SUMMARY
We undertook this study to determine the role of microsomal PGE synthase-1 (mPGES-1) and mPGES-1-generated prostaglandin (PG) E2 on dendritic cell (DC) phenotype and function. Using mPGES-1 knockout (KO) mice, we generated bone marrow derived DCs and determined their eicosanoid production profile, cell surface marker expression, and cytokine production. We also assessed DC migratory and functional capacity in vivo. Compared to wild-type, mPGES-1 deficient DCs exhibited a markedly attenuated increase in PGE2 production upon LPS stimulation, and displayed preferential shunting towards PGD2 production. mPGES-1 KO DCs did not display deficiencies in maturation, migration or ability to sensitize T cells. However, mPGES-1 deficient DCs generated reduced amounts of the Th1 cytokine IL-12, which may in part be due to increased PGD2 rather than decreased PGE2. These findings provide useful information on the effects of inducible PGE2 on the innate immune system, and have important implications regarding potential consequences of pharmacologic mPGES-1 inhibition.
INTRODUCTION
Prostaglandins (PGs) are lipid molecules that mediate numerous homeostatic and pathologic processes in numerous organ systems [1]. PGE2, the most abundant and well-studied prostanoid, is generated by many cell types, both under basal conditions and in response to a diverse array of inflammatory stimuli, via immediate and delayed biosynthetic pathways [2, 3]. In response to inflammation, much higher levels of PGE2 are synthesized by the coordinated induction of COX-2 and microsomal PGE synthase-1 (mPGES-1). mPGES-1 is upregulated in a number of human diseases and animal models of inflammation [4, 5]
Research has classically focused on the inflammatory properties of PGE2, especially given the widespread use of prostaglandin-inhibiting non-steroidal anti-inflammatory drugs (NSAIDs). However, there has been increasing recognition of its immunomodulatory properties. There has been interest in the role of PGE2 as a component of the innate immune response that can significantly shape the acquired immune response [8, 9]. In particular, the effects of PGE2 on dendritic cell (DC) phenotype and function have been the subject of investigation, but the conclusions have been difficult to interpret. As the most potent antigen presenting cells, DCs provide a link between the innate and acquired arms of the immune system [10]. DCs reside in tissues in an immature state, and undergo activation and maturation in response to antigens encountered in the context of a variety of danger signals, many of which are known to induce PGE2 production. DCs then migrate via afferent lymphatics to lymphoid organs, where they interact with naïve and memory CD4+ T cells, promoting maturation towards a Th1, Th2, Th17 or Treg phenotype that influences the final nature of the immune response. This process is determined in large part by the milieu that DCs are exposed to at various points during their maturation, as well as by intrinsic characteristics of different subsets of DCs [11].
DCs both produce PGE2 and express all four receptors [12]. Research investigating the relationship between PGE2 and DCs has generated highly variable data, largely dependent on the type and species of precursor cell being studied, the specific culture conditions and stimulatory molecules used, and the timing of exposure to PGE2. In general, PGE2 is felt to have a potential role in DC migration; this has been most strongly supported by studies with EP4 knockout (KO) mice, who exhibit decreased Langerhans cell accumulation in regional lymph nodes after cutaneous sensitization [13]. Additionally, in vitro studies have shown that DCs matured/stimulated with PGE2-containing cocktails gain migratory capacity towards chemokines [14, 15]. What is less clear is the effect of PGE2 on the cytokine production profile of DCs, and their subsequent downstream effects on T cells. Early studies suggested that PGE2 shifted DC cytokine production towards a Th2 promoting profile [9]. More recently, there has been evidence for the role of PGE2 in supporting Th17 promoting DC responses [16, 17]. However, it is critical to differentiate between studies exposing DCs to exogenous PGE2, which approximate paracrine/endocrine effects, with inhibitor studies, which attempt to define the autocrine role of PGE2 upon DCs. Additionally, inhibitor studies do not specifically evaluate PGE2 alone, as the non-specific or COX-2-specific NSAIDs used in these studies also block synthesis of other PGs. Since PGE2 production is often stimulated in situations where an immune response is needed, it is not unreasonable to suspect that the high levels of inducible PGE2 exert a differential immunomodulatory effect than basal PGE2. Thus, as the primary and specific upstream synthase of inducible PGE2, determining the role of mPGES-1 in DC immunobiology is an important addition to the literature.
Studies using mPGES-1 KO mice have demonstrated a role for mPGES-1 in inflammatory arthritis, wound healing, nociception, and central fever regulation [18], but few have explored the underlying immunologic mechanisms [19]. We now utilize this model to evaluate the immunoregulatory effects associated with the genetic deletion of mPGES-1, specifically evaluating the phenotype and function of DCs.
MATERIALS AND METHODS
Mice
mPGES-1 heterozygote mice were generated as previously described on an inbred DBA/1lacJ background [20]. Breeding colonies were maintained in microisolator cages in specific pathogen free barrier facilities at the University of Michigan and the University of Kentucky. Wild-type (WT) and KO mice were generated by matings between heterozygous breeding pairs and used between 8–12 weeks of age. All experiments were approved by the relevant institutional committees for animal use at the University of Michigan, the University of Kentucky, and Mount Sinai School of Medicine.
Reagents
The following monoclonal antibodies (mAbs) and their appropriate controls (BD Pharmingen, San Diego, CA) were used for FACS analysis: CD16/32 (rat), FITC- and PE- CD11c (hamster), PE-Cy5- CD3 (rat), PE-Cy5- CD45R/B220 (rat), PE- CD80 (hamster), PE I-A/I-E (rat), PE CD86 (rat), PE CD40 (rat). Langerin staining was carried out using goat polyclonal anti-Langerin antibody (Santa Cruz), followed by PE-conjugated donkey anti-goat IgG (Jackson Immunoresearch). Recombinant murine IL-4 was purchased from RDI (Flanders, NJ); conditioned media from a GM-CSF expressing B16 cell line [21] was a kind gift from Dr. Jerold G. Woodward (Department of Microbiology, Immunology and Molecular Genetics, University of Kentucky). LPS (O55:B5), FITC and Optiprep density gradient was purchased from Sigma (St. Louis, MO); collagenase D from Roche (Indianapolis, IN); EDTA from Cambrex (Walkersville, MD); and non essential amino acids, sodium pyruvate, RPMI 1640, HBSS, fetal calf serum, L-glutamine, penicillin/streptomycin, and 2-mercaptoethanol from Gibco/Invitrogen (Carlsbad, California). Rabbit anti-human mPGES-1 polyclonal antibody (pAb), rabbit anti-mouse COX-2 pAb, rabbit anti-human mPGES-2 pAb, rabbit anti-human cPGES pAb, rabbit anti-mouse PGI synthase (PGIS) pAb, rabbit anti-mouse hematopoietic PGD synthase (H-PGDS) pAb, rabbit anti-human TX synthase (TXS) pAb, rabbit anti-mouse COX-1 pAb, PGE2 and PGD2 were purchased from Cayman Chemicals, Ann Arbor, MI; horseradish peroxidase-linked donkey anti-rabbit Ig was purchased from Amersham Biosciences, Little Chalfont, England. Recombinant mPGES-1 positive control was a kind gift from Dr. Per Jakobsson (Karolinska Institutet, Stockholm, Sweden).
DC generation and stimulation
Bone marrow-derived DCs (BMDCs) were generated in vitro as described by Lutz et al. [22] with a few modifications. Briefly, bone marrow cells from mPGES-1 deficient and WT mice were cultured in complete media plus GM-CSF conditioned media and IL-4 (10 ng/ml). On day 10, nonadherent cells were collected and utilized as immature DCs. There was no difference in DC yield between wild type and mPGES-1 deficient mice (data not shown). DCs were stimulated for 6 or 18 hours with LPS (1 μg/ml), and then cells and supernatant were collected for further analysis.
Western blotting of COXs and PG synthases
DCs were lysed in Tris-buffered saline (TBS) containing 0.1% sodium dodecyl sulfate (SDS). Murine abdominal skin was homogenized in lysis buffer (1x PBS (pH 7.4) with 5mM EDTA, 1% NP40, 0.1% SDS, 0.5% deoxycholic acid, 1 μg/ml leupeptin, 10 mcg/ml aprotinin, 1mM phenylmethylsulfonyl fluoride); lysis debris was spun down (2000g × 15 minutes at room temperature) and supernatants collected and frozen at −80°C until use. Western blotting was then performed as previously described [23].
Phenotypic analysis
5×105 cells were first incubated for 10 minutes at room temperature with anti-CD16/CD32 to block FcγII/III receptor-mediated nonspecific antibody binding. Then cells were stained with fluorochrome-conjugated mAbs or appropriate isotype matched control antibodies for 30 minutes at 4°C, washed, and fixed in 4% w/v paraformaldehyde. Analysis was performed on a FACScalibur (BD Biosciences).
Eicosanoid and cytokine measurements
Eicosanoids were measured in cell-free supernatant and from homogenized skin samples using commercial ELISA kits (Cayman Chemicals, Ann Arbor, MI) as per manufacturer’s protocol. As TXA2 and PGI2 are rapidly broken down, we measured supernatant levels of their stable metabolites (TXB2 and 6-keto-PGF1a, respectively). The detection limits for PGE2, TXB2, PGD2 and 6-keto-PGF1α are 15, 11, 1.95 and 11 pg/ml, respectively. Cytokine ELISAs were performed using OptEIA kits (TNFα; BD Pharmingen, San Diego, CA) and ELISA Ready-SET-Go! kits (IL-12p70, IL-23, and IL-10; eBioscience, San Diego, CA) per manufacturer’s protocols. Detection limits for TNFα, IL12p70, IL-23 and IL-10 are 15.6, 15, 30, and 30 pg/ml, respectively.
In vivo skin DC migration assay
Skin sensitization with FITC and DC migration were assessed as previously described [24]. Briefly, FITC dissolved in an acetone:dibutyl phthalate vehicle was applied to the shaved dorsal skin of WT and mPGES-1 deficient mice (5 per group); 18 hours later, draining lymph nodes were harvested, processed, and stained for CD11c, CD86 and Langerin.
Cutaneous PGE2 determination
Ventral abdominal skin was shaved and either vehicle or FITC solution (15 mg/ml) was applied; 18 hours later the skin was dissected out. Tissue was processed and PGE2 levels measured using an ELISA kit according to the manufacturer’s protocol (Cayman Chemicals, Ann Arbor, MI).
Contact hypersensitivity assay
Shaved abdominal skin of WT and mPGES-1 deficient mice was sensitized with 400 μl of FITC solution. Five days later, baseline bilateral ear thicknesses were measured using a micrometer, and then 20 μl of FITC solution was applied to either side of each mouse’s right ear; vehicle was applied to the left. 24 hours later ear thickness was re-measured. Ears were fixed in 10% formalin, paraffin embedded, sectioned, and H&E stained.
Statistical Analysis
The data are expressed as mean ± SEM. Student’s t-tests were used to calculate all p values.
RESULTS
Protein expression of COXs and terminal PG synthases in mPGES-1 WT and KO BMDCs
We performed Western blotting of WT and mPGES-1 deficient BMDCs, both with and without LPS stimulation (Fig. 1). WT DCs did not basally express mPGES-1, but upregulated expression in response to LPS. As expected, KO DCs did not express mPGES-1, either with or without stimulation. COX-2 was also not expressed basally, but increased to a similar degree in both WT and KO DCs after stimulation. All other synthases were present basally and did not increase expression after stimulation with LPS.
Figure 1. Protein expression of COX and terminal PG synthases in mPGES-1 WT and KO DCs.

mPGES-1 WT and KO DCs were harvested at 18 hrs post LPS stimulation, and protein expression of mPGES-1, COX-2, mPGES-2, cPGES, PGIS, H-PGDS, TXS, COX-1 and β-actin was determined by Western blotting. Representative data are shown from 4 separate experiments.
Profile of prostanoid expression in mPGES-1 deficient BMDCs
We first determined the role of mPGES-1 in BMDC PGE2 production under basal and stimulated conditions. The level of PGE2 under unstimulated conditions was similarly low, but not absent, when comparing WT and mPGES-1 KO mice (Fig. 2). LPS stimulation resulted in a significantly greater increase in PGE2 production by WT BMDCs compared with mPGES-1 KO BMDCs (P<0.05). To determine if there was compensatory shunting of AA into other eicosanoid pathways, we measured levels of PGD2 and the stable metabolites of thromboxane and prostacyclin (TXB2 and 6-keto-PGF1α,), the other major eicosanoid products of murine DCs. WT and mPGES-1 deficient BMDCs displayed similar baseline levels of TXB2 and 6-keto-PGF1α, that increased significantly upon LPS stimulation, but with no significant differences between genotypes. However, LPS stimulation resulted in significantly higher PGD2 levels in KO (P<0.01) but not WT BMDCs.
Figure 2. Eicosanoid profile of BMDCs.
BMDCs were generated by culturing bone marrow cells in complete media supplemented with GM-CSF/IL-4, and then stimulated with LPS or vehicle for 18 hours. Supernatants were analyzed by ELISA for PGE2, PGD2, 6-keto-PGF1α and TXB2. Stimulated mPGES-1 deficient DCs fail to strongly upregulate PGE2; however, they do significantly upregulate PGD2 upon stimulation with LPS. Results are the mean of 10 separate experiments. *= p<0.05, **=p<0.01 and ***=p<0.001; by student’s t-test.
Cell surface marker expression of mPGES-1 deficient BMDCs
Next, we analyzed the cell surface expression after LPS stimulation of MHC Class 2, CD40, CD80, and CD86, antigen presentation molecules which DCs upregulate upon maturation. While LPS treatment did increase all examined markers, there were no significant differences in maturation marker expression between BMDCs from WT and mPGES-1 deficient mice, both before and after LPS stimulation (Table 1).
Table 1. Cell surface marker expression of BMDCs.
Cultured BMDCs were stimulated with LPS (1 μg/ml) or vehicle for 18 hours, stained with fluorochrome-conjugated antibodies, and analyzed by flow cytometry. mPGES-1 deficient BMDCs are phenotypically similar to WT DCs. Results are presented as percent positivity (%), mean fluorescent intensity (MFI), and geometric mean of fluorescent intensity (geoMFI), and are the mean of three experiments; there were no statistically significant differences between WT and KO cells.
| Un-treated | LPS-treated | ||||
|---|---|---|---|---|---|
| Phenotypic marker | WT | KO | WT | KO | |
| CD11c | % | 82.98 | 77.92 | 85.80 | 84.04 |
| MFI | 2296.71 | 2381.21 | 1582.23 | 1590.32 | |
| geoMFI | 1364.69 | 1380.76 | 1025.19 | 1016.90 | |
| CD40 | % | 3.71 | 4.09 | 56.65 | 52.43 |
| MFI | 508.40 | 652.18 | 768.94 | 574.83 | |
| geoMFI | 256.36 | 275.12 | 423.73 | 390.83 | |
| CD80 | % | 78.43 | 79.66 | 91.07 | 89.77 |
| MFI | 907.41 | 919.86 | 3390.57 | 3226.23 | |
| geoMFI | 511.78 | 545.06 | 2122.61 | 2012.78 | |
| CD86 | % | 35.78 | 41.53 | 78.43 | 80.10 |
| MFI | 1556.90 | 1384.18 | 3503.80 | 3578.89 | |
| geoMFI | 642.36 | 610.19 | 1896.69 | 1995.19 | |
| MHC class2 | % | 49.13 | 53.94 | 70.41 | 74.20 |
| MFI | 3298.21 | 3201.17 | 5005.41 | 4830.55 | |
| geoMFI | 1197.12 | 1192.18 | 2249.21 | 2163.31 | |
Cytokine expression by mPGES-1 deficient BMDCs
DCs secrete a number of cytokines that mediate some of their effects on other immune cells. In particular, DCs secrete IL-12, which is considered to be the critical Th1 polarizing cytokine [25], and TNFα; they also secrete IL-10, a Th2 associated cytokine, and IL-23, important for Th17 responses. We measured cytokine release from WT and mPGES-1 deficient LPS-stimulated BMDCs. There was a significant reduction of IL-12 production by mPGES-1 deficient BMDCs (Fig. 3; p<0.001). TNFα and IL-23 were similar between WT and KO mice at both 6h and 18h after stimulation. IL-10 levels were 30% higher in KO at 6h, but this difference did not reach statistical significance. There was no difference between WT and KO mice at the 18h time point.
Figure 3. Cytokine production by BMDCs.
Cultured mPGES-1 DCs stimulated with LPS (1 μg/ml) for 6 and 18 hours display significantly decreased IL-12 (A) production compared to WT DCs, as measured by ELISA (*=p<0.001 by student’s t-test). There is no significant difference in the levels of IL-23 (B), TNFα (C), or IL-10 (D) comparing WT and KO BMDCs. Results are expressed as the percent difference from the corresponding stimulated wild type production (designated as 100%) and are the mean of 3–6 experiments.
Effect of exogenous PGE2 and PGD2 on IL-12 production in LPS-stimulated DCs
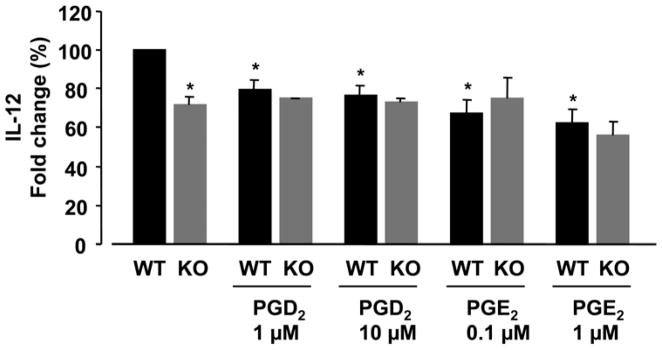
We wished to determine whether the observed diminishment in IL-12 production in the absence of mPGES-1 was a function of the altered eicosanoid profile displayed by the KO DCs, as well as further explore the difference between autocrine vs. exogenously supplied PGE2. To this end, WT and KO BMDCs were stimulated with LPS in the presence or absence of PGE2 or PGD2, and IL-12 production was determined (Fig. 4). We discovered that adding PGE2 to KO DCs did not increase IL-12 levels. However, adding PGD2 to WT DCs resulted in decreased IL-12 production (p<0.001), similar to levels exhibited by mPGES-1 deficient BMDCs. Also, adding PGE2 to WT DCs resulted in decreased IL-12 production (p<0.001). These data suggest that increased PGD2, not decreased PGE2, may contribute to the diminished IL-12 production in stimulated mPGES-1 deficient DCs. Additionally, inducible and exogenous PGE2 appear to exert differential effects on IL-12 production from WT DCs.
Figure 4. Effect of exogenous PGE2 and PGD2 on IL-12 production in LPS-stimulated DCs.
DCs from mPGES-1 WT and KO were cultured with or without PGE2 or PGD2 in the presence of LPS (1 μg/ml) for 18 hours. The levels of IL-12 in supernatant were measured by ELISA. Addition of both exogenous PGD2 and PGE2 to WT DCs results in diminished IL-12 production. Addition of exogenous PGE2 to KO DCs does not alter IL-12 production to resemble that of WT. Results are expressed as the percent difference from the corresponding stimulated WT production (designated as 100%) and are the mean of 4 experiments. *=p<0.001 vs. WT stimulated with LPS alone (student’s t-test). Raw values for the stimulated WT after LPS stimulation are 922.5 pg/ml ± 265.1
DC migration in mPGES-1 deficient mice
To evaluate the paracrine effects resulting from the absence of inducible PGE2 upon the migration of DCs in vivo, we employed a FITC sensitization model; upon application of a FITC solution to the skin of mice, cutaneous DCs phagocytose the FITC and migrate to regional lymph nodes. First, we verified that this model results in upregulated mPGES-1 and inducible PGE2 production at the site of sensitization, to ensure that WT cutaneous DCs are exposed to a higher PGE2 environment than KO mice (Fig. 5). Skin from mPGES-1 KO mice, as expected, displayed absent mPGES-1 protein expression and low PGE2 levels, both with and without FITC sensitization. In contrast, WT skin displayed mPGES-1 expression both basally and after sensitization; cutaneous PGE2 levels were higher in unsensitized (p<0.05) and sensitized (p<0.002) WT skin compared with KO. Next, migration of cutaneous DC was determined by flow cytometry. The frequency of CD11c+ FITC+ (migratory) DCs in regional draining lymph nodes was not significantly different between WT and mPGES-1 KO mice (Fig. 6B). To specifically look at Langerhans cells (a specialized population of migratory DCs), CD11c + cells were gated for Langerin. As demonstrated in Fig. 6C, this subset of migratory DCs was also not differentially present in WT and KO lymph nodes. Of note, the density of Langerhans was similar prior to FITC staining between the two genotypes; epicutaneous application of FITC reduced the density of Langerhans cells in the epidermis by about 20% at 18 h for each genotype (data not shown). Lastly, we determined if the maturation status of the migratory DCs in the lymph node was altered in the absence of mPGES-1. Levels of CD86 were not different between WT and mPGES-1 KO DCs in the lymph node (Fig. 6D), suggesting that maturation of migratory DCs in vivo is not dependent upon inducible PGE2.
Figure 5. Effect of FITC sensitization upon cutaneous mPGES-1 and PGE2 expression.
Shaved WT skin displayed baseline mPGES-1 expression, and basal PGE2 levels higher than KO. Application of a FITC solution for 18 hours results in upregulation of mPGES-1 protein and PGE2 production in WT, but not mPGES-1 deficient, murine skin. Panel 5A shows a representative Western blot of mPGES-1 expression in WT and KO skin, with and without FITC sensitization, as well as the ratio of mPGES-1 to β-actin expression, normalized to WT. +control is a recombinant mPGES-1 protein used as a positive control. Panel 5B shows the mean cutaneous PGE2 production from 3 mice per treatment group; levels are normalized to total protein expression. *=p<0.05; **=p<0.002 by student’s t-test.
Figure 6. In vivo DC migration.
After epicutaneous application of a FITC solution for 18 hours, draining lymph nodes were harvested, stained and analyzed by flow cytometry. Panel 6A shows the percent of CD11c+FITC+ (migratory) DCs in representative histograms of WT and mPGES-1 KO lymph nodes; 6B shows the number of FITC+ DCs in the lymph nodes of 5 mice per genotype. 6C shows the proportion of Langerhans cells in representative histograms of each genotype. 6D shows the expression of CD86 on FITC+ migratory DCs. There were no differences between WT and mPGES-1 deficient mice in the frequency of migratory DCs found in lymph nodes (both total DCs and Langerhans cells specifically) or their maturation status (as determined by CD86 expression).
Contact hypersensitivity in mPGES-1 KO mice
Finally, we determined whether mPGES-1 plays a role in the immunomodulatory properties of DCs by performing a contact hypersensitivity assay. Contact hypersensitivity requires functional DCs that not only uptake antigen and migrate correctly, but interact with T cells that can subsequently generate rapid inflammatory responses upon re-exposure to the antigen. We sensitized and subsequently challenged WT and KO murine skin with FITC applied to the ears. We saw no difference in the degree of contact hypersensitivity elicited between the two genotypes, as ascertained by measuring changes in ear swelling pre-and post FITC challenge (Fig. 7a). Histologically, there were no differences in the amount or type of inflammatory infiltrate by H&E staining (Fig. 7b).
Figure 7. Contact hypersensitivity assay.
Mice were sensitized to FITC solution and subsequently challenged with FITC or vehicle application to their ears. 24 hours later the mice were sacrificed and the change in ear swelling measured. mPGES-1 KO mice display the same contact hypersensitivity response as WT mice. There were no significant differences clinically or histologically between WT and mPGES-1 KO mice, with either vehicle or FITC application. Results are the mean of 7–8 mice per group, and are reported as the change in ear thickness pre-and post FITC or vehicle challenge, ± SEM.
DISCUSSION
In this study we report several novel findings. First, mPGES-1 deficient BMDCs do not markedly increase PGE2 production in response to LPS stimulation, and display shunting of prostanoid synthesis towards PGD2. Second, mPGES-1 deficient BMDCs produce less IL-12 than WT DCs upon stimulation; this appears not to be a function of decreased PGE2, but due to increased PGD2 levels. Finally, the absence of mPGES-1 does not impair migration of cutaneous DCs or their ability to sensitize T cells in vivo.
We have demonstrated that absence of mPGES-1 does not change the expression of other PG synthases, including the constitutive PGE2 synthases cPGES and mPGES-2. Also, mPGES-1 deficiency does not alter COX-2 expression; this implies that neither mPGES-1 nor its associated PGE2 regulate their upstream synthase in a feedback loop. Thus, the synthetic machinery for other PGs remains intact. We have also shown that mPGES-1 is required for the markedly increased production of PGE2 in response to LPS by BMDCs. This correlates with similar findings in other tissues and cells such as macrophages and embryo fibroblasts [23, 26, 27]. There is a much smaller increase in PGE2 production upon LPS stimulation of mPGES-1-deficient DCs. Potentially, this may arise through the actions of other PGES isozymes, which can couple with COX-2 in certain situations [28].
Interestingly, we saw evidence for increased production of PGD2 by BMDCs from mPGES-1 KO mice; this appears to be due to shunting of PG precursors down the PGD2 synthetic pathway in the absence of mPGES-1. Shunting of prostanoid synthesis towards PGD2 could potentially be one mechanism of immunomodulation in the absence of mPGES-1. Dendritic cells express the DP1 receptor [29], thereby allowing for both autocrine and paracrine effects. Studies have demonstrated a potential role for PGD2 in DC cytokine production and migration [30, 31]. However, the autocrine effects of PGD2 are largely unexplored.
Our results for eicosanoid shunting in BMDCs are unlike what has been reported in other knockout cells. These other studies differed from each other, however, in terms of which prostanoids were found to be preferentially upregulated. Our data support the growing body of evidence that there exists considerable variability and cell specificity to prostanoid diversion in the absence of mPGES-1 [32]. Additionally, we saw no evidence for diminished basal PGE2 production in the absence of mPGES-1 in unstimulated BMDCs, as has been previously described [23, 27]. This may be the result of mPGES-1 functionally coupling to upstream synthases in a cell-specific manner, thus differentially contributing to basal PGE2 production.
Exogenous PGE2 is a potent maturation agent for human monocyte-derived DCs [33] and is used in cocktails for enhancing the efficacy of DC tumor vaccines [34]. However, the contribution of endogenous PGE2 to DC maturation is less well understood. Our data suggest that the absence of inducible PGE2 alone does not affect BMDC maturation in an autocrine fashion. These findings do not eliminate a role for paracrine effects of mPGES-1-induced PGE2 on BMDC maturation. Higher PGE2 levels generated by surrounding cells could accelerate or increase maturation beyond what is seen in isolated cultured BMDCs exposed only to their own endogenous PGE2, and thus systemic mPGES-1 deficiency might result in a “less” mature phenotype than possible in WT BMDCs.
Exogenous PGE2 has been shown to inhibit production of inflammatory chemokines and cytokines by DC [35, 36]. More specifically, in vitro exposure of immature DCs to exogenous PGE2 decreased production of IL-12, the classical Th1 promoting cytokine [37, 38]. This suggests that the presence of PGE2 at the initial peripheral site of damage (where the immature DC sentinels resides) could potentially mature DCs that promote a downstream Th2 response, and that inhibition of PGE2 might possibly skew towards a subsequent Th1 response (via increased IL-12 production). However, a recent study demonstrated that in vitro differentiation of DCs in the presence of exogenous PGE2 alters the IL-12/IL-23 balance, with resultant promotion of Th17 differentiation [39]. Additionally, prostaglandin inhibitor studies have generated differing data. Some studies have found that inhibition of endogenous prostanoids using selective and non-selective COX-2 inhibitors did not alter cytokine production [41, 42]. Other groups have found otherwise [17, 36, 43]. Some of these varying results may in part be due to technical differences with variable culture conditions and stimulatory molecules, making direct comparison between studies difficult. Murine genetic background plays a role in the amount and effect of PGE2 production and subsequent Th1/Th2 effects [40] and may cause differences between various mouse models studied.
Our data revealed a significant decrease in IL-12 production after LPS stimulation in mPGES-1 deficient BMDCs compared with WT, a finding apparently in contradiction to published data showing downregulation of IL-12 in the presence of PGE2. As exogenous PGE2 has been shown to downregulate IL-12 production via a cAMP dependent mechanism [44], we measured cAMP levels basally and after stimulation in WT and KO BMDCs. We found no differences between the genotypes (data not shown), indicating that the machinery for response to PGE2 is intact in the absence of mPGES-1. Additionally, we found that adding exogenous PGE2 does cause our WT BMDCs to decrease IL-12 production. This illustrates one potentially important difference between the effect of exogenous PGE2 vs. the effect of autocrine PGE2 and/or its synthase.
However, the diminished IL-12 production by mPGES-1 deficient BMDCs does not appear to be the result of diminished autocrine inducible PGE2 levels, as adding exogenous PGE2 to KO DCs did not increase IL-12 and restore the WT phenotype. In fact, the altered IL-12 profile may at least in part be due to increased PGD2 produced by KO cells, as adding PGD2 to WT DCs downregulated IL-12. This is consistent with other studies that have shown exogenous PGD2 to decrease IL-12 production by murine BMDCs [30, 45].
Studies have suggested a primary role for PGE2 in controlling DC migration. Exogenous PGE2 enhances the function of CCR7, the key migratory chemokine receptor for DCs [14, 46], and stimulates migration [15, 47]. However, these in vitro studies demonstrated that PGE2 is able to confer a migratory phenotype to DCs; they did not preclude the possibility of other mediators having similar capabilities in the absence of PGE2. More compellingly, mice deficient in the EP4 receptor for PGE2 demonstrated impaired migration of sensitized cutaneous DCs to regional lymph nodes [13]. However, certain subsets of human DCs have been shown to not require PGE2 in order to obtain a migratory capacity [15, 48].
Our results demonstrate that, while WT cutaneous DCs are clearly exposed to higher levels of PGE2 than KO mice in a well-established FITC sensitization model [49], no impairment of DC migration was demonstrable in mPGES-1 KO mice, despite the absence of inducible PGE2 in the inflammatory milieu and likely in the DCs themselves. Potentially, basal PGE2 exposure and production is adequate to maintain DC migration in the presence of other inflammatory signals, possibly via the EP4 receptor, which has been shown to be important utilizing the same FITC sensitization model we used [13]. Alternately, there may be redundancy with regards to which mediators confer migratory capacity; in the absence of inducible PGE2, other compounds may be sufficient. Differences in inbred mouse strains used may also account for some of the discrepancy between our data and the EP4 knockout findings. Inducible PGE2 also does not appear to affect the maturation status of migratory DCs, as we saw no difference in CD86 expression between WT and KO DCs. This addresses the issue of the paracrine effects of PGE2 on DC maturation; WT cutaneous DCs are exposed to much higher PGE2 levels than mPGES-1 deficient DCs, and yet do not exhibit higher maturation marker expression upon arrival to the lymph nodes. Additionally, migratory DCs from the mPGES-1 deficient animals were capable of engaging T cells in the lymph nodes appropriately and eliciting functional downstream immune responses, as we saw equivalent contact hypersensitivity responses between the two genotypes. This assay also gives us indirect insight into the effect of inducible PGE2 on T cells, as KO mice mounted appropriate T cell-mediated delayed type hypersensitivity.
Much of the literature regarding the effects of PGE2 on DC phenotype and function, especially using human derived cells, has been conducted in order to understand and refine DC vaccine use in immunotherapy. To that end, many studies have been performed ex vivo with exogenous PGE2 administration. Advantages of using the mPGES-1 knockout model include better study of in vivo physiologic and pathologic effects, as well as evaluation of the autocrine effects of inducible PGE2 without eliminating alternate PGs. Our study finds that while mPGES-1 is essential for increased PGE2 production in BMDCs, there is a minimal role for mPGES-1 generated PGE2 on DC maturation and migration.
In addition to expanding our understanding of the specific role of mPGES-1 and mPGES-1 induced PGE2 on DC biology, these data provide information on the potential consequences of mPGES-1 inhibition in a therapeutic setting. This is of particular importance since mPGES-1 is an attractive pharmacologic target, given its specificity for inducible, inflammatory PGE2. However, as has been demonstrated by the adverse effects associated with COX-2-specific NSAIDs [50], inducible prostaglandins also have a number of physiologic functions, and it is critical to understand the full spectrum of biologic effects associated with absence or inhibition of mPGES-1 in order to determine the ultimate clinical usefulness of this enzyme as a therapeutic target.
Acknowledgments
We thank Dr. Mariana Kaplan and Dr. Mike Denny for helpful discussions, and Min Qian, Hemal Mehta, Dr. Martin R Ward and Dr. Lihua Yang for technical assistance.
This work was supported in part by an American College of Rheumatology Research and Education Foundation Physician-Scientist Development Award (S.U.M.), a National Institutes of Health grants AI49653 (G.J.R.), an Established Investigator Award from the American Heart Association (G.J.R.) and a National Institutes of Health grants AR049010 (L.J.C.).
Footnotes
CONFLICT OF INTEREST STATEMENT
Dr. Crofford has had grant funding from Pfizer. She is not a consultant and not a participant in any Speaker’s Bureau and does not own stock.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Miller SB. Prostaglandins in health and disease: an overview. Semin Arthritis Rheum. 2006;36:37–49. doi: 10.1016/j.semarthrit.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006;119:229–40. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Hara S, Kamei D, Sasaki Y, Tanemoto A, Nakatani Y, Murakami M. Prostaglandin E synthases: Understanding their pathophysiological roles through mouse genetic models. Biochimie. 2010;92:651–9. doi: 10.1016/j.biochi.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Westman M, Korotkova M, af Klint E, et al. Expression of microsomal prostaglandin E synthase 1 in rheumatoid arthritis synovium. Arthritis Rheum. 2004;50:1774–80. doi: 10.1002/art.20286. [DOI] [PubMed] [Google Scholar]
- 5.Subbaramaiah K, Yoshimatsu K, Scherl E, et al. Microsomal prostaglandin E synthase-1 is overexpressed in inflammatory bowel disease. Evidence for involvement of the transcription factor Egr-1. J Biol Chem. 2004;279:12647–58. doi: 10.1074/jbc.M312972200. [DOI] [PubMed] [Google Scholar]
- 6.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 7.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Rocca B, FitzGerald GA. Cyclooxygenases and prostaglandins: shaping up the immune response. Int Immunopharmacol. 2002;2:603–30. doi: 10.1016/s1567-5769(01)00204-1. [DOI] [PubMed] [Google Scholar]
- 9.Harizi H, Gualde N. The impact of eicosanoids on the crosstalk between innate and adaptive immunity: the key roles of dendritic cells. Tissue Antigens. 2005;65:507–14. doi: 10.1111/j.1399-0039.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 11.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol. 2005;26:289–307. doi: 10.1007/s00281-004-0167-1. [DOI] [PubMed] [Google Scholar]
- 12.Harizi H, Grosset C, Gualde N. Prostaglandin E2 modulates dendritic cell function via EP2 and EP4 receptor subtypes. J Leukoc Biol. 2003;73:756–63. doi: 10.1189/jlb.1002483. [DOI] [PubMed] [Google Scholar]
- 13.Kabashima K, Sakata D, Nagamachi M, Miyachi Y, Inaba K, Narumiya S. Prostaglandin E2-EP4 signaling initiates skin immune responses by promoting migration and maturation of Langerhans cells. Nat Med. 2003;9:744–9. doi: 10.1038/nm872. [DOI] [PubMed] [Google Scholar]
- 14.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–61. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 15.Luft T, Jefford M, Luetjens P, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–72. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 16.Sheibanie AF, Khayrullina T, Safadi FF, Ganea D. Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum. 2007;56:2608–19. doi: 10.1002/art.22794. [DOI] [PubMed] [Google Scholar]
- 17.Yao C, Sakata D, Esaki Y, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–40. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 18.Sampey AV, Monrad S, Crofford LJ. Microsomal prostaglandin E synthase-1: the inducible synthase for prostaglandin E2. Arthritis Res Ther. 2005;7:114–7. doi: 10.1186/ar1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima F, Kapoor M, Yang L, et al. Defective generation of a humoral immune response is associated with a reduced incidence and severity of collagen-induced arthritis in microsomal prostaglandin E synthase-1 null mice. J Immunol. 2008;180:8361–8. doi: 10.4049/jimmunol.180.12.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trebino CE, Stock JL, Gibbons CP, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A. 2003;100:9044–9. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storozynsky E, Woodward JG, Frelinger JG, Lord EM. Interleukin-3 and granulocyte-macrophage colony-stimulating factor enhance the generation and function of dendritic cells. Immunology. 1999;97:138–49. doi: 10.1046/j.1365-2567.1999.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 23.Kapoor M, Kojima F, Qian M, Yang L, Crofford LJ. Shunting of prostanoid biosynthesis in microsomal prostaglandin E synthase-1 null embryo fibroblasts: regulatory effects on inducible nitric oxide synthase expression and nitrite synthesis. FASEB J. 2006;20:2387–9. doi: 10.1096/fj.06-6366fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–68. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 25.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 26.Trebino CE, Eskra JD, Wachtmann TS, Perez JR, Carty TJ, Audoly LP. Redirection of eicosanoid metabolism in mPGES-1-deficient macrophages. J Biol Chem. 2005;280:16579–85. doi: 10.1074/jbc.M412075200. [DOI] [PubMed] [Google Scholar]
- 27.Boulet L, Ouellet M, Bateman KP, et al. Deletion of microsomal prostaglandin E2 (PGE2) synthase-1 reduces inducible and basal PGE2 production and alters the gastric prostanoid profile. J Biol Chem. 2004;279:23229–37. doi: 10.1074/jbc.M400443200. [DOI] [PubMed] [Google Scholar]
- 28.Murakami M, Nakashima K, Kamei D, et al. Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J Biol Chem. 2003;278:37937–47. doi: 10.1074/jbc.M305108200. [DOI] [PubMed] [Google Scholar]
- 29.Herve M, Angeli V, Pinzar E, et al. Pivotal roles of the parasite PGD2 synthase and of the host D prostanoid receptor 1 in schistosome immune evasion. Eur J Immunol. 2003;33:2764–72. doi: 10.1002/eji.200324143. [DOI] [PubMed] [Google Scholar]
- 30.Faveeuw C, Gosset P, Bureau F, et al. Prostaglandin D2 inhibits the production of interleukin-12 in murine dendritic cells through multiple signaling pathways. Eur J Immunol. 2003;33:889–98. doi: 10.1002/eji.200323330. [DOI] [PubMed] [Google Scholar]
- 31.Angeli V, Faveeuw C, Roye O, et al. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J Exp Med. 2001;193:1135–47. doi: 10.1084/jem.193.10.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scholich K, Geisslinger G. Is mPGES-1 a promising target for pain therapy? Trends Pharmacol Sci. 2006;27:399–401. doi: 10.1016/j.tips.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–42. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 34.Lee AW, Truong T, Bickham K, et al. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20(Suppl 4):A8–A22. doi: 10.1016/s0264-410x(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 35.Jing H, Vassiliou E, Ganea D. Prostaglandin E2 inhibits production of the inflammatory chemokines CCL3 and CCL4 in dendritic cells. J Leukoc Biol. 2003;74:868–79. doi: 10.1189/jlb.0303116. [DOI] [PubMed] [Google Scholar]
- 36.Harizi H, Norbert G. Inhibition of IL-6, TNF-alpha, and cyclooxygenase-2 protein expression by prostaglandin E2-induced IL-10 in bone marrow-derived dendritic cells. Cell Immunol. 2004;228:99–109. doi: 10.1016/j.cellimm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 38.von Bergwelt-Baildon MS, Popov A, Saric T, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–37. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- 39.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–35. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuroda E, Yamashita U. Mechanisms of enhanced macrophage-mediated prostaglandin E2 production and its suppressive role in Th1 activation in Th2-dominant BALB/c mice. J Immunol. 2003;170:757–64. doi: 10.4049/jimmunol.170.2.757. [DOI] [PubMed] [Google Scholar]
- 41.Jozefowski S, Bobek M, Marcinkiewicz J. Exogenous but not endogenous prostanoids regulate cytokine secretion from murine bone marrow dendritic cells: EP2, DP, and IP but not EP1, EP3, and FP prostanoid receptors are involved. Int Immunopharmacol. 2003;3:865–78. doi: 10.1016/S1567-5769(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 42.Teloni R, Giannoni F, Rossi P, Nisini R, Gagliardi MC. Interleukin-4 inhibits cyclo-oxygenase-2 expression and prostaglandin E production by human mature dendritic cells. Immunology. 2007;120:83–9. doi: 10.1111/j.1365-2567.2006.02482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whittaker DS, Bahjat KS, Moldawer LL, Clare-Salzler MJ. Autoregulation of human monocyte-derived dendritic cell maturation and IL-12 production by cyclooxygenase-2-mediated prostanoid production. J Immunol. 2000;165:4298–304. doi: 10.4049/jimmunol.165.8.4298. [DOI] [PubMed] [Google Scholar]
- 44.Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–8. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theiner G, Gessner A, Lutz MB. The mast cell mediator PGD2 suppresses IL-12 release by dendritic cells leading to Th2 polarized immune responses in vivo. Immunobiology. 2006;211:463–72. doi: 10.1016/j.imbio.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 46.Scandella E, Men Y, Legler DF, et al. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood. 2004;103:1595–601. doi: 10.1182/blood-2003-05-1643. [DOI] [PubMed] [Google Scholar]
- 47.Legler DF, Krause P, Scandella E, Singer E, Groettrup M. Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J Immunol. 2006;176:966–73. doi: 10.4049/jimmunol.176.2.966. [DOI] [PubMed] [Google Scholar]
- 48.Jefford M, Schnurr M, Toy T, et al. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: differential regulation of function by specific classes of physiologic stimuli. Blood. 2003;102:1753–63. doi: 10.1182/blood-2002-12-3854. [DOI] [PubMed] [Google Scholar]
- 49.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–28. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 50.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]