Abstract
Introduction
Adenoviral gene therapy has been widely applied for cancer therapy, however, transient gene expression as result of humoral immuno-neutralization response to adenovirus limits its effect. The purpose of this study is to determine whether DOTAP:cholesterol liposome could shield adenovirus from neutralizing antibody and permit the use of multiple cycles of intravenous L-A-5-RIP-TK with GCV to enhance its effect.
Methods
The effect of multiple cycles of systemic L-A-5-RIP-TK/GCV therapy was evaluated in grouped PANC-1 SCID mice treated with different number of cycles. Humoral immune response to A-5-RIP-TK or L-A-5-RIP-TK was assessed using C57/B6J mice challenged with adenovirus or liposome adenovirus complex.
Results
The minimal residual tumor burden (3.2±0.6mm3) and longest survival time (153.0±5.6d) were obtained in the mice receiving 4 cycles and 3 cycles of therapy, respectively. Toxicity to islet cells associated with RIP-TK/GCV therapy was observed after four cycles. DOTAP:chol-encapsulated adenovectors were able to protect adenovectors from the neutralization of high titer of anti-adenoviral antibodies induced by itself.
Conclusion
Multiple treatment cycles of L-A-5-RIP-TK/GCV effectively ablate human PANC-1 cells in SCID mice, however, the mice become diabetic and have significant mortality after the 4th cycle. Liposome-encapsulated adenovirus is functionally resistant to the neutralizing effects of anti-adenoviral antibodies, suggesting feasibility of multiple cycles of therapy. Liposome-encapsulation of the adenovirus is a promising strategy for repeated delivery of systemic adenoviral gene therapy.
Keywords: Adenovirus, gene therapy, rat insulin promoter, thymidine kinase, multiple treatments
Introduction
Pancreatic cancer (PC) is one of the most aggressive malignancies, with poor survival despite the significant advances in understanding, diagnosis, and access to conventional therapy. The only treatment option with curative potential is surgical intervention by means of a pancreatic resection. However, due to the high percentage of patients with locally advanced metastatic disease at diagnosis, only 10-20% of patients are eligible for curative surgery(1). The median survival of these most optimally treated patients is only 17 months(2). Thus, it is clear that novel therapeutics for patients with pancreatic cancer are urgently needed.
Adenoviral gene therapy has for many years been regarded as a potential new treatment modality. High efficiency of transduction of a large range of cell types in all stages of cell division is its major advantage(3). In addition, their relative inability to insert into the host genome limits the risk of insertional mutagenesis, but as a drawback also limits their therapeutic life-time(4). The limitations include low levels of CAR(5, 6) (the receptor on the pancreatic cancer cells to which the natural tropism of adenovirus-5 is directed), harm to healthy tissue when systemic adenoviral vectors are used, and the innate immune response towards the vector which can lead to neutralization of the adenoviral vectors and to a severe systemic inflammatory response (7, 8).
Many attempts have been made to overcome above limitations such as using pancreatic cancer-specific promoter-directed suicide gene therapy to exert a specific cytotoxic effect on pancreatic cancer(9-12). Using rat insulin promoter (RIP) directed viral thymidine kinase (TK) gene with ganciclovir (GCV) (RIP-TK/GCV), we have obtained significant and specific therapeutic effect on human pancreatic cancer in vivo(13). We have shown that overexpression of the transcription factor, pancreaticoduodenal homeobox-1 (PDX-1) in pancreas cancer cells specifically activates the RIP promoter, thus leading to expression of TK. In turn, expression of TK leads to susceptibility of the pancreas cancer cell to cytotoxic GCV. The delivery system is essential to success of this gene therapy. Adenoviral-5-RIP-TK vector was developed using serotype 5 adenovirus, which effectively delivered RIP-TK to human pancreatic cancer cells in SCID mice using intravenous injection. However, theoretically, the adenoviral vector could only be used once due to the host's immune response to adenovirus, which would be another limitation to obtain an effective treatment in immunocompetent animals. Neutralizing antibodies present in circulation serum after the initial exposure to adenovirus limit further cycles of adenovirus therapy. One approach to solve this problem is the use of liposome-encapsulated adenovirus to protect the adenovirus from neutralization circulating antibodies. In addition, liposomes also facilitate adenovirus binding to the cell surface, particularly on CAR-deficient cells to increase the transduction efficiency(14-17).
In the past 10 years, multiple cycles of gene therapy have been tried in order to resolve the important issue regarding transient transgene expression. Repeated administration of adenovectors by direct intratumoral injection has been successfully applied in basic and clinical studies(18-25); however, the adenovirus is readily neutralized by circulating antibodies(26, 27). Repeated cycles of systemically delivered adenoviral vector have been tried in immunocompetent mice and shown to cause resistance to the adenovirus via high titer of immune neutralizing antibody(28). In the present study, we performed a study using multiple cycles using systemically-delivered, liposome-coated A-5-RIP-TK/GCV to test its efficacy against PDX-1-expressing human pancreatic cancer cells in SCID mice. The humoral immune response was also evaluated in immune competent mice as well. The study provides strong evidence to support the hypothesis that multiple cycles with liposome-coated A-5-RIP-TK/GCV are effective against PDX-1-expressing human pancreatic cancer cells in mice.
Materials and methods
Cell Lines, Adenovectors and Antibodies
Human pancreatic cancer cell line PANC-1 was purchased from the American Type Culture Collection (ATCC, Bethesda, MD), and was maintained in DMEM medium (Invitrogen, MD) supplemented with 100,000 units/L of penicillin, 100,000 ug/L of streptomycin and 10% fetal bovine serum. A-5-RIP-TK construct preparation was described as before(13) A-5-CMV-LacZ was produced by Dr. Davis. Rabbit anti-HSV-TK antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Cy3 conjugated anti-rabbit IgG antibodies were purchased from Sigma (St. Louis, MO).
Preparation of adenovirus–liposome complexes
Liposome–virus complexes were prepared fresh at room temperature. Formulated liposomes (DOTAP or DOTAP:chol) (20 mM) were diluted to a 4 mM final concentration in a 300μl final volume with 5% dextrose in water (D5W). Adenovirus was diluted from 5×1012 vp in 300μl and mixed with equal volume of 4 mM DOTAP to give a final concentration of 103 vp/cell for in vitro. 5×108 vp/mouse was used in vivo. Infections were performed in OPTI-MEM (invitrogen) at 37°C. Cells were washed 6 hours post infection with 4 ml of PBS and fresh DEME medium supplemented with 10% FCS.
Animals and gene delivery
SCID mice were housed in a BL-4 facility and cared for under the guidelines in The Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources, the Commission on Life Science, the National Research Council, and the Animal Research Committee of Baylor College of Medicine (BCM). Male ICR/SCID mice at age 8 to 10 weeks (wks) were inoculated with 0.5× 105 PANC-1 cells intraperiteoneal to develop intraperitoneal tumor within 2 wks. This model of metastatic pancreas cancer has been developed in used in our previous studies(13). These mice were then randomized to 5 groups to receive: (1) 1 cycle of A-5-RIP-TK/GCV, (2) 1 cycle of A-5-RIP-TK/GCV+1 cycle of liposome-coated A-5-RIP-TK/GCV (L-A-5-RIP-TK), (3) 1 cycle of A-5-RIP-TK/GCV+2 cycles of L-A-5-RIP-TK/GCV, (4) 1 cycle of A-5-RIP-TK/GCV+3 cycles of L-A-RIP-TK/GCV and (5) 4 cycles of empty vector and GCV (control). Each group contained 30 mice. Each cycle lasted 3 wks: A-5-RIP-TK or L-A-5-RIP-TK were injected via the tail vein at 108 viral particles/mouse, followed by 2 weeks of GCV (40 mg/kg body weight twice daily) and 1 wk of rest with no therapy.
Tumor evaluation and survival analysis
Necropsy and tumor evaluation were performed at 7, 14, 21 and 120 d after treatment. At least five mice were sacrificed each time point. Tissues were saved for further pathological immunohistochemical analyses. Tumor volume was evaluated at each time point. Peritoneal tumors were macroscopically and microscopically evaluated and the larger (A) and smaller (B) diameters measured and recorded. Tumor volume (V; a rotational ellipsoid) was calculated according to the formula: V (mm3)= A(mm) ×B2(mm)2/2. Mice were classified according to presence or absence of tumor. Mouse survival was measured from initial treatment to date of death or sacrifice
Immunohistochemical staining
At the time of necropsy, pancreata and tumors were removed and fixed in 4% paraformaldehyde at 4°C for 4h. After process, tissue blocks were embedded in paraffin and tissue sections were prepared. H and E staining was performed. For immunostaining, sections were deparaffinized in xylene and hydrated gradually through graded alcohol. Slides were then placed in a humidified chamber, overlaid with diluted antibodies against anti-HSV-TK antibody and incubated overnight at 4°C. Dilution factors were 100. After washing with PBS, slides were incubated with Cy3-conjugated rabbit antibody for PP for 1h at RT. Slides were then washed with PBS and mounted with cover slides.
Detection of apoptosis in tumor xenografts and islet cells of mice
Apoptosis in tumor and pancreatic specimens were determined with TUNEL assay (FragEL DNA Fragmentation Detection Kit, Colorimetric-TdT Enzyme; Calbiochem, La Jolla, CA) according to the manufacturer's protocol and expressed as the ratio of apoptotic cancer cells to the total number of endothelial cells in 10 fields at ×100 magnification. To evaluate the effect of L-A-5-RIP-TK/GCV on the endocrine pancreas, at least ten islets per specimen were evaluated.
Insulin and glucose measurements
At the time of sacrifice, 50μl whole blood samples were collected and spun to separate the serum. Serum samples were stored at -20°C until completion of experiments. Glucose levels and insulin levels were measured as previously reported(29).
Neutralizing antibody titers measurements
The serum was collected from pre-immune adenovirus-injected SCID or C57/B6J mice for their ability to inhibit adenovirus infection. Dilutions of each serum were incubated with reporter gene A-5-CMV-LacZ and incubated them for 1 hour at 37°C. Each adenovirus serum mixture was then added to PANC-1 cells (2–4 104 cells per well at 24 well plates) and incubated them at 37° for 24 hours. LacZ gene expression was analyzed by X-gal staining, which determined the titer by the highest dilution at which the serum inhibited > 70% of infectivity compared to the control well without serum.
X-gal staining
Twenty-four hours after reporter gene transfection, cells were washed twice with PBS and fixed in 0.25% glutaradehyde solution for 15 min at 37°C. Cells were then washed three times with PBS as before and staining solution (1M MgCl2, 30 mM potassium ferricyanide, 30 mM potassium ferrocyanide, and 2% X-gal solution) was added to each well. The staining reaction was carried out 6 to 24 h at 37°C for color development. Microscopic observation was performed and photography was carried out via digital camera (Diagnostic Instruments Inc., Sterling Heights, MI). Stained blue cells and unstained cells were counted under microscope one by one vision area. The positive cell rate was calculated using following formula: blue cell number/total cell number.
Detection of gene expression in liver after injection of A-5-CMV-LacZ or L-A-5-CMV-LacZ adenovirus
8-10-wk-old C57BL/6J mice from The Jackson Laboratory were divided into 2 groups, 30 mice for each group to receive 1) A-5-CMV-LacZ once2) L-A-5-CMV-LacZ at dose of 5×108 vp. On day 21 after first injection, 5 mice received A-5-CMV-LacZ and 5 received L-A-5-CMV-LacZ again in each group. The expression in liver was evaluated at 7, 21 and 30d after first injection and at 7d after second delivery. X-gal staining for liver was performed as previously described(11). Stained the liver tissues were processed and embedded in paraffin. The sections were cut at 10μm for each and counterstained with eosin. LacZ gene expression was reported as ratio of blue staining cells to the total number of liver cells in 10 fields at ×100 magnification.
Statistical analysis
The unpaired Student's t-test was used for statistical analyses of tumor volume, glucose, and insulin levels, with P< 0.05 indicating significance. The χ2 test was used for rate comparison. Rank-log was used for mice survival comparison. Kaplan-Meier in SPSS 15.0 for Windows was used to plot survival curves.
Results
Thymidine kinase gene expression in tumor xenografts in SCID mice
To determine whether A-5-RIP-TK or L-A-5-RIP-TK were expressed in PANC-1 tumor cells in SCID mice with multiple systemically delivered cycles, TK protein expression was monitored in tumor cells following each delivery by immunohistochemistry. As shown in the Fig 1A, there was strong TK expression (71.8%) in the tumor cells at 7d after first gene delivery. Expression was then reduced to 40.2% on day 14, and 8.7% on day 21. Re-expression of TK was observed after second gene delivery as shown in figure 1, (65.5% on day 7, 38.4% on day 14, and 11.2% on day 21). Similarly, the third L-A-5-RIP-TK administration resulted in the TK expression of 68.9% on day 7, 43.6% on day 14, and 7.8% on day 21. There was no significant difference of gene expression among three cycles of gene delivery at same time point, indicating systemic delivery of both A-5-RIP-TK and L-A-5-RIP-TK results in effective, but transient transgene expression in PANC-1 cells in SCID mice.
FIG. 1.
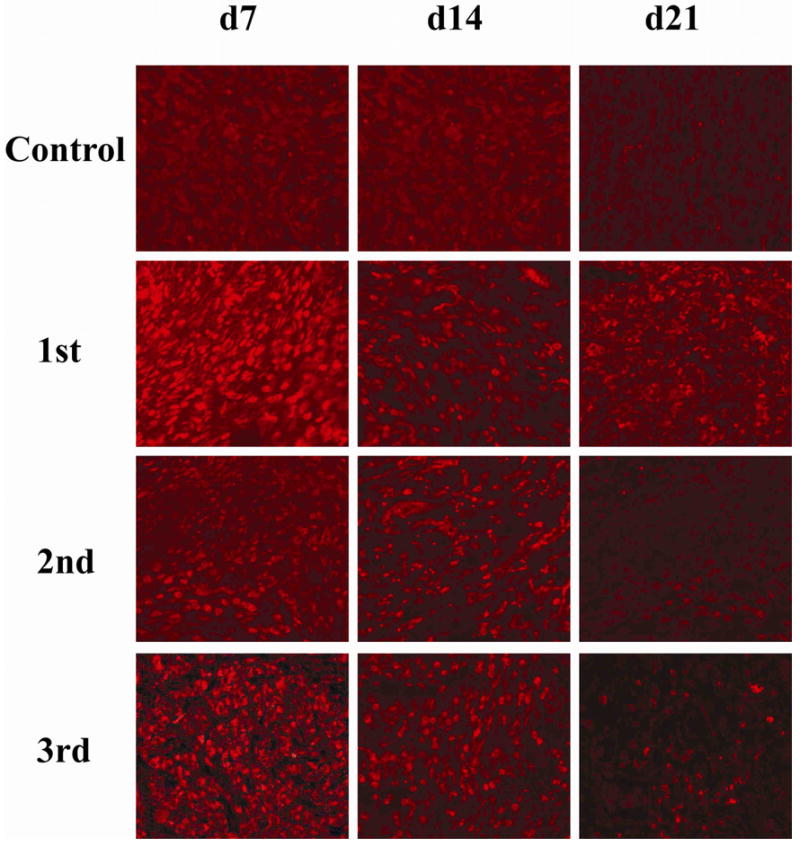
Adenoviral type 5 RIP-TK expression in PANC-1 tumors.
A-5-RIP-TK was intravenously administered to SCID mice 2 mo after inoculation with PANC-1 human pancreatic cancer cells. L-A-5-RIP-TK was repeated administrated on day 22, 43 after initial delivery, three gene deliveries in total. Mice were sacrificed on day 7, 14, 21d after each A or L-A-5-RIP-lacZ administration, respectively. The tumor tissues were processed and sectioned as usual. Immunostaining was performed using anti-HSV-TK antibody. Red staining cells indicate strong expression of HSV-TK in tumor cells. The figure showed the TK gene expression profile in control group (top row), first gene delivery (next to top row), second delivery (third row from top) and third delivery (bottom row) (x200)
Inhibitory effect of L-A-5-RIP-TK on xenograft tumor growth in SCID mice is associated with the number of cycles
To evaluate the effect of multiple cycles, two, three and four cycles of therapy were compared to mice treated with one cycle or GCV control. The tumor burden was assessed at the time of necropsy four months after completion of therapy. Tumor growth was maximally suppressed by four cycles of L-A-RIP-TK/GCV with 67.0% of mice free of tumor, followed by three cycles, two cycles, and one cycle of showing 60.0%, 54.5% and 40.0% of mice free of tumor, respectively(Fig 2A). Among them, both four and three cycles of therapy had statistically significant increase in rate of mice free of tumor compared to one cycle of therapy, however there was no significant difference between four and three cycles, and between two and one cycles of therapy. Residual tumor volume was also evaluated. Minimal residual tumor was observed in the group of mice receiving 4 cycles of therapy with mean tumor volume of 3.2±0.6mm3, followed by those with three, two, and one cycle(s) of RIP-TK/GCV therapy, with a mean tumor volume of 4.0±1.2, 8.9±3.9, 19.3±2.5, respectively (Fig 2B). Tumor volume in empty vector GCV control group was 618.9±326.5 mm3 (Fig 2B). Similar to the observation above, there was no significant difference in tumor volume between the mice with four and three cycles, and between one and two cycles, but both three and four cycles of therapy resulted in significant reduced the tumor volume as compared to that of mice receiving one and two cycles of therapy. Taken together, these results demonstrate a correlation between the effect of inhibition of tumor growth and the number of cycles of therapy.
FIG. 2.
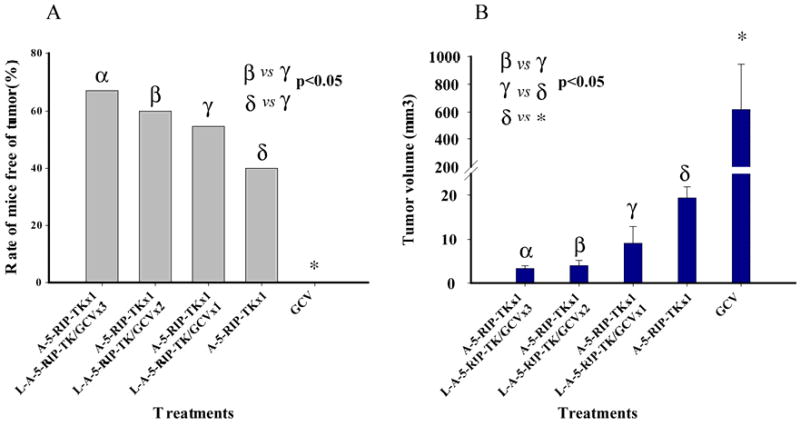
Multiple treatment cycles of A or/and L-A-5-RIP-TK/GCV treatment suppresses growth of human pancreas cancer xenografts. Two weeks after i.p. injection of PANC-1 cells, SCID mice were divided into five groups (30 animals/group) and treated as follows: (1) 4 cycles of GCV, (2) 1 cycle of A-5-RIP-TK/GCV, (3) 1 cycle of A-5-RIP-TK/GCV+1 cycles of L-A-5-RIP-TK/GCV, (4) 1 cycle of A-5-RIP-TK/GCV+2 cycles of L-A-5-RIP-TK/GCV, (5) 1 cycle of A-5-RIP-TK/GCV+3 cycles of L-A-RIP-TK/GCV. At 4 months after treatment, at least five mice were sacrificed. Percentages of tumor-free animals in different groups were compared using χ2-test significance (A)Tumor size was measured and compared using Student's t-test (B) with P< 0.05 representing significance. Tumor growth was significantly suppressed in all treated mice compared with controls.
Multiple cycles of L-A-5-RIP-TK/GCV therapy prolong survival of tumor-bearing SCID mice
We next evaluated the effect of multiple cycles of L-A-5-RIP-TK/GCV on mouse survival. Five groups of mice were analyzed by rank-log survival analysis and Kaplan-Meier survival curves were plotted (Fig. 3). Overall, multiple cycles of therapy had a survival advantage over single cycle of therapy, however three cycles of L-A-RIP-TK/GCV resulted in the longest survival (median survival 153.0±5.6 d) in PANC-1 tumor-bearing animals when compared with others one cycle =124.0 ±1.1d), two cycles=133.0±6.6d, and four cycles=126.0±4.8d (P < 0.05 vs three cycles), respectively. All treatment groups had significant prolonged survival as compared to the empty vector/GCV control group (69.0±5.4 d). Survival time between one, two and four cycles of therapy was comparable. These data indicate that one or multiple cycles of therapy effectively prolong survival in SCID mice, however the greatest survival was seen after three cycles of therapy.
FIG. 3.
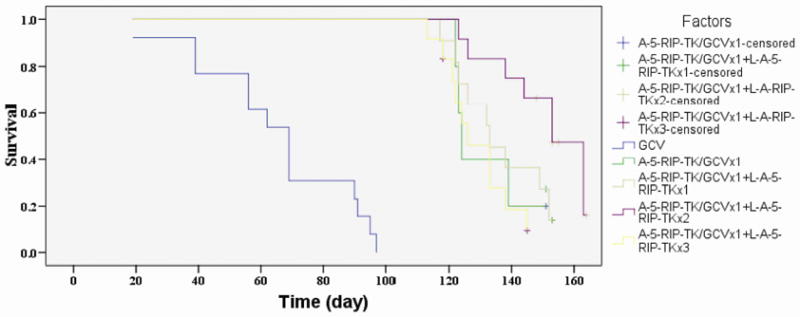
Multiple treatments with A or/and L-A-5-RIP-TK/GCV prolongs survival of PANC-1 tumor bearing mice. PANC-1 tumor mice were grouped to receive (1) 4 cycles of GCV, (2) 1 cycle of A-5-RIP-TK/GCV, (3) 1 cycle of A-5-RIP-TK/GCV+1 cycles of L-A-5-RIP-TK/GCV, (4) 1 cycle of A-5-RIP-TK/GCV+2 cycles of L-A-5-RIP-TK/GCV, (5) 1 cycle of A-5-RIP-TK/GCV+3 cycles of L-A-RIP-TK/GCV. Mice survival was estimated by using the Kaplan-Meier and log rank tests. All the treatment group had significant prolonged survival as compared to control group treated with GCV. Three cycle of A and L-A-5-RIP-TK/GCV treatment obtained longest survival among all of groups (P<0.05).
Multiple cycles of L-A-5-RIP-TK gene therapy causes diabetes associated with islet cell apoptosis
We have previously shown that single treatment cycle with A-5-RIPTK/GCV caused pancreatogenic diabetes in SCID mice (13). We wanted to determine whether the effect of multiple cycles of therapy with L-5-RIP-TK/GCV would further adversely affect insulin levels and glucose regulation. Glucose and insulin levels were monitored during each treatment cycle. Glucose levels increased after each cycle, reaching 287.8 mg/dl on day 7 after the 4th cycle, whereas insulin levels decreased after each cycle, with a nadir of 0.3ug/ul, after the 4th cycle (Fig 4). The changes of insulin and glucose levels correlated with islet cell apoptosis after each cycle of therapy, as shown in the Fig.4 bottom. 8.2%±1.4%%% after 1st cycle, 14.2%±3.4% after 2nd, 42.8%±11.7% after 3rd, and 56.2%±18.2% after 4th cycle; all were significantly higher than in control mice (2.1%±0.6%). The data confirm that multiple cycles of therapy sequentially increase islet apoptosis and have a deleterious effect on insulin levels and glucose regulation, thus representing a toxicity of this therapy.
FIG. 4.
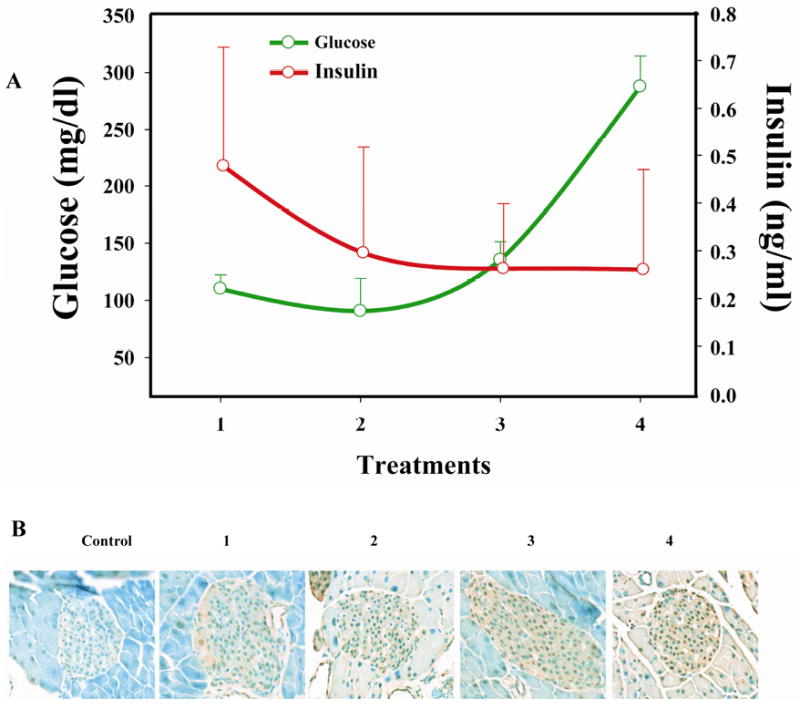
Multiple cycles of L-A-5-RIP-TK/GCV treatments cause diabetic. Fasting serum was collected on day 7 after each L-A-RIP-TK/GCV gene therapy. The glucose (green line) and insulin (red line) levels are shown at different L-A-5-RIP-TK/GCV treatment cycles. Glucose levels increased while insulin levels decreased (A). The changes of insulin and glucose levels were correlation with islet cell apoptosis in pancreas of treated mice (B)
Humoral immune response in C57BL/6J with A- or L-A-5-RIP-TK vectors
Multiple cycles of L-A-5-RIP-TK/GCV have shown a greater inhibitory effect on tumor growth in SCID mice than did a single cycle, however, the effect was observed in the absence of normal immune response, since the study was performed in SCID mice. In order to assess whether the liposome-coated adenoviral complexes would induce lower levels of immune response in immunocompetent mice, the levels of adenoviral specific neutralizing antibody in serum were measured following intravenous injection with L-A-5-RIP-TK complexes, and compared to that induced by A-5-RIP-TK. C57BL/6J mice and SCID mice without prior exposure to adenovirus were treated with L-A-5-RIP-TK or A-5-RIP-TK displaying a similar neutralized antibody titer at 1/16, respectively. Similarly, repeated injections of either L-A-5-RIP-TK or A-5-RIP-TK induced anti-adenovirus antibody, but were higher than that with the first injection. The 3rd injection of A-5-RIP-TK resulted in a 2 fold higher titer (1/256) than did the 3rd injection of L-A-5-RIP-TK (1/128) (Fig 5). No difference was found the levels of antibody between the 2nd and 3rd injections of L-A-5-RIP-TK. Conversely, no antibody was found in the SCID mice following each injection with either adenovirus alone or adenovirus liposome complex (Data not shown). The data suggest that both L-A-5-RIP-TK and A-5-RIP-TK induce high levels of antibody titers and lower titers of neutralized antibody in the mice without prior exposure to adenovirus.
FIG. 5.
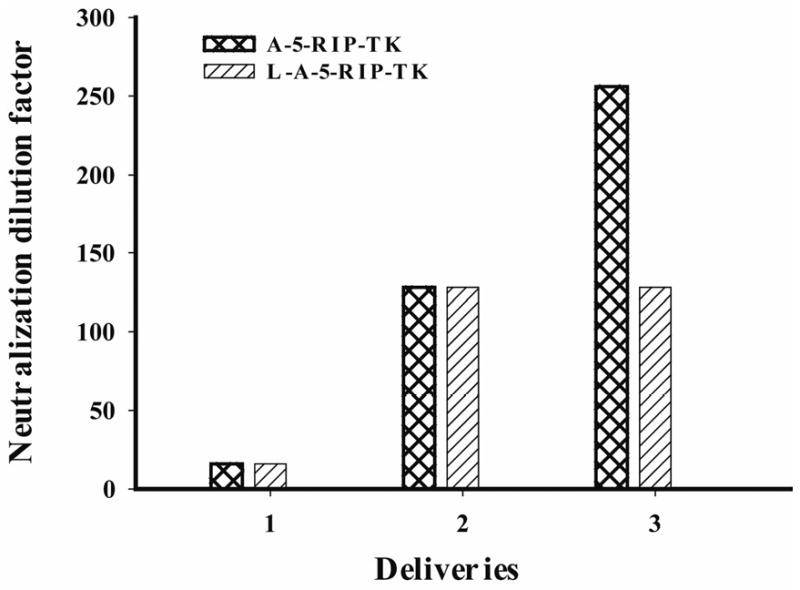
Repeated L-A-5-RIP-TK administration induced anti-adenovirus antibodies. C57BL/6J mice without prior exposure to adenovirus were treated with naked A-5-RIP-TK or L-A-5-RIP-TK. Repeated naked A-5-RIP-TK or L-A-5-RIP-TK was administrated in a 3 weeks interval, 3 doses in total. 7days after each administration, the serum was collected for measurement of neutralized antibody. Either A-5-RIP-TK or L-A-5-RIP-TK induced high titer of anti adenoviral antibody. Repeated administration of adenovirus resulted in increased antibody titers.
Liposome protects adenovirus from neutralized antibodies in vivo after repeated adenovirus administrations
To determine whether encapsulated adenoviral vectors protect adenovirus from high titers of neutralized antibody in the circulation serum in vivo, a reporter assay was performed by i.v. injection of C57B6J mice with A-5-CMV-LacZ or L-A-5-CMV-LacZ vectors. β-gal expression in liver cells was evaluated on day 7, 21 and 30 following adenovirus administration. We have previously shown that systemically-delivered A-5-CMV-LacZ is highly expressed in liver cells and not islets (13). On day 7, 62.5% vs 68.2% (P>0.05) of liver cells were positive for β- gal expression in the mice receiving A-5-CMV-LacZ and L-A-5-CMV-LacZ, respectively. Expression levels were significantly reduced to 5.3% vs 8.5% (P>0.05) at 21 day, and further decreased by 0.4% vs 2.8% (P<0.05) on day 30, respectively. No significant difference was found in β-gal expression between naked adenovirus and liposome-coated adenovirus on days 7 and 21, but with an exception on day 30 (p<0.05). Repeat doses of adenovirus were performed on day 21 after first injection and β- gal expression was determined on day 7. Only 2.1% and 3.2% of liver cells were positive for β-gal in A-5-CMV-LacZ and L-A-5-CMV-LacZ immunized mice (P>0.05), respectively. In contrast, liposome coated A-5-CMV-LacZ re-administration resulted in 43.2% vs 50.8% (P<0.05) of liver cells expressing β-gal in A-5-CMV-LacZ and L-5-A-CMV-LacZ immunized mice, respectively. These data suggest that liposomal encapsulation protects adenovirus from neutralization in the presence of high titer neutralizing antibody.
Discussion
The advantage of adenoviral gene therapy is its high efficiency of transduction and low risk of integrating into host genome(4, 13, 30, 31). However, a major limitation is that transgene expression is transient. Therefore, multiple cycles of therapy would be necessary for a long term therapeutic effect. While multiple cycles of adenoviral gene therapy would be an attractive therapeutic strategy, adenovirus induced humoral immune neutralizing response to repeated applications of adenovirus is a significant hurdle to overcome. In the current study, we studied the multiple cycles of systemically-delivered liposome-coated A-RIP-TK/GCV in a human pancreatic cancer cell SCID mouse model, as well as studying immune status and transgene expression after multiple deliveries of liposome-coated adenoviral gene therapy in immune competent mice.
The effect of L-A-RIP-TK/GCV gene therapy was evaluated using a human PANC-1 cell xenograft SCID mouse model in this study, since our experience demonstrated that the model provides a precise assessment of therapeutic effect in human cancer cells without interference of immunological factors(13, 32). Conversely, the drawback of the model is absence of an immunological response and analyses. Therefore, similar studies were performed in an immunocompetent mouse model to determine the immune response to multiple cycles of adenoviral gene therapy and whether liposomal coating would affect the immune response. Therefore, both models give a complementary understanding of multiple cycles of L-A-RIP-TK/GCV gene therapy.
We designed the treatment regimen on the based upon the considerations that more than one treatment of gene therapy is necessary to have a beneficial effect for cancer patients and that transgene expression in vivo, which lasts 30 days, but is significant reduced by the third week(19, 23, 25). The present study showed benefit of more than one cycle. Furthermore, a three-week-cycle is reasonable to maintain a high transgene expression levels in tumor cells; there was remarkable reduction of vTK expression 21 days after gene delivery, indicating that it is necessary to scheduled gene delivery at an interval of 21 days. 2 weeks of GCV treatment following gene delivery was selected according to previous studies(13). Three treatment cycles appears to be optimal in our mouse model, as a higher mortality was observed after fourth cycle of gene therapy, although better effect was obtained.
While the current study indicates that three treatments every three weeks with liposomal A-5-RIP-TK followed by 2 weeks of daily GCV is the optimal regimen for treatment of our SCID mouse metastatic human pancreatic cancer cell model, the study demonstrated that four cycles of L-A-5-RIP-TK/GCV induced maximal tumor inhibitory effect on PANC-1 tumor. This is was consistent with Vlachaki, et al's study, in which they obtained smallest tumor size in treatment of a mammary tumor by three cycles of HSV-TK/GCV with intratumoral injection(19). However, in another study, no significant reduction of ovarian tumor volume was observed by three cycles of intraperitoneal injection of HSV-TK/GCV as compared to that of one treatment(33). A possible explanation is induction of humoral immune response to adenovirus with repeated administration, although local concentrations of anti-adenoviral–specific antibodies were considerably lower compared to the serum levels in our study. Our study demonstrates the effect of multiple cycles of intravenous L-A-RIP-TK/GCV enhances inhibition of tumor growth in the absence of immune response. Furthermore, there is reversal correlation between the number of treatment cycles and tumor size, as seen in the Vlachaki's study, indicating an accumulative treatment effect of multiple cycles. This could be explained by periodic delivery of vTK gene into tumor cells, resulting in persistent expression of vTK protein that contributes to continuous cytotoxic effect on tumor cells. However, as seen in the present study and others, not all the tumor was ablated despite multiple treatments, most likely due to incomplete gene transduction after every gene delivery
While the concept of multiple cycles of therapy is important, there appears to be a limit on the number of cycles possible, since therapy related toxicity resulted in increased mortality. Three cycles of therapy resulted in the longest survival, which is consistent with previous reports showing that the longest survival in the treatment of mammary tumors and ovarian tumors was after three treatment cycles. In contrast, four cycles did not prolong survival due to high mortality after the fourth cycle of treatment. The decrease in survival compared to 3 cycles could not be simply attributed to the toxicity of liposome-coated adenoviral complex, since there was no evidence of impairment of heart, liver, kidney, brain and gastrointestinal tract on the basis of pathological analysis (data not shown). It is possible that increased mortality was due to impairment of function of endocrine pancreas, as high levels of serum glucose, low levels of serum insulin and apoptosis of the islets were observed in the mice. Adenoviral expression was seen in the islets at 30 days. The development of pancreatogenic diabetes after A-5-RIP-TK/GCV treatment has been described in our previous study. This was anticipated since both islets and pancreas cancer express PDX-1, which we have shown to be the transcription factor that activates the RIP promoter at the A box site on the promoter(32). The toxicity to the endocrine pancreas could potentially limit the effectiveness of L-RIP-TK/GCV therapy for pancreas cancer, however it is to be noted that pancreatogenic diabetes can be treated with insulin and oral hypoglycemics.
The success of using adenoviral vectors for gene therapy is often limited by the host's immune response to the virus (34). The induction of humoral immune response still remains a significant challenge to the use of more than one cycle of adenoviral therapy. The effectiveness of the re-administration of adenoviral vectors is limited by the large-scale production of adenoviral specific neutralizing antibodies by the host (35). One method used to prevent the neutralization of the adenoviral vectors is to bind cationic polymers or liposome to the virus (36-41). Dr. Yatnda's studies had shown that bilamellar cationic liposomes not only protect adenovectors from preexisting humoral immune responses in vitro and in vivo but also overcome the limitation of absence of CAR expression on the cancer cells surface, which is required for adenovirus targeting(42). Further more, liposomal encapsulated adenovirus reduce the liver's uptake of adenovirus when systemic adenovirus is administrated(43). To quantify neutralizing antibody titers, we used bioassay instead of others such as ELISA to enable to precisly evaluation of viral titer and neutralizing activity simulteneously. This approch has been proved to be liable and used by others(42). Our data showed that high titer of neutralizing antibody in immunocompetent mice after initial exposure to adenovirus and increased following second and third injection, which was consistent with other's finding(36-41). The minor difference was lower titer of antibody in L-A-5-RIP-TK re-injection followed by L-A-5-RIP-TK injection, as compared to that in L-5-RIP-TK re-injection followed by A-5-RIP-TK injection. This may contribute to a relative low neutralizing immune response to adenovirus, but did not appear to be a major factor to affect the gene transfer and expression after repeated injections of adenovirus. The fact is that liposome actually shields adenovirus from neutralizing antibodies, since naked adenovirus re-injection shows little transgene expression. While an adenovirus vector can be repeatedly injected to tumor locally, this study supports the concept that liposome-coated adenovirus gene therapy is necessary for the multiple treatment cycles of systemic therapy.
In conclusion, our study demonstrated that multiple cycles of L-A-RIP-TK/GCV significantly increased the cytoablative effect on metastatic human pancreatic cancer cells in SCID mice, suggesting that liposomal coating of the adenovirus could permit multiple cycles of systemic gene therapy. In this study, it appears that three cycles of therapy was most effective due to toxicity after the fourth cycle, however the specific cause of toxicity could be due to pancreatogenic diabetes, as opposed to an immune reaction to the fourth cycle. These data support the hypothesis that liposome-coated adenovirus is functionally resistant to the neutralizing effects of anti-adenoviral antibodies. We conclude that liposome-encapsulation of adenoviral RIP-TK/GCV gene therapy is a promising strategy for pancreatic cancer using repeated delivery of systemic pancreas cancer-specific adenoviral gene therapy.
FIG. 6.
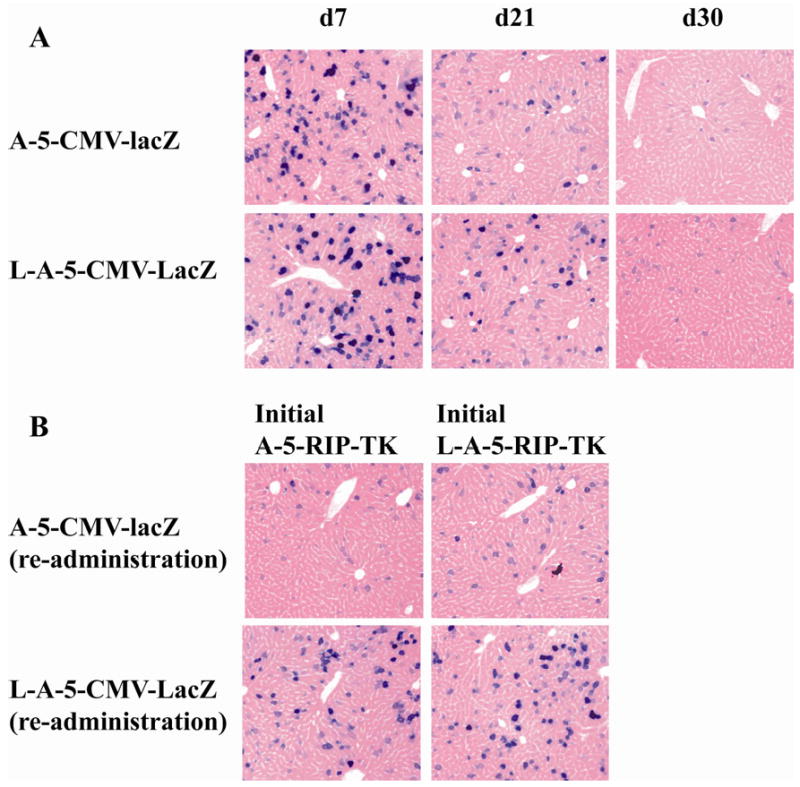
Liposome shield adenovirus from neutralizing antibody. C57BL/6J mice without prior exposure to adenovirus were treated with naked adenoviral LacZ (A-5-CMV-lacZ) and liposome encapsulated adenoviral lacZ (L-A-5-CMV-lacZ). Repeated A-5-CMV-lacZ or L-A-5-CMV-LacZ was administrated on day 21 after initial injection of naked A-5-RIPTK or L-A-RIP-TK vector. The β- gal expression was evaluated at 7, 21 and 30d following initial adenoviral lacZ administration and day 7 after repeated adenovirus administration by x-gal staining for liver cells as previous described. Blue staining cells indicate positive for β-gal expression. (X200)
Acknowledgments
We would like to thank our colleagues working in Dr. Brunicardi's laboratory for their support and suggestions. We also thank Katie Elsbury for her continuous assistance and editorial support. The work was supported by National Institutes of Health (NIH) grants, NIDDK R01-DK46441 and NCI R01 – CA095731, the Vivian L. Smith Foundation, the MD Anderson Foundation, the Elkins Pancreas Center at Baylor College of Medicine, and the generosity of Mr. and Mrs. Lloyd Kirchner.
Footnotes
Conflict of interest: There is no conflict of interest on this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 2.Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549–58. doi: 10.1016/j.ejca.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–58. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 4.Prince HM, Dessureault S, Gallinger S, Krajden M, Sutherland DR, Addison C, et al. Efficient adenovirus-mediated gene expression in malignant human plasma cells: relative lymphoid cell resistance. Exp Hematol. 1998;26:27–36. [PubMed] [Google Scholar]
- 5.Pearson AS, Koch PE, Atkinson N, Xiong M, Finberg RW, Roth JA, et al. Factors limiting adenovirus-mediated gene transfer into human lung and pancreatic cancer cell lines. Clin Cancer Res. 1999;5:4208–13. [PubMed] [Google Scholar]
- 6.Kim M, Zinn KR, Barnett BG, Sumerel LA, Krasnykh V, Curiel DT, et al. The therapeutic efficacy of adenoviral vectors for cancer gene therapy is limited by a low level of primary adenovirus receptors on tumour cells. Eur J Cancer. 2002;38:1917–26. doi: 10.1016/s0959-8049(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 7.Crystal RG, Harvey BG, Wisnivesky JP, O'Donoghue KA, Chu KW, Maroni J, et al. Analysis of risk factors for local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of comorbid conditions. Hum Gene Ther. 2002;13:65–100. doi: 10.1089/10430340152712647. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai H, Kawabata K, Sakurai F, Nakagawa S, Mizuguchi H. Innate immune response induced by gene delivery vectors. Int J Pharm. 2008;354:9–15. doi: 10.1016/j.ijpharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Ding Q, Li Y, Miller SA, Abbruzzese JL, Hung MC. Suppression of pancreatic tumor progression by systemic delivery of a pancreatic-cancer-specific promoter driven Bik mutant. Cancer Lett. 2005 doi: 10.1016/j.canlet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Deharvengt S, Wack S, Aprahamian M, Hajri A. Transcriptional tumor-selective promoter targeting of E. coli purine nucleoside phosphorylase for pancreatic cancer suicide gene therapy. J Gene Med. 2005;7:672–80. doi: 10.1002/jgm.701. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Olmsted-Davis E, Davis A, Liu S, Li Z, Yang J, et al. Specific targeting of pancreatic islet cells in vivo by insulin-promoter-driven adenoviral conjugated reporter genes. World J Surg. 2006;30:1543–52. doi: 10.1007/s00268-005-0688-3. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Wang XP, Brunicardi FC. Enhanced cytotoxicity of RIPTK gene therapy of pancreatic cancer via PDX-1 co-delivery. J Surg Res. 2007;137:1–9. doi: 10.1016/j.jss.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 13.Liu SH, Davis A, Li Z, Ballian N, Davis E, Wang XP, et al. Effective ablation of pancreatic cancer cells in SCID mice using systemic adenoviral RIP-TK/GCV gene therapy. J Surg Res. 2007;141:45–52. doi: 10.1016/j.jss.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 14.Fasbender A, Zabner J, Chillon M, Moninger TO, Puga AP, Davidson BL, et al. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. J Biol Chem. 1997;272:6479–89. doi: 10.1074/jbc.272.10.6479. [DOI] [PubMed] [Google Scholar]
- 15.Byk T, Haddada H, Vainchenker W, Louache F. Lipofectamine and related cationic lipids strongly improve adenoviral infection efficiency of primitive human hematopoietic cells. Hum Gene Ther. 1998;9:2493–502. doi: 10.1089/hum.1998.9.17-2493. [DOI] [PubMed] [Google Scholar]
- 16.Sung MW, Lee SG, Yoon SJ, Lee HJ, Heo DS, Kim KH, et al. Cationic liposome-enhanced adenoviral gene transfer in a murine head and neck cancer model. Anticancer Res. 2000;20:1653–6. [PubMed] [Google Scholar]
- 17.Toyoda K, Nakane H, Heistad DD. Cationic polymer and lipids augment adenovirus-mediated gene transfer to cerebral arteries in vivo. J Cereb Blood Flow Metab. 2001;21:1125–31. doi: 10.1097/00004647-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Li Y, Li L, Zhang Y, Gao N, Zhang Z, et al. Phase I study of repeated intraepithelial delivery of adenoviral p53 in patients with dysplastic oral leukoplakia. J Oral Maxillofac Surg. 2009;67:1074–82. doi: 10.1016/j.joms.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 19.Vlachaki MT, Chhikara M, Aguilar L, Zhu X, Chiu KJ, Woo S, et al. Enhanced therapeutic effect of multiple injections of HSV-TK + GCV gene therapy in combination with ionizing radiation in a mouse mammary tumor model. Int J Radiat Oncol Biol Phys. 2001;51:1008–17. doi: 10.1016/s0360-3016(01)01698-4. [DOI] [PubMed] [Google Scholar]
- 20.Shimada H, Matsubara H, Shiratori T, Shimizu T, Miyazaki S, Okazumi S, et al. Phase I/II adenoviral p53 gene therapy for chemoradiation resistant advanced esophageal squamous cell carcinoma. Cancer Sci. 2006;97:554–61. doi: 10.1111/j.1349-7006.2006.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagliaro LC, Keyhani A, Williams D, Woods D, Liu B, Perrotte P, et al. Repeated intravesical instillations of an adenoviral vector in patients with locally advanced bladder cancer: a phase I study of p53 gene therapy. J Clin Oncol. 2003;21:2247–53. doi: 10.1200/JCO.2003.09.138. [DOI] [PubMed] [Google Scholar]
- 22.McVey D, Hamilton MM, Hsu C, King CR, Brough DE, Wei LL. Repeat administration of proteins to the eye with a single intraocular injection of an adenovirus vector. Mol Ther. 2008;16:1444–9. doi: 10.1038/mt.2008.124. [DOI] [PubMed] [Google Scholar]
- 23.Lambright ES, Force SD, Lanuti ME, Wasfi DS, Amin KM, Albelda SM, et al. Efficacy of repeated adenoviral suicide gene therapy in a localized murine tumor model. Ann Thorac Surg. 2000;70:1865–70. doi: 10.1016/s0003-4975(00)01815-4. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- 24.Fujiwara T, Tanaka N, Kanazawa S, Ohtani S, Saijo Y, Nukiwa T, et al. Multicenter phase I study of repeated intratumoral delivery of adenoviral p53 in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:1689–99. doi: 10.1200/JCO.2005.03.4116. [DOI] [PubMed] [Google Scholar]
- 25.Benihoud K, Saggio I, Opolon P, Salone B, Amiot F, Connault E, et al. Efficient, repeated adenovirus-mediated gene transfer in mice lacking both tumor necrosis factor alpha and lymphotoxin alpha. J Virol. 1998;72:9514–25. doi: 10.1128/jvi.72.12.9514-9525.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng X, Szabo S, Khomenko T, Jadus MR, Yoshida M. Gene therapy with adenoviral plasmids or naked DNA of vascular endothelial growth factor and platelet-derived growth factor accelerates healing of duodenal ulcer in rats. J Pharmacol Exp Ther. 2004;311:982–8. doi: 10.1124/jpet.104.071464. [DOI] [PubMed] [Google Scholar]
- 27.Steel JC, Cavanagh HM, Burton MA, Abu-Asab MS, Tsokos M, Morris JC, et al. Increased tumor localization and reduced immune response to adenoviral vector formulated with the liposome DDAB/DOPE. Eur J Pharm Sci. 2007;30:398–405. doi: 10.1016/j.ejps.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Lee EM, Hong SH, Lee YJ, Kang YH, Choi KC, Choi SH, et al. Liposome-complexed adenoviral gene transfer in cancer cells expressing various levels of coxsackievirus and adenovirus receptor. J Cancer Res Clin Oncol. 2004;130:169–77. doi: 10.1007/s00432-003-0521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Ballian N, Belaguli NS, Patel S, Li M, Templeton NS, et al. PDX-1 acts as a potential molecular target for treatment of human pancreatic cancer. Pancreas. 2008;37:210–20. doi: 10.1097/MPA.0b013e31816a4a33. [DOI] [PubMed] [Google Scholar]
- 30.Kuhlmann KF, Gouma DJ, Wesseling JG. Adenoviral gene therapy for pancreatic cancer: where do we stand? Dig Surg. 2008;25:278–92. doi: 10.1159/000145981. [DOI] [PubMed] [Google Scholar]
- 31.Appledorn DM, Seregin S, Amalfitano A. Adenovirus vectors for renal-targeted gene delivery. Contrib Nephrol. 2008;159:47–62. doi: 10.1159/000125581. [DOI] [PubMed] [Google Scholar]
- 32.Tirone TA, Wang XP, Templeton NS, Lee T, Nguyen L, Fisher W, et al. Cell-specific cytotoxicity of human pancreatic adenocarcinoma cells using rat insulin promoter thymidine kinase-directed gene therapy. World J Surg. 2004;28:826–33. doi: 10.1007/s00268-004-7291-x. [DOI] [PubMed] [Google Scholar]
- 33.Al-Hendy A, Magliocco AM, Al-Tweigeri T, Braileanu G, Crellin N, Li H, et al. Ovarian cancer gene therapy: repeated treatment with thymidine kinase in an adenovirus vector and ganciclovir improves survival in a novel immunocompetent murine model. Am J Obstet Gynecol. 2000;182:553–9. doi: 10.1067/mob.2000.104837. [DOI] [PubMed] [Google Scholar]
- 34.Mack CA, Song WR, Carpenter H, Wickham TJ, Kovesdi I, Harvey BG, et al. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum Gene Ther. 1997;8:99–109. doi: 10.1089/hum.1997.8.1-99. [DOI] [PubMed] [Google Scholar]
- 35.Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000;81:2573–604. doi: 10.1099/0022-1317-81-11-2573. [DOI] [PubMed] [Google Scholar]
- 36.Chillon M, Lee JH, Fasbender A, Welsh MJ. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Ther. 1998;5:995–1002. doi: 10.1038/sj.gt.3300665. [DOI] [PubMed] [Google Scholar]
- 37.O'Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE, et al. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther. 1999;10:1349–58. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- 38.Natsume A, Mizuno M, Ryuke Y, Yoshida J. Cationic liposome conjugation to recombinant adenoviral vector reduces viral antigenicity. Jpn J Cancer Res. 2000;91:363–7. doi: 10.1111/j.1349-7006.2000.tb00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worgall S, Worgall TS, Kostarelos K, Singh R, Leopold PL, Hackett NR, et al. Free cholesterol enhances adenoviral vector gene transfer and expression in CAR-deficient cells. Mol Ther. 2000;1:39–48. doi: 10.1006/mthe.1999.0013. [DOI] [PubMed] [Google Scholar]
- 40.Croyle MA, Chirmule N, Zhang Y, Wilson JM. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther. 2002;13:1887–900. doi: 10.1089/104303402760372972. [DOI] [PubMed] [Google Scholar]
- 41.Mizuno M, Ryuke Y, Yoshida J. Cationic liposomes conjugation to recombinant adenoviral vectors containing herpes simplex virus thymidine kinase gene followed by ganciclovir treatment reduces viral antigenicity and maintains antitumor activity in mouse experimental glioma models. Cancer Gene Ther. 2002;9:825–9. doi: 10.1038/sj.cgt.7700503. [DOI] [PubMed] [Google Scholar]
- 42.Yotnda P, Chen DH, Chiu W, Piedra PA, Davis A, Templeton NS, et al. Bilamellar cationic liposomes protect adenovectors from preexisting humoral immune responses. Mol Ther. 2002;5:233–41. doi: 10.1006/mthe.2002.0545. [DOI] [PubMed] [Google Scholar]
- 43.Yotnda P, D A, Hicks MJ, Templeton NS, Brenner MK. Liposomal enhancement of the antitumor activity of conditionally replication-competent adenoviral plasmids. Mol Ther. 2004;9:489–95. doi: 10.1016/j.ymthe.2004.01.018. [DOI] [PubMed] [Google Scholar]


