Abstract
Background
Lymphatic vascular endothelial hyaluronan receptor-1 (LYVE-1) is a selective marker for lymphatic endothelium and a homolog of CD44, the hyaluronan (HA) receptor. HA in the extracellular matrix plays roles in tissue remodeling, development, and homeostasis, and as an HA receptor, LYVE-1 mediated HA metabolism might regulate these events. Currently, little is known about the lymphatic character within the human placenta. This study therefore determined LYVE-1 and other lymphatic markers in human placentas.
Methods and Results
Placentas and villous tissue were fixed and immunostained for human LYVE-1 and CD44 and examined by RT-PCR. LYVE-1 was expressed at both protein and mRNA levels in trophoblast cells (TC) and in villous core endothelium (VCE). Predominant protein expression for LYVE-1 was observed in syncytiotrophoblast cells (TCs) of preterm placentas. Neither mRNA or protein for CD44 was expressed. Other blood and lymphatic-lineage molecules (VEGF-A, -C, and -D, Flt-1, KDR, Flt-4, and Prox-1) were examined by RT-PCR. VEGF-A, VEGF-D, and Flt-1 mRNA were observed in TCs and VCEs, while mRNA for VEGF-C, KDR, and Flt-4 was mainly observed in VCEs. Prox-1 was found at the mRNA, but not protein level in TCs and VCEs. Our findings indicate (1) the importance of LYVE-1, but not CD44, in regulation of HA metabolism in the maternal—-fetal interface and fetal circulation, and (2) possible dual blood and lymphatic phenotypic characteristics in fetal endothelium. These results provide new insights into HA metabolism and lymphatic-lineage molecule expression in the human placenta.
INTRODUCTION
Hyaluronan (hyaluronic acid, HA) is a major extracellular matrix (ECM) proteoglycan. It plays an important role in maintaining tissue integrity and regulating cell migration and differentiation during embryogenesis. During several stages of development, changes in matrix HA levels could induce condensation of mesenchymal cells, and lead to the onset of chondrogenesis and myogenesis.1 HA also facilitates cell migration during processes of inflammation and wound healing. A recent study by Banerji et al.2 found that the lymphatic vascular endothelial HA receptor (LYVE-1) is a major receptor for HA on lymphatic endothelial cells (LEC). By comparing blood endothelial cells to isolated LEC, they demonstrated that LYVE-1 is only expressed in lymphatic endothelial cells. Therefore, LYVE-1 is now considered as a selective marker for the lymphatic endothelium.2
The placenta is a heterogeneous organ, which consists of trophoblasts and villous core tissue that is composed of stromal tissue and fetal blood vessels. In adult tissue, the blood circulatory system needs the lymphatic system to collect and transfer macromolecules, lymphocytes, and protein-rich fluid back to the blood circulation. The lymphatic vascular system provides an exclusive microenvironment not only in maintaining homeostasis but also playing critical roles in immune responses and tumor metastasis. Developmentally, blood and lymphatic endothelial cells share many structural and functional markers, including Von Willebrand factor/factor VIII, heparin sulfate proteoglycan, collagen type IV, PECAM-1 (CD31), and other characteristics.3 However, nothing is known about the lymphatic character in the human placenta, or how fetal vessel endothelia resemble or differ from blood vessel endothelia.
Since LYVE-1 is a specific marker for lymphatic endothelial cells, a study of LYVE-1 in the human placenta may provide new insights on the lymphatic vascular character of the placenta, a highly specialized organ of pregnancy and fetal development. For this purpose, we collected human placentas delivered by preterm and term pregnant women. Placental villous sections were immunohistochemical stained with LYVE-1 and CD44 antibodies. CD44 is a transmembrane receptor for the extracellular matrix glycosaminoglycan HA. Gene (mRNA) expression for LYVE-1 and CD44 was examined in isolated placental trophoblast cells and placental villous vessel endothelial cells. To further study the lymphatic-lineage molecules in the human placenta, mRNA expression for VEGF-A, -C, and -D, Flt-1, KDR, Flt-4, and Prox-1 were examined by RT-PCR in isolated trophoblast cells and villous core vessel endothelial cells. We found that both syncytiotrophoblast cells and villous core vessel endothelial cells express LYVE-1. These results provide evidence of LYVE-1 in regulation of HA metabolism in trophoblasts and the possibility of lymphatic-lineage specific molecules in the placental vasculature.
MATERIALS AND METHODS
Placental sample collection
Placentas were obtained after delivery from term and preterm deliveries and processed immediately. Term placentas were delivered by normal pregnant women between 38 and 40 weeks of gestation. Preterm placentas were delivered by women with gestational age between 33 and 34 weeks. A total of eight placentas were used in this study. None of these pregnancies were complicated with preterm rupture of membranes. Institutional approvals were obtained to conduct this study.
Immunohistochemical staining
Fresh villous tissues were fixed with Zamboni buffer containing 2% formaldehyde, 0.1% M phosphate buffer and 15% saturated picric acid (pH 7.0) and embedded with Immuno-Bed solution (Polysciences Inc. Warrington, PA). Villous tissue sections of 5 µm thickness were applied to polylysine-coated slides. Endogenous peroxide was blocked by 0.3% (v/v) hydrogen peroxide. The sections were then incubated with polyclonal antisera against human LYVE-1 and Prox-1 with dilution of 1:100 in phosphate-buffered saline (PBS). Rabbit polyclonal antisera against LYVE-1 and Prox-1 were generated by Proteintech Group, Inc. (Chicago, IL). Rabbits were immunized with KLH conjugated peptide. The peptide sequence for LYVE-1 is: C-PNEESKKTDKNPEESK. The peptide sequence for Prox-1 is C-DSTDSENDEDGNLSE. Antiserum was tested by ELISAs to assess reactivity with LYVE-1 peptide or Prox-1 peptide, respectively. Immunohistochemical ABC staining system was used according to manufacturer’s instructions (Santa Cruz, San Diego, CA). Tissue sections were also stained with antibodies against CD44 (an HA receptor) (Santa Cruz), CD31 (a marker for endothelial cells) (Dako, Fort Collins, CO), and cytokeritin 7 (a marker for trophoblasts) (Santa Cruz). Stained slides were counterstained with hematoxylin/eosin. Tissue sections stained with a secondary antibody only served as negative control. Stained slides were reviewed by a microscope, Olympus IX71 (Olympus, Japan). Images were captured by a digital camera with computer software Picturefram (Optronics, Goleta, CA).
Isolation of placental trophoblast cells and villous vessel endothelial cells
Trophoblast cells were isolated by Dispase digestion and Percoll gradient centrifugation as previously described.4 Endothelial cells were isolated as described previously 5–7 with modifications. Percoll gradients were made from 45% to 15% Percoll in 5% steps of 5.0 mL each except 9.0 mL in the 15% step. Endothelial cell containing section was recovered in the density of the gradient between 30% and 35%. The isolated endothelial cell section was washed twice in endothelial cell growth medium (BioWittiker, Walkersville, MD) and further purified by CD31 magnetic beads. Preparation of magnetic beads conjugated to anti-CD31 was performed as previously described.8 Briefly, anti-human CD31 mAb (M0823, Dako) was mixed with paramagnetic beads coated with sheep anti-mouse IgG (1181, Amac, Westbrook, Maine) at a concentration of 5 µg IgG per mg of beads in 200 µL PBS containing bovine serum albumin (BSA, 0.2 mg/mL). The mixture of beads conjugated to anti-CD31 with endothelial section from Percoll gradient was incubated on ice for 20 min, and beads were separated from the supernatant by a magnet. The magnetic pellet was washed before plating cell pellets into the culture plate. We usually repeated the CD31 magnetic bead selection procedure more than once in order to obtain pure endothelial cells during subculture if necessary. Cultured endothelial cells were examined by LDL uptake. Endothelial expressions of Factor VIII/vWF and VE-cadherin were also used as markers of endothelial cells.
Messenger RNA expression
Total RNA was isolated from trophoblast cells and endothelial cells by Tri Reagent (Molecular Research Center, Inc., Cincinnati, OH). mRNA expression for LYVE-1 was determined by reverse transcript polymerase chain reaction (RT-PCR). Two µg of total RNA from trophoblast cells and villous fetal vessel endothelial cells were used for the reverse transcription by using SuperScript™ first-strand synthesis system purchased from Invitrogen Life Technologies (Carlsbad, CA). The RT products were subjected to PCR reaction using primers specific for the LYVE-1 and CD44. Messenger RNA expressions for VEGF-A, VEGF-C, VEGF-D, Flt-1, KDR, Flt-4, and Prox-1 were also determined by RT-PCR. VEGF PCR primer set, which contains primers for VEGF189, VEGD165, VEGF121, Flt-1, KDR, and GAPDH, was purchased from Maxim Biotech Inc. (San Francisco, CA). PCR Primers for LYVE-1, CD44, VEGF-C, VEGF-D, and Prox-1 were synthesized by Ransom Hill Bioscience, Inc (Ramona, CA). Gene names, primer sequences, and accession number are presented in Table 1. The PCR products were analyzed on 2.0% agarose gel.
TABLE 1.
Gene Name, Primer Sequence, RT-PCR Product, and Accession Number
| Gene | Primer sequence | PCR products | Accession # |
|---|---|---|---|
| LYVE-1 | 5′-ATG GCC AGG TGC TTC AGC CTG GTG TTG-3′ | 471bp | NM_006691 |
| 5′-GTG TTG CAG TTT GAG TGT TGA ATA TGG-3′ | |||
| CD44 | 5′-ATG ACA CAT ATT GCT TCA ATG CTT-3′ | 446bp | M59040 |
| 5′-CCT TAT AGG ACC AGA GGT TGT GTT-3′ | |||
| VEGF-C | 5′-CAG CTA AGG AAA GGA GGC TGG CAA CAT AAC-3′ | 469bp | X96216 |
| 5′-TTC GCT GCC TGA CAC TGT GGT AGT GTT GCT-3′ | |||
| FIGF (VEGF-D) | 5′-CAG GCT GAG GCT CAA AAG TT-3′ | 383BP | D89630 |
| 5′-GCT GTT GGC AAG CAC TTA CA-3′ | |||
| Flt-4 | 5′-TAC GTG TTC GTG AGA GAC TTT GAG-3′ | 420bp | NM_002020 |
| 5′-GTA GTC CCA GTC AAA GGT GAC A-3′ | |||
| Prox-1 | 5′-CCG ACG CAA GTT GAC GGC TCT CGA CTA-3′ | 461bp | AF100755 |
| 5′-TTG CCT TAA GCA TTA CCA GGT AAT CAT-3 |
RESULTS
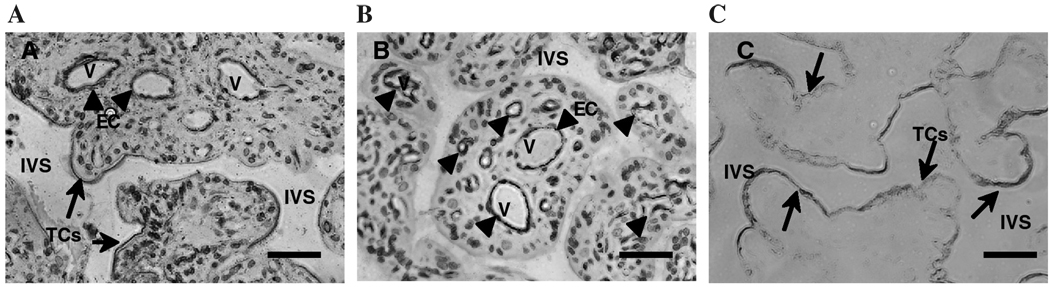
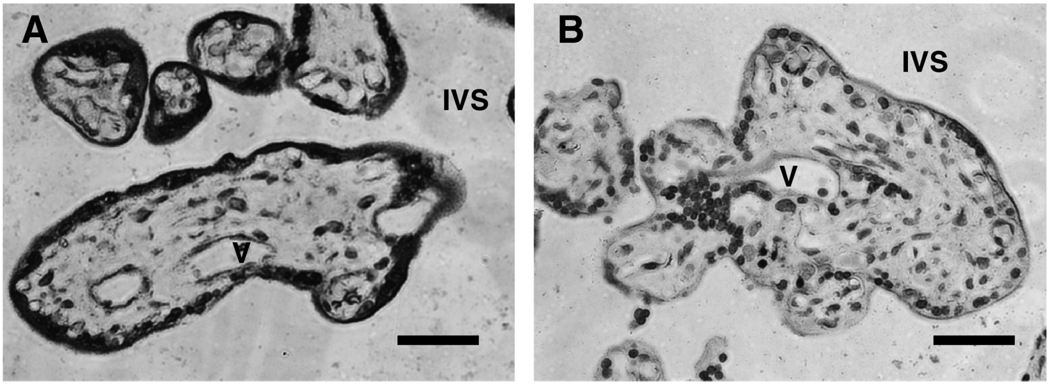
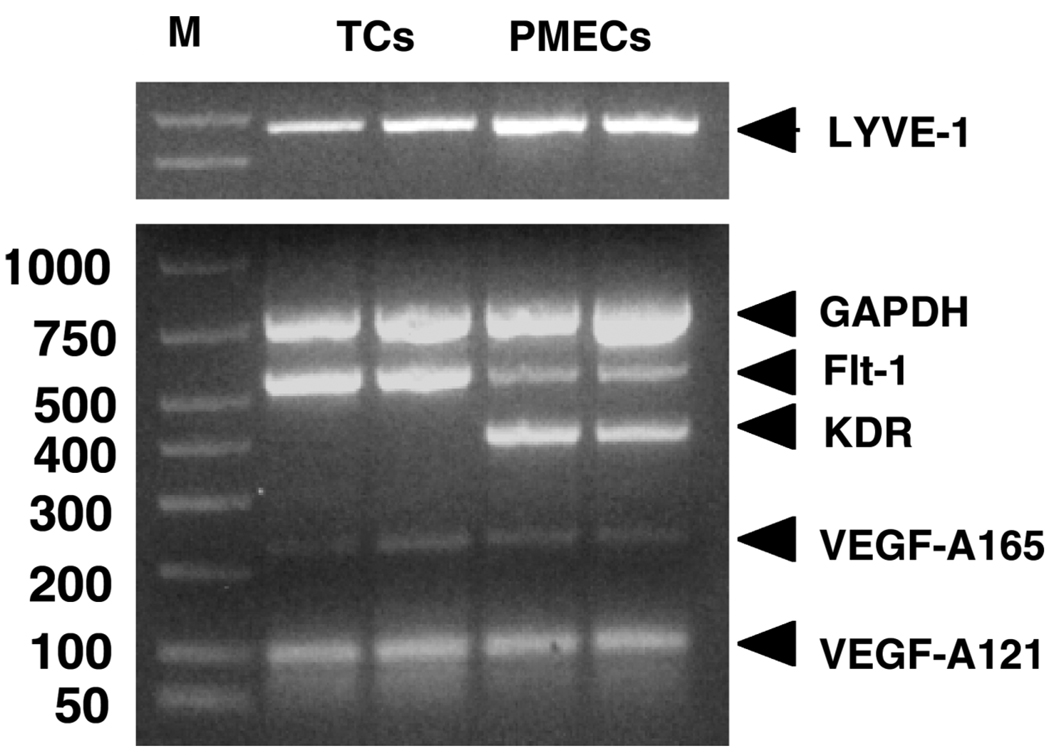
Figure 1 shows representative immunohistochemical staining results of placental villous tissue sections with anti-LYVE-1, anti-CD31, and anti-cytokeritin. We found that positive staining of LYVE-1 was not only in the villous core fetal endothelium but also in the syncytiotrophoblast layer of the placenta villous tissue (Fig. 1A). Positive expression of CD31 is only observed in villous core vessel endothelium (Fig. 1B) and cytokeritin expression is only shown in syncytiotrophoblast layer (Fig. 1C). We further found that LYVE-1 staining was much enhanced in the syncytiotrophoblast layer of preterm placental villous tissue (Fig. 2A) compared to that in the term placental villous tissue (Fig. 2B). Gene expression for LYVE-1 was determined by RT-PCR in isolated placental trophoblasts and villous core vessel endothelial cells. As shown in Figure 3, both trophoblast cells and villous endothelial cells express LYVE-1. However, CD44 expression was not detectable either at mRNA or at protein levels (data not shown).
FIG. 1.
Representative LYVE-1, CD31, and cytokeritin expression in placental villous tissue. (A) villous stained with anti-LYVE-1; (B) villous stained with anti-CD31; and (C) villous stained with anti-cytokeritin 7. Arrowheads indicate villous fetal vessel endothelial layer (EC) and arrows indicate syncytiotrophoblast layer (TCs). A and B, not C, were counterstained with hematoxylin/eosin. IVS: intervillous space; V: vessel. Bar = 10 µm.
FIG. 2.
Representative LYVE-1 expression in placental villous tissue from preterm and term placentas. (A) villous tissue from a 33-weeks preterm delivery; (B) villous tissue from a 40-weeks term delivery. LYVE-1 staining was much more intensive in the syncytiotrophoblast layer of the preterm placenta than that of the term placenta. V: vessel; IVS: intervillous space. Bar = 10 µm.
FIG. 3.
mRNA expressions of LYVE-1, VEGF-A, Flt-1, and KDR in trophoblast cells and placental villous microvascular endothelial cells. TCs: trophoblast cells; PMECs: placental villous microvascular endothelial cells.
To further examine the lymphatic-lineage molecules in the human placenta, mRNA expressions for VEGF-A, -C, -D, and VEGF receptors, Flt-1, KDR, Flt-4, were also determined. VEGF-A (as examined by its isoform VEGF165, VEGF121) and Flt-1 are positively expressed in both isolated trophoblast cells and villous vessel endothelial cells, but KDR is only expressed in villous vessel endothelial cells (Fig. 3).
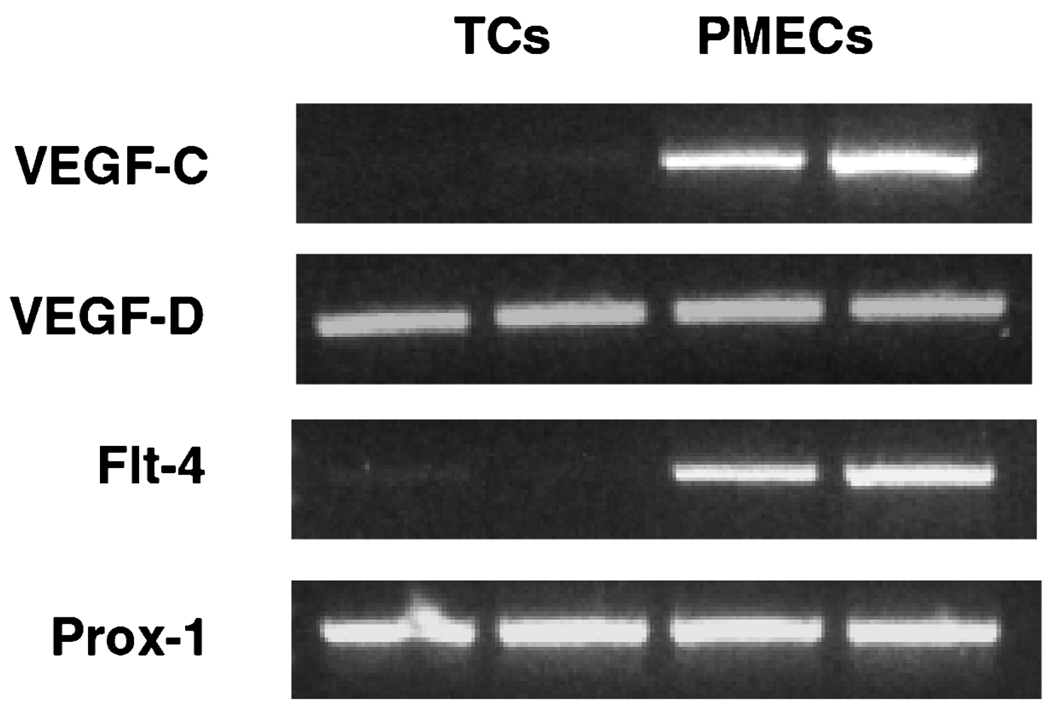
Figure 4 shows gene expressions for VEGF-C, VEGF-D, Flt-4, and Prox-1. VEGF-C and Flt-4 are mainly expressed in villous vessel endothelial cells, whereas both trophoblast cells and villous vessel endothelial cells express VEGF-D and Prox-1. Prox-1 protein expression was not detectable by immunohistochemical staining of placental villous tissue sections (data not shown).
FIG. 4.
mRNA expressions of VEGF-C, VEGF-D, Flt-4, and Prox-1 in trophoblast cells and placental villous microvascular endothelial cells. TCs: trophoblast cells; PMECs: placental villous microvascular endothelial cells.
DISCUSSION
HA is a large mucopolysaccharide copolymer of N-acetyl D-glucosamine and D-glucuronic acid that is abundant in the extracellular matrix of tissues throughout the body. HA has been implicated in numbers of biological processes including cell adhesion, migration, and proliferation.9 To date, two HA receptors, CD44 and LYVE-1, and several HA binding proteins, such as extracellular binding protein ‘stabilin-1’ and intracellular HA-binding proteins (IHABPs), have been identified. CD44 is considered to be a major receptor for HA and it is widely expressed on epithelial, mesenchymal, and lymphoid cells.10 Like CD44, LYVE-1 is a transmembrane glycoprotein, which binds to both immobilized and to soluble HA. Stabilin-1 is a member of the link protein family like CD44 and LYVE-1, but its expression and function are largely uncharacterized. In the present study, we, for the first time, found that LYVE-1 is expressed in both trophoblast cells and villous fetal vessel endothelial cells. We further observed enhanced LYVE-1 expression in the syncytiotrophoblast layer of the preterm placentas compared to that in term placentas. However, CD44 expression was not detectable at either protein or mRNA levels by immunohistochemical staining or by RT-PCR, respectively.
Although both CD44 and LYVE-1 are receptors for HA, the 59% difference of deduced amino acid sequences between the two HA receptors2 may explain the cell type- or organ-specific differences in HA metabolism in HA-CD44 or HA- LYVE-1 signaling activation pathways. Our results implicate that LYVE-1, but not CD44, is a functional HA receptor in the placenta, and thus that LYVE-1, but not CD44 regulates HA metabolism at both the maternal—fetal interface and the fetal vascular system during pregnancy. To date, except for positive expression of LYVE-1 in the lymphatic vascular endothelium, gene expression for LYVE-1 was also detected positive in the lung and liver tissues.11 The lung is an organ specialized for gaseous exchange while the liver is a manufacturing and processing organ, generating and releasing digestive enzymes, filtering toxic agents, and producing numerous growth factors and hormones. Interestingly, the placenta functions like both the lung and the liver. In the placenta, trophoblast cells and microvillous fetal endothelial cells are the two layers of cell components directly related to oxygen transfer from the maternal circulation to the fetal compartment. Placental trophoblasts are also important for generating growth factors and hormones required for placental and fetal development during pregnancy. The presence of LYVE-1 in the trophoblasts and villous fetal endothelial cells indicate that LYVE-1 may perform special functions in the placenta beyond those described in lymphatic endothelial cells. Although the precise function and signaling regulation in HA- LYVE-1 pathway in the placenta are not known, the enhanced LYVE-1 protein expression in the syncytiotrophoblast layer of the preterm placenta indicates a unique physiological significance of LYVE-1 during placental and fetal development.
Based on the prior study by Banerji et al.2 that showed exclusive expression of LYVE-1 on the luminal face of the lymph vessel wall, with LYVE-1 being absent from blood vessels, LYVE-1 has been considered to be a lymphatic-specific receptor for HA in lymphatic vascular endothelial cells. Therefore, detection of LYVE-1 has been employed to identify lymphangiogenesis such as in inflammatory diseases and tumor metastasis.12,13 Although villous trophoblast cells are not of endothelial or lymphatic origin, they come into direct contact with maternal blood in the intervillous space. Trophoblast cells are the structural and biochemical barriers between the maternal and the fetal compartments during pregnancy. They exert effector and/or modulator functions including bi-directional exchanges in macromolecules, growth factors, and hormones between the maternal and the fetal compartment. Evidence has shown that trophoblast cells express many endothelial lineage molecules. For example, VEGF, placental growth factor (PlGF), VEGFR-1 (Flt-1), and VEGFR-3 (Flt-4) are expressed in invasive cytotrophoblasts in early gestation.14,15 The expression of LYVE-1 observed in our study supports the idea that LYVE-1 may play a role in invasive phenotypic cells such as trophoblasts in the placenta.
To further study the lymphatic-lineage molecules in the human placenta, mRNA expressions for VEGF-A, -C, and -D, Flt-1, KDR, Flt-4, and Prox-1 were examined by RT-PCR in isolated trophoblasts and villous core vessel endothelial cells. Positive mRNA expression for VEGF-A, VEGF-D, and Flt-1 were observed in both isolated trophoblasts and endothelial cells, while mRNA expressions for VEGF-C and Flt-4 were mainly expressed in villous core vessel endothelial cells. Consistent with previous reports, KDR was expressed only in endothelial cells. Prox-1 is another marker for lymphatic endothelial cells. Surprisingly, Prox-1 was expressed at the mRNA, but not at the protein level in both trophoblast cells and endothelial cells. Based on the data of positive gene expressions for lymphatic markers of VEGF-C, Flt-1, LYVE-1, and Prox-1 in villous core vessel endothelium, the possibility of lymphatic-linage phenotype exists in the fetal vessel endothelial cells of the human placenta. However, our current data could not discriminate whether a separate true lymphatic vascular system is present in the placenta.
In summary, we, for the first time, demonstrated that placental syncytiotrophoblasts and villous fetal vessel endothelial cells express HA receptor, LYVE-1. The expression and localization of LYVE-1, but not CD44, in the placenta point out a possible unique function of LYVE-1 in HA metabolism and its physiological significance in the maternal—fetal interface and in the fetal circulation during fetal development. Further studies are needed to find out if trophoblasts synthesize HA, and how HA receptor-LYVE-1 is regulated, and to delineate the HA- LYVE-1 signal pathways and metabolism at the maternal-fetal interface and their importance during pregnancy.
Acknowledgments
This study was funded in part by grants from National Institute of Health, National Institute of Child Health Development (NICHD), HD36822 and National Heart Blood Lung Institute (NHBLI), HL 65997.
REFERENCES
- 1.Knudson CB, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis and disease. FASEB J. 1993;7:1233–1241. [PubMed] [Google Scholar]
- 2.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erhard H, Rietveld FJ, Brocker EB, de Waal RM, Ruiter DJ. Phenotype of normal cutaneous microvasculature. Immunoelectron microscopic observations with emphasis on the differences between blood vessels and lymphatics. J Invest Dermatol. 1996;106:135–140. doi: 10.1111/1523-1747.ep12329708. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Walsh SW. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in preeclampsia. Placenta. 2001;22:206–212. doi: 10.1053/plac.2000.0608. [DOI] [PubMed] [Google Scholar]
- 5.Marks RM, Czerniecki M, Penny R. Human dermal microvascular endothelial cells: An improved method for tissue culture and a description of some singular properties in culture. In Vitro Cell Devel Biol. 1985;21:627–635. doi: 10.1007/BF02623295. [DOI] [PubMed] [Google Scholar]
- 6.Leach L, Bhasin Y, Clark P, Firth JA. Isolation of endothelial cells from human term placental villi using immunomagnetic beads. Placenta. 1994;15:355–364. doi: 10.1016/0143-4004(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 7.Schutz M, Friedl P. Isolation and cultivation of endothelial cells derived from human placenta. Eur J Cell Biol. 1996;71:395–401. [PubMed] [Google Scholar]
- 8.Springhorn JP. Isolation of human capillary endothelial cells. In: Spector DL, Goldman RD, Leinwand LA, editors. Cells: A Laboratory Manual. Vol 1: Culture and Biochemical Analysis of Cells. New York: Cold Spring Harbor Laboratory Press; 1998. pp. 6.1–6.10. [Google Scholar]
- 9.Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol. 2000;12:581–586. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- 10.Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- 11.Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–19430. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Edwards JR, Espinosa O, Banerji S, Jackson DG, Athanasou NA. Expression of a lymphatic endothelial cell marker in benign and malignant vascular tumors. Hum Pathol. 2004;35:857–861. doi: 10.1016/j.humpath.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Goydos JS, Gorski DH. Vascular endothelial growth factor C mRNA expression correlates with stage of progression in patients with melanoma. Clin Cancer Res. 2003;9:5962–5967. [PubMed] [Google Scholar]
- 14.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunk C, Ahmed A. Expression of VEGF-C and activation of its receptors VEGFR-2 and VEGFR-3 in trophoblast. Histol Histopathol. 2001;16:359–372. doi: 10.14670/HH-16.359. [DOI] [PubMed] [Google Scholar]