngo J, Engelen A, Molag M, et al. A review of the use of information and communication technologies for dietary assessment. Br J Nutr. 2009;101(Suppl 2):S102–12. doi: 10.1017/S0007114509990638. [DOI] [PubMed] [Google Scholar]
- Pehowich DJ, Gomes AV, Barnes JA. Fatty acid composition and possible health effects of coconut constituents. West Indian Med J. 2000;49(2):128–33. [PubMed] [Google Scholar]
- Phukan RK, Chetia CK, Ali MS, Mahanta J. Role of dietary habits in the development of esophageal cancer in Assam, the north-eastern region of India. Nutr Cancer. 2001;39(2):204–9. doi: 10.1207/S15327914nc392_7. [DOI] [PubMed] [Google Scholar]
- Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269(2):305–14. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Phromnoi K, Yadav VR, Chaturvedi MM, Aggarwal BB. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010;76(11):1044–63. doi: 10.1055/s-0030-1250111. [DOI] [PubMed] [Google Scholar]
- Psota TL, Gebauer SK, Kris-Etherton P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am J Cardiol. 2006;98(4A):3i–18i. doi: 10.1016/j.amjcard.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Rahman K, Lowe GM. Garlic and cardiovascular disease: a critical review. J Nutr. 2006;136(3 Suppl):736S–40S. doi: 10.1093/jn/136.3.736S. [DOI] [PubMed] [Google Scholar]
- Ramasastri BV. Calcium, iron and oxalate content of some condiments and spices. Plant Foods Hum Nutr. 1983;33:11–5. [Google Scholar]
- Rastogi T, Reddy KS, Vaz M, et al. Diet and risk of ischemic heart disease in India. Am J Clin Nutr. 2004;79(4):582–92. doi: 10.1093/ajcn/79.4.582. [DOI] [PubMed] [Google Scholar]
- Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009;77(6):937–46. doi: 10.1016/j.bcp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Ghosh S, Bhattacharjee S. Allium vegetables in cancer prevention: an overview. Asian Pac J Cancer Prev. 2004;5(3):237–45. [PubMed] [Google Scholar]
- Shelef L. Antimicrobial effects of spices. J Food Safety. 1984;6:29–44. [Google Scholar]
- Slimestad R, Fossen T, Vagen IM. Onions: a source of unique dietary flavonoids. J Agric Food Chem. 2007;55(25):10067–80. doi: 10.1021/jf0712503. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food Chem Toxicol. 2002;40(8):1091–7. doi: 10.1016/s0278-6915(02)00037-6. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Lee E, Lee JM. Chemoprotective properties of some pungent ingredients present in red pepper and ginger. Mutat Res. 1998;402(1-2):259–67. doi: 10.1016/s0027-5107(97)00305-9. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Lee SS. Capsaicin in hot chili pepper: carcinogen, co-carcinogen or anticarcinogen? Food Chem Toxicol. 1996;34(3):313–6. doi: 10.1016/0278-6915(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Thimmayamma BVS, Rau P, Radhaiah G. Use of spices and condiments in the dietaries of urban and rural families. Indian Journal of Nutrition and Dietetics. 1983;20:153–61. [Google Scholar]
- Tsitouras PD, Gucciardo F, Salbe AD, Heward C, Harman SM. High omega-3 fat intake improves insulin sensitivity and reduces CRP and IL6, but does not affect other endocrine axes in healthy older adults. Horm Metab Res. 2008;40(3):199–205. doi: 10.1055/s-2008-1046759. [DOI] [PubMed] [Google Scholar]
- Uma Pradeep K, Geervani P, Eggum BO. Common Indian spices: nutrient composition, consumption and contribution to dietary value. Plant Foods Hum Nutr. 1993;44(2):137–48. doi: 10.1007/BF01088378. [DOI] [PubMed] [Google Scholar]
- Verschoyle RD, Steward WP, Gescher AJ. Putative cancer chemopreventive agents of dietary origin-how safe are they? Nutr Cancer. 2007;59(2):152–62. doi: 10.1080/01635580701458186. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund (WCRF)/ American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer:A Global Perspective. AICR; Washington DC: 2007. [Google Scholar]