Abstract
Objective
To examine the association between memory for previously-encoded emotional faces and depression symptoms assessed over four years in adolescent girls. Investigating the interface between memory deficits and depression in adolescent girls may provide clues about depression pathophysiology.
Method
Participants were 213 girls recruited from a longitudinal, community-based study; the majority was African-American. Scores on depressive screening measures at age 8 were used to increase the base rate of depression. Depression symptoms and diagnoses were assessed annually for four years. In year four, when the girls were 12-13 years old, a face emotion encoding task was administered during which ratings were generated in response to sad, fearful, angry, and happy faces. A surprise memory task followed where participants identified which of two faces, displaying neutral expressions, they had seen previously.
Results
Girls with higher depression symptom levels from ages 9-12 years evidenced lower accuracy in identifying previously-encoded emotional faces. Controlling for IQ, higher depression symptom level was associated with a memory deficit specific to previously-encoded sad and happy faces. These effects were not moderated by race.
Conclusions
Individual differences in face memory deficits relate to individual differences in emerging, early adolescent depression, and may be vulnerability markers for depression.
Keywords: depression, memory, face emotion, adolescence
Introduction
Major depressive disorder (MDD) is highly prevalent in adolescents, with estimates ranging from 14-20%.1 Depressive disorders are also the most common and impairing of mental and medical disorders among females. Between ages 15-50 years, 15-20% of women experience MDD.2 Confirming the well-documented sex and age effects on MDD3-4, a meta-analysis of epidemiologic studies demonstrated that MDD rates double from childhood to adolescence particularly among girls.5 Thus, peri-adolescence is a critical period for understanding vulnerabilities for depression in females.6
Contextual factors such as race and poverty further influence the emergence of depression. Nearly 35% of African-American children and adolescents live in poverty, roughly three times the rate for European-American youth.7 Among both adults and adolescents the mental health outcome most consistently linked to stressful life contexts, such as poverty, is depression.8 Poverty and inequality contribute a range of stressors that lead to significant racial and socio-economic disparities in mental health, and individuals from minority groups are unlikely to receive mental health services.9-11 Low-income urban African-American youth, therefore, face heightened risk for recurrent and severe depression.12
Cognitive theories of depression and associated empirical evidence indicate that one marker of vulnerability to depression is memory perturbation.13-15 Indeed, a well-replicated finding in adult depression is memory dysfunction16-18, including poor overall memory19 and memory bias.20-21 Perturbed memory in adults has been documented in older adults afflicted with MDD.19, 21 The age at which depression-related memory abnormalities typically manifest, however, is unclear. Potential cognitive vulnerability markers should be evident prior to disorder onset and in those at high risk for the disorder.22 Stressful life events are suggested to further increase risk for depression by interacting with vulnerabilities.23 Thus, a critical question is whether memory perturbations can be identified reliably in female adolescents, particularly in nonclinical samples with heightened contextual risk for depression.
Of particular relevance to adolescent depression is emotional memory, the capacity to remember stimuli of various emotional valences. Three sets of findings guide this focus. First, depression and risk for depression in youth are linked to memory biases for negative versus positive information24, negative self-description25, impaired memory for negative events26, and overgeneral memory biases.27-29 Second, risk for depression coincides with emotional memory maturation during adolescence.30-31 Third, neuroimaging studies of emotional memory implicate brain structures common to MDD, including the amygdala, hippocampus, and prefrontal cortices.32-33 This is particularly salient given that adolescence is characterized by protracted maturation of cognitive-regulatory neural circuits and hyper-reactivity to emotional stimuli in affective neural circuits.34-35
Adolescent depression has been associated with memory deficits following emotional-face processing. Results from one study demonstrated reduced memory for fearful faces in adolescents with remitted MDD whose parents had MDD compared to those offspring with no MDD.36 A second study, in which the same task was administered in the context of neuroimaging, suggested that amygdala dysfunction might account for the previously found memory deficit in adolescent MDD.37 Both studies, however, had limitations. In neither study were sad facial displays used, a stimulus relevant to depression. In both studies relatively complicated encoding and recognition strategies were utilized, yielding both ceiling37 and floor effects.36 Finally, both studies used clinic-based samples, in which various pathophysiological findings are difficult to replicate.38 The current study addresses these limitations.
In the present study, face-memory function was investigated in a sub-study of girls at risk for depression drawn from a community sample that was oversampled based on neighborhood poverty and enriched for depression risk based on high scores on depressive screening measures collected in preadolescence. Over half of the sample was African-American. We used a face-memory task to assess adolescents’ ability to form representations of individuals depicting high-valence emotions. We then assessed the ability to later recognize these individuals when depicting neutral expressions. We hypothesized that memory deficits of previously encoded emotional faces would be associated with higher depression symptom levels across preadolescence, and we expected that memory for previously encoded sad faces would be most compromised in relation to depression. In addition, we examined whether race moderated depression and memory associations based on evidence that African-American girls face higher risk for depression than European-American girls39 and race differences in certain risk factors are associated with depression.40
Method
Participants
Participants were girls and their biological mothers from the Pittsburgh Girls Study (PGS, N=2,451), a prospective study of the development of Conduct Disorder. The PGS recruited 5-8 year old girls living in the city of Pittsburgh and oversampled girls living in low income neighborhoods. The oversampling led to relatively equal numbers of African-American and European-American girls (52% were African-American) in the PGS. From among the youngest PGS participants, girls were selected to participate in the PGS-Emotions (PGS-E) sub-study who either screened high on standardized depressive screening measures at age 8, or were from a random selection of the remaining PGS girls.39 This approach was used to increase the base rate of depression as the girls entered adolescence. Depression screeners included the Short Moods and Feelings Questionnaire41 and the Child Symptom Inventory.42 Girls scoring ≥75th percentile by self-report, mother’s report, or both comprised the screen-high group (n=135), which included significantly more African-American than European-American girls. From the remaining PGS participants scoring <75th percentile on the depression screeners, 136 girls were randomly selected and matched to the screen-high group on race. Of the 271 eligible families for the PGS-E, 8 were not recruited (e.g., the family moved). Of the remaining 263 families, 232 (88.2%) participated, 25 (9.5%) declined participation, and 6 (2.3%) could not be scheduled for an assessment. The first PGS-E assessment occurred at age 9 years. Retention rates remained high across annual assessments (e.g., Year 02=97.8%; Year 03=97.0%). In Year 04, 219 (94.4%) participated, 5 (2.2%) declined participation, and 8 (3.4%) could not be scheduled. The current study includes 213 girls who completed the face-emotion task in Year 04. Of 219 girls assessed in Year 04, 4 completed a phone assessment but not the face-emotion task and 2 had unusable task data.
Mothers’ average age was 35.7 years (SD=7.0). Most of the mothers (71.9%) were single parents, 16.4% had <12 years of education, and 52% had received public assistance. Families of African-American girls (63.1%) were more likely than families of European-American girls (26.1%) to be public assistance recipients (χ2=2.65, p<.001).
Procedures
Girls and their mothers completed an annual assessment of DSM-IV depression symptoms. In Year 04, when the girls were on average 12 years of age, the Emotional Faces Task was administered. Written informed consent was obtained. The University of Pittsburgh Institutional Review Board approved all study procedures.
Measures
Depression
Current depression symptoms (i.e., past month) were measured using the Kiddie-Schedule for Affective Disorders and Schizophrenia for School-Age-Children-Present- and Lifetime-Version (K-SADS-PL), a semi-structured diagnostic interview administered to the girl and her mother.43 Depression symptoms were assessed rigorously for being present most of the time (i.e., interviewers are trained to probe for information to determine whether a behavior meets symptom criteria). All depression symptoms were assessed, including mood disturbance (i.e., sadness, irritability) or anhedonia. Depression diagnoses were generated from combining caregiver and child assessments and according to symptom threshold, duration, and frequency for DSM-IV-based major and minor depression. Inter-rater reliability was assessed by having a second interviewer code the interview, using 25% of interviews randomly selected for this purpose. Average ICCs were .96 and .95 for total number of symptoms from youth- and caregiver-reported data, respectively. Average kappa coefficients for minor/major depressive disorders from youth- and caregiver-reported data were .89 and .91, respectively.
Emotional faces task
Past work on adolescent depression and face memory has not included sad facial stimuli and previously used tasks generated either extremely high or low memory scores. To address this, we adapted earlier versions of an emotional-faces task.36-37 First, we included sad face stimuli, in addition to task instructions that directed participants’ attention to subjective and objective perceptions of sadness during face encoding. Although our focus is on memory, by including both encoding and recognition tasks we could assess the degree to which deficits emerged at face encoding or face recognition. Second, we modified the face-recognition procedures (see below).
The task involved viewing 32 faces (8 sad, 8 fearful, 8 angry, 8 happy) presented pseudo-randomly for 4 s within each of three instruction sets (displayed for 3 s) four times. Participants rated each face from 1=not at all to 5=very much so on: “How sad is this person?” “How sad does this face make you feel?” and “How wide is the nose?” Reaction time (RT) was recorded per rating. Inter-trial interval was 750-1250 msec. A surprise recognition task was administered 20 minutes later. Thirty-two neutral face pairs were presented side-by-side. One face was previously viewed at encoding and one was novel. Of the two neutral faces, participants identified which one they had seen previously by pressing 1 if the recognized face was on the left side of the screen or 2 if the recognized face was on the right side. RT was also recorded. Prior task versions used presentations of one isolated neutral face per trial.
Stimuli were from three standardized sets of gray-scale photographs of 56 actors.44-46 Participants viewed 32 actors, with each actor randomly assigned to display one of the four expressions. An actor might be randomly chosen to portray “anger” across the task for one participant; this same actor might portray “sad” for another subject and “happiness” for yet another. This design controlled for variability in non-emotional features of the actors (e.g., hair color). Actors were racially and sexually diverse; 33% of the actors were African-American or Asian-American.
Race
Sixty-one girls (28.6%) were identified as European-American, 143 (67.1%) as African-American, and 9 (4.2%) as African-American/Multi-racial. The 9 African-American/Multi-racial girls differed from European-American girls in depression symptoms (p=.007), but did not differ from African-American girls not identified as multiracial (p=.48). These 9 girls were grouped with the African-American girls, yielding 152 African-American/Multi-racial and 61 European-American girls.
IQ
Verbal IQ was assessed with Vocabulary and Similarities subtests of the Wechsler Intelligence Scale for Children-III-R 47 administered by trained interviewers in the home when the girls were age 10 years. This short-form has excellent psychometric properties relative to the full-length Verbal Scale.48-49
Pubertal stage
Pubertal stage was measured using the Physical Development Scale (PDS) and associated scoring50-51, which includes four questions about growth spurt, body hair, breast development, and changes in skin rated on 4-point scales and one yes/no question about menstruation onset. We used maternal report, which more closely relates with physical exam scoring than does child self-report.52 Based on age 12 pubertal status, girls were classified into three pubertal groups: pre-pubertal/beginning (n=14), mid-pubertal (n=42), and advanced/post-pubertal (n=147). Pubertal status data was unavailable for 10 girls. This approach has been widely used in other studies3, is consistent with the normal sequence of pubertal development identified by Tanner53, has shown good reliability and validity54, and convergent validity with Tanner staging by physical exam.55
Data Analysis
To test within-group differences in relation to depression on the encoding and recognition tasks, respectively, depression groups were categorized as: full MDD in ≥1 year (n=45); subthreshold MDD in ≥1 year but never having met full criteria for MDD (n=36); and no history of subthreshold/full MDD (n=132) (Table 1). Initial analyses tested potential depression-group and race-group effects to determine task performance variation based on face-emotion or instruction set. Reaction time serves as a measure of overall stimulus processing by indexing the capture of attention and encoding. Two 3 (depression group) x 2 (race) x 3 (instruction-set) x 4 (face-emotion) repeated measures analyses of variance evaluated differences in ratings and RT per face-emotion and instruction-set. A set of similarly constructed ANOVAs was conducted for recognition accuracy and recognition RT per face-emotion. IQ was covaried in all analyses. These analyses tested for significant 2- or 3-way interactions with depression group or race.
Table 1.
Sample Descriptive Information by Depression Group
| No MDD (n=130) |
Subthreshold MDD (n=36) |
MDD (n=45) |
|
|---|---|---|---|
| African American Race, % (n) | 68.3 (90) | 77.8 (28) | 75.6 (34) |
| Age 12 Pubertal Status, % (n)a | |||
| Pre- or beginning pubertal | 9.6 (12) | 0 (0) | 4.4 (2) |
| Mid-pubertal | 20.8 (26) | 30.3 (10) | 13.3 (6) |
| Advanced or post pubertal | 66.9 (87) | 69.6 (23) | 82.2 (37) |
| Depressive Symptoms Year 1, M (SD) | 1.54 (1.52) | 2.58 (1.48) | 4.78 (1.72) |
Note: Groups were based on presence of subthreshold major depressive disorder (MDD) or MDD diagnosis in at least one of four annual assessments.
Age 12 pubertal status was unavailable for 8 girls (5 with no MDD; 3 with subthreshold MDD).
Primary analyses used average depression symptom counts and variability (SD) of depression symptom counts across four annual assessments. We used symptoms given low base-rates of MDD diagnoses and to test whether associations between memory and depression symptoms could be observed before onset of depressive disorders. Spearman’s ρ coefficients were computed to examine associations of key variables with depression symptoms. Primary analyses used hierarchical multiple regression to predict emotional-face memory by mean depression symptom counts and variability of depression symptom counts, across four annual assessments. Face memory accuracy was calculated as the percent of faces correctly recognized overall and per emotion. IQ correlated with mean depression symptom counts (ρ=−.39, p<.001), variability of depression symptom counts (ρ=−.23, p<.01) and overall recognition accuracy (ρ=.28, p<.001). Puberty status correlated with mean depression symptoms (ρ=.18, p<.05) and race (ρ=.21, p<.01) but not with any of the task performance measures; thus, puberty was not included in further analyses given our interest in predicting face memory accuracy. IQ was centered on the sample mean and included as a covariate in all analyses; two participants were missing IQ scores. We applied a theoretically-driven approach in designing our models to assess contributions from known individual-level correlates and hypothesized depression correlates to variance in face memory accuracy. Thus, IQ and race were entered on step one and the depression variable in step two to evaluate its unique contribution to memory indices. An interaction term was added in a third step to evaluate the potential moderating effect of race on depression in predicting face memory accuracy.
Results
Depression Diagnosis
Encoding task
Depression group and race did not influence ratings or RT. No significant interactions emerged between depression group or race and face-emotion or instruction-set. All girls, regardless of depression group and race, encoded the faces similarly, regardless of face-emotion or instruction-set, based on their ratings and RTs (Table 2).
Table 2.
Encoding Task Performance by Face-Emotion, Instruction-Set, and Depression Group
| Rating (1-5), mean(SE)a | Reaction Time (ms), mean(SE)b | |||||
|---|---|---|---|---|---|---|
| No MDD (n=130) |
Subthreshold MDD (n=36) |
MDD (n=45) |
No MDD (n=130) |
Subthreshold MDD (n=36) |
MDD (n=45) |
|
| How sad is this person? | ||||||
| Sad | 3.22±.08 | 3.18±.16 | 3.19±.14 | 2156.78±88.37 | 2209.03±181.33 | 1989.15±157.09 |
| Fearful | 2.57±.09 | 2.72±.18 | 2.48±.16 | 1999.00±70.26 | 2024.07±144.19 | 1998.29±124.91 |
| Angry | 2.65±.11 | 3.06±.23 | 2.52±.20 | 2012.47±66.93 | 1989.20±137.34 | 2023.34±118.97 |
| Happy | 1.21±.05 | 1.11±.09 | 1.20±.08 | 1439.28±49.24 | 1461.43±101.05 | 1496.85±87.54 |
| How sad does this face make you feel? | ||||||
| Sad | 2.35±.09 | 2.29±.19 | 2.53±.17 | 1826.40±70.51 | 2003.53±144.69 | 1681.63±125.35 |
| Fearful | 1.99±.09 | 1.98±.19 | 2.05±.16 | 1781.87±71.75 | 1692.32±147.23 | 1651.41±127.55 |
| Angry | 2.05±.11 | 2.12±.22 | 2.17±.19 | 1772.82±71.08 | 1726.47±145.86 | 1686.86±126.35 |
| Happy | 1.30±.05 | 1.19±.10 | 1.24±.09 | 1374.48±55.86 | 1374.96±114.63 | 1445.86±99.31 |
| How wide is their nose? | ||||||
| Sad | 2.42±.07 | 2.25±.14 | 2.52±.12 | 1947.57±69.63 | 1756.75±142.88 | 1901.09±123.78 |
| Fearful | 2.55±.07 | 2.37±.15 | 2.61±.13 | 1890.46±61.66 | 1879.57±126.52 | 1809.75±109.61 |
| Angry | 2.91±.07 | 2.74±.15 | 2.84±.13 | 1934.78±58.36 | 1907.53±119.76 | 1824.38±103.74 |
| Happy | 2.77±.08 | 2.67±.16 | 2.83±.13 | 1861.57±60.21 | 1941.70±123.55 | 1800.74±107.03 |
Note: Groups were based on presence of subthreshold major depressive disorder (MDD) or MDD diagnosis in at least one of four annual assessments.
Ratings were 1=not at all, 5=very much so.
Higher RT=longer latency to rate. Mean IQ was covaried.
A significant face-emotion x instruction-set effect was found on ratings (F[6, 1224]=5.78, p<.001). These variations followed expectable patterns suggesting that participants performed the task appropriately (e.g., sad faces were rated as most sad, happy faces as least sad). Similarly, a significant face-emotion x instruction-set effect was found on RT (F[6, 1224]=3.18, p<.01).
Recognition task
A significant group effect was found on recognition accuracy, F(2,204)=3.49, p<.05. Girls with MDD in at least one of four assessment years had a lower accuracy for identifying previously-encoded emotional faces relative to girls with no MDD history (M=.81 versus .76, p<.01). There were no significant differences in accuracy between girls with a history of threshold and subthreshold MDD, or between girls with no MDD history and subthreshold MDD. None of the within-group effects were significant nor did they interact with group.
A similar repeated-measures ANOVA was conducted for RT to indicate recognition of whether a face was old or new. Neither within- or between-group effects were significant; the groups responded in a similar amount of time when indicating whether they recognized each of the different emotion faces.
Depressive Symptoms
Correlations between predictors and emotional-face memory indices are presented in Table 3. IQ, race, accuracy recognizing faces overall, and accuracy recognizing previously-encoded happy and sad faces correlated significantly with depression symptoms averaged over four years. These associations indicated that lower IQ scores, African-American race, and lower accuracy recognizing previously-encoded faces, particularly those depicting happiness and sadness, were associated with higher depression symptom levels. IQ and race were associated with depression symptom variability across time; specifically, lower IQ and African-American race were associated with greater variability.
Table 3.
Spearman Associations between IQ, Race, Face Memory, and Depression Symptoms across Four Years (N=213)
| Depression symptoms (Mean) | Depression symptoms (SD) | |
|---|---|---|
| IQ | −.392*** | −.239*** |
| Race (0=European-American; 1=African-American) |
.337*** | .169* |
| Face Memory Accuracy | ||
| Overall | −.177** | −.106 |
| Sad | −.242*** | −.093 |
| Fearful | −.063 | −.032 |
| Angry | −.075 | −.050 |
| Happy | −.145* | −.097 |
Note:
p<.05
p<.01
p<.001
Mean symptom levels
When entered on step one, IQ accounted for 8% of the variance in overall accuracy. Race did not significantly account for additional variance (Table 4). Depression symptom count across time was entered on step two, explaining an additional 2% of variance in overall accuracy. Specifically, a higher level of depression symptoms was associated with lower accuracy in remembering previously encoded faces. The race x depression interaction was entered on step three. Race did not moderate the association between depression and accuracy.
Table 4.
Standardized (S) and Unstandardized (US) Coefficients for Hierarchical Regression Analyses Predicting Face Memory Overall and by Emotion (N=211)
| Predictors |
||||||
|---|---|---|---|---|---|---|
| Step 1 |
Step 2 |
Step 3 |
||||
| Face Memory Accuracy |
IQ | Race | Depression Symptoms |
Depression Symptoms x Race |
R2 | FΔ |
| Overall | ||||||
| Step 1 β S/US | .28/.002 | .005/.001 | .08 | 8.49*** | ||
| Step 2 β S/US | .22/.001 | .04/.009 | −.18/−.01 | .10 | 5.85* | |
| Step 3 β S/US | .21/.001 | −.003/−.001 | −.25/−.02 | .11/.005 | .11 | 1.03 |
| Sad | ||||||
| Step 1 β S/US | .19/.002 | .03/.01 | .03 | 3.29* | ||
| Step 2 β S/US | .11/.001 | .07/.02 | −.23/−.02 | .08 | 9.99** | |
| Step 3 β S/US | .12/.001 | .11/.04 | −.17/−.02 | −.10/−.006 | .08 | 0.74 |
| Fearful | ||||||
| Step 1 β S/US | .24/.002 | −.03/−.01 | .07 | 7.26** | ||
| Step 2 β S/US | .25/.002 | −.03/−.01 | .03/.004 | .07 | 0.21 | |
| Step 3 β S/US | .24/.002 | −.11/−.04 | −.11/−.01 | .22/.02 | .08 | 3. 78ŧ |
| Angry | ||||||
| Step 1 β S/US | .12/.001 | −.03/−.01 | .02 | 1.96 | ||
| Step 2 β S/US | .09/.001 | −.02/−.006 | −.08/−.01 | .02 | 1.19 | |
| Step 3 β S/US | .09/.001 | −.03/−.01 | −.11/−.01 | .04/.003 | .03 | .10 |
| Happy | ||||||
| Step 1 β S/US | .17/.002 | .04/.02 | .03 | 2.71ŧ | ||
| Step 2 β S/US | .12/.001 | .08/.03 | −.18/−.02 | .05 | 6.03* | |
| Step 3 β S/US | .11/.001 | .04/.01 | −.26/−.03 | .11/.009 | .06 | 1.02 |
Note: Step 1 df=2, 208; Step 2 df=3, 207; Step 3 df=4, 206.
p<.05
p<.01
p<.001
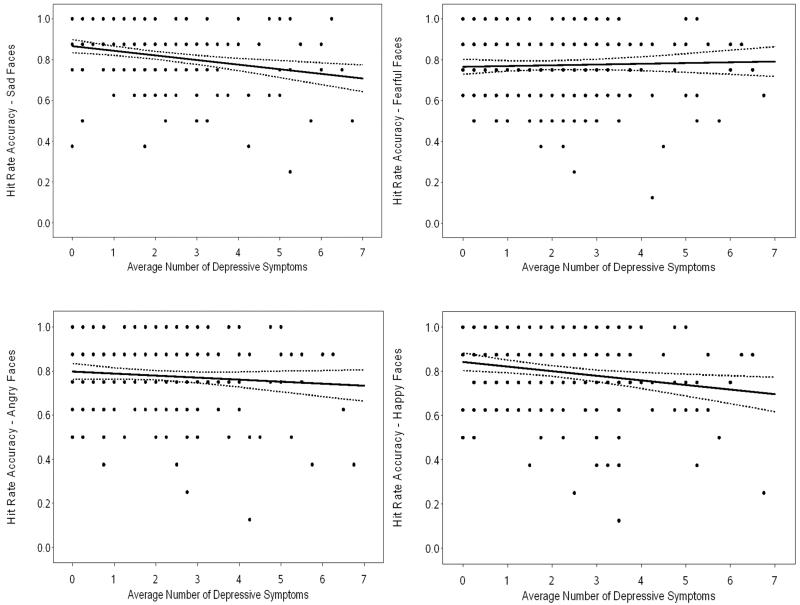
Regression analyses were similarly conducted for each emotion (Table 4). IQ significantly accounted for variance in recognition accuracy for sad faces. When depression symptoms were added on step two, it was the only factor that remained significantly associated with accuracy in recognizing previously encoded sad faces, accounting for an additional 5% of variance. Similar results were found for happy faces. IQ significantly accounted for variance in recognition accuracy on step one. When IQ, race, and depression symptoms were entered on step two, only depression symptoms remained associated with accuracy, accounting for an additional 2% of variance. In both models, higher depression symptom levels predicted lower accuracy when remembering previously encoded sad and happy faces, which is about a 15% decrease in accuracy from the lowest to highest levels of symptoms. For fearful faces, IQ was the only significant predictor. There were no significant predictors of accuracy in recognizing angry faces. Race did not moderate depression and accuracy for any emotion-specific memory indices. Figure 1 depicts the associations between depression symptoms and accuracy in recognizing previously-encoded faces depicting each emotion, adjusted for IQ and race.
Figure 1.
Age 12-13 Accuracy for Recognizing Emotional Faces Predicted by Age 9-12 Depressive Symptoms
Symptom variability
A similar set of regressions tested whether variability in depression symptoms over time, and the potential moderating effect of race, predicted face memory. IQ was the only significant predictor associated with overall accuracy and accuracy for fearful and sad faces after depression variability and race x depression variability were added. None of the predictors were associated with memory performance. Thus, depression symptom variability was not associated with memory function and race did not moderate depression variability and memory.
Effects of RT
Correlations tested associations between RT, face memory, and depression. Overall RT for recognizing whether a face was old or new (regardless of emotion) was not associated with IQ, race, depression symptoms, or depression symptom variability. RT for happy faces was associated with depression variability (ρ=.16, p=.02), whereby higher variability in depression symptoms was associated with a longer latency to indicate recognition of previously-encoded happy faces. A partial correlation (using a Spearman correlation matrix) between accuracy in recognizing faces that had depicted happiness and average depression symptoms, controlling for RT to indicate recognition, remained significant (r=−.14, p<.05). This suggests that a slowed RT for recognizing faces that had depicted happiness did not mediate the association between memory deficits for happy faces and depression symptoms.
Discussion
We tested hypotheses about associations between emerging depression and memory for faces that previously depicted various emotions in a large, well-characterized sample of girls. As predicted, the results indicated that girls’ depression symptoms during preadolescence were associated with compromised ability to recognize neutral faces of actors who depicted high-valence emotional expressions at encoding. These associations appeared specifically for sad and happy faces; depression symptoms were not associated with memory for faces that had shown fear or anger. Race did not moderate associations between face memory and emerging depression.
Results from the present study extend the existing data on memory deficits and depression in several ways. First, our novel face encoding and recognition task offers advantages over other paradigms. Many emotional memory tasks present emotional content using words; however, variability in reading and language abilities may be influenced by depression56 and verbal stimuli may have reduced emotional salience to adolescents.57 Using facial stimuli in the current task offset these methodological issues.
Second, facial stimuli can depict various emotions, allowing for emotion to be manipulated and other factors held constant. We designed the current task to include sad faces, which have not been used in previous iterations of this task. By doing so, we could assess response to stimuli that mimic the experience of depression. The current task also included different encoding instructions than in previously used renditions of this paradigm. These instructions cued participants to subjective and objective aspects of sadness. Again, the aim was to direct attention to sadness and assess whether deficits emerged during encoding or recognition. The task appeared successful, as illustrated by the finding that all participants, regardless of race or depression, encoded the stimuli similarly whereas face memory varied by depression symptom levels.
Third, prior studies indicated difficulty with variants of this recognition paradigm, with participants showing better abilities to identify novel faces as novel, possibly since all photographs viewed at recognition are truly novel. In contrast, recognizing photographs of previously seen actors, now depicting neutral expressions, is more difficult.36 In the current study’s task, we combined both recognition presentations, whereby face-pairs shown per trial included a previously seen face and a novel face and both faces displayed neutral expressions, an expression not seen during encoding.
Finally, the present study used a unique sample enriched for depression risk from a larger, community-based study not commonly found in cognitive neuroscience. Participants’ depression histories were well documented from 9-12 years of age. Moderate stability in depression symptoms during childhood has been observed in this sample and half of the sample was African-American; thus, the girls face high risk for MDD. In addition, the sample was well characterized on depression using a DSM-IV-based interview with high inter-rater reliability. Thus, depression symptom assessments were both clinically-sound and relevant to diagnosed depression, which is useful given the typically low base-rates of diagnosed depression during this peri-adolescent period.39
While the current methods center on memory for previously-encoded facial emotions, it is unlikely that the results reflect broad perturbations in face emotion processing. Relative to the current and prior findings on face-emotion memory, other work finds no association between adolescent MDD and face-emotion labeling deficits.58 This suggests that different neuropsychological processes may be implicated in different mood disorders during adolescence. For adolescent depression, memory for faces that had depicted different emotions is impaired whereas emotion labeling and initial encoding of emotion appears unaffected, as supported by our results indicating no MDD group differences in encoding.
Our results showing reduced memory for happy faces align with evidence of individual differences in positive emotionality and depression in youth. For example, a recent study showed that deficits in generating specific memories to positive cues, but not to negative cues, during an autobiographical memory test were associated prospectively with depression symptoms.59 Cross-sectional neuroimaging studies of adolescent depression have revealed blunted striatal and OFC response in depressed adolescents during reward processing, possibly reflecting lower motivation for positive stimuli.60 In addition to valence, stimulus arousal level may influence recognition memory. For example, happy and sad faces may be perceived as less arousing than angry and fearful faces, which suggests that the recognition deficits found here may occur for less arousing stimuli. Further work is needed that accounts for the arousal level of to-be-remembered stimuli.
The current study has some limitations. First, the encoding task had a small number of trials when parsed by emotion-type and instruction-set. Future work might include alternative designs with fewer emotion types, allowing more emotion replicates or more trials overall. Alternative viewing designs could also further specify the psychological processes engaged during encoding. However, pilot data showed poor memory unless adolescents repeatedly viewed each face, but rating the same face repeatedly reduced adolescents’ attention with each viewing, suggesting that performing the same rating multiple times does not guarantee invariant psychological processes across viewings. Thus, varying instructions helps maintain participants’ attention throughout the task.
Second, it was not possible to calculate a measure of signal detection threshold (d’) because the recognition task presented face stimuli in pairs. The d’ measure represents the degree to which an individual differentiates true targets from distractor items.61 This difference in assessing memory limits direct comparison to previous work that measured memory performance with d’. Third, the potential role of comorbid conditions (e.g., anxiety, disruptive behaviors) was not tested. We have previously reported that early emerging depression symptoms are stable39 and are the best predictors, in comparison to anxiety symptoms, of adolescent-onset depression.62 Nonetheless, co-occurring anxiety and depression symptoms may yield a unique profile of face-emotion memory performance. Fourth, other memory tests were not collected; as such, future work would benefit by their inclusion to test for memory deficits specific to emotional faces. Finally, memory performance was assessed at one time-point and depression symptoms at four annual time-points. More research is needed to assess depression and memory performance at multiple time-points in order to prospectively track their association across adolescence.
Our findings have clinical implications. Targeting symptoms and sub-syndromal forms of depression in preadolescence before development of the full disorder in adolescence may be one avenue toward reducing the morbidity associated with this disorder. Research that provides further evidence for the validity of early emerging symptoms as targets of intervention, and reveals processing deficits associated with early emerging symptoms may lead to opportunities to propose and test novel prevention strategies. Results from the present study have the potential to improve identification of early vulnerability markers and endophenotypes of depression in females, particularly for those at heightened risk due to stressful living situations.
In summary, adolescent girls at risk for depression demonstrate overall and emotion-specific memory deficits for previously-encoded faces that were associated with depression symptoms ascertained longitudinally. This association remained after accounting for IQ and race, and was not moderated by race. These findings lend themselves to further hypotheses about the neurophysiological mechanisms linking memory deficits and depression in adolescent females. For example, future neuroimaging work could use this face-emotion task to test whether functional neural alterations account for the observed associations.
Acknowledgments
This study was supported by National Institute of Mental Health (NIMH) grant R01 MH66167 (KK), the NIMH Intramural Research Program, and NIMH grant K99 MH080076 (AG).
We thank the families participating in the Learning about Girls’ Emotions Study. We also thank Harvey Iwamoto and Stephen Fromm from the NIMH for task development and programming assistance, as well as Michal Rischal, Susan Klostermann, and Amanda Hinze from the University of Pittsburgh for data collection assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Guyer, Grimm, Pine, and Keenan and Ms. Choate report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Dr. Amanda E. Guyer, University of California-Davis
Ms. Victoria R. Choate, National Institute of Mental Health
Dr. Kevin J. Grimm, University of California-Davis
Dr. Daniel S. Pine, National Institute of Mental Health
Dr. Kate Keenan, University of Chicago
References
- 1.Kessler RC, Avenevoli S, Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001 Jun 15;49(12):1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993 Oct-Nov;29(2-3):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 3.Ge X, Conger RD, Elder GH., Jr. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001 May;37(3):404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- 4.Hankin BL, Abramson LY. Development of gender differences in depression: an elaborated cognitive vulnerability-transactional stress theory. Psychol Bull. 2001 Nov;127(6):773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- 5.Costello EJ, Erkanli A, Angold A. Is there an epidemic of child or adolescent depression? J Child Psychol Psychiatry. 2006;47(12):1263–1271. doi: 10.1111/j.1469-7610.2006.01682.x. [DOI] [PubMed] [Google Scholar]
- 6.Keenan K, Hipwell AE. Preadolescent clues to understanding depression in girls. Clin Child Fam Psychol Rev. 2005 Jun;8(2):89–105. doi: 10.1007/s10567-005-4750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeNavas-Walt C, Proctor BD, Smith J, U.S. Census Bureau . In: Current Population Reports, P60-233, Income, Poverty, and Health Insurance Coverage in the United States: 2006. Office USGP, editor. Washington, DC: 2007. [Google Scholar]
- 8.Grant KE, Compas BE, Thurm AE, McMahon SD, Gipson PY. Stressors and child and adolescent psychopathology: measurement issues and prospective effects. J Clin Child Adolesc Psychol. 2004 Jun;33(2):412–425. doi: 10.1207/s15374424jccp3302_23. [DOI] [PubMed] [Google Scholar]
- 9.Williams DR, Gonzalez HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007 Mar;64(3):305–315. doi: 10.1001/archpsyc.64.3.305. [DOI] [PubMed] [Google Scholar]
- 10.Pastor PN, Reuben CA, Falkenstern A. Parental reports of emotional or behavioral difficulties and mental health service use among U.S. school-age children. In: Manderscheid RW, Berry JT, editors. Mental Health, United States. U.S. Department of Health and Human Services; Rockville: 2004. [Google Scholar]
- 11.Sen B. Adolescent propensity for depressed mood and help seeking: race and gender differences. J Ment Health Policy Econ. 2004 Sep;7(3):133–145. [PubMed] [Google Scholar]
- 12.Grant KE, Lyons AL, Finkelstein JAS, et al. Gender differences in rates of depressive symptoms among low-income, urban, African American youth: A test of two mediational hypotheses. Journal of Youth and Adolescence. 2004 Dec;33(6):523–533. [Google Scholar]
- 13.Joormann J, Teachman BA, Gotlib IH. Sadder and less accurate? False memory for negative material in depression. J Abnorm Psychol. 2009 May;118(2):412–417. doi: 10.1037/a0015621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingram RE, Miranda J, Segal ZV. Cognitive vulnerability to depression. Guilford Press; New York: 1998. [Google Scholar]
- 15.Jacobs RH, Reinecke MA, Gollan JK, Kane P. Empirical evidence of cognitive vulnerability for depression among children and adolescents: a cognitive science and developmental perspective. Clin Psychol Rev. 2008;28:759–82. doi: 10.1016/j.cpr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas BW, Canli T. Emotional memory function, personality structure and psychopathology: a neural system approach to the identification of vulnerability markers. Brain Res Rev. 2008 Jun;58(1):71–84. doi: 10.1016/j.brainresrev.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- 18.Gotlib IH, Gilboa E, Sommerfield B. Cognitive functioning in depression: Nature and origins. In: Davidson RJ, editor. Anxiety, depression, and emotion. Oxford University Press; New York: 2000. pp. 133–163. [Google Scholar]
- 19.Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000 Oct 1;48(7):674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- 20.Bradley BP, Mogg K, Williams R. Implicit and explicit memory for emotion-congruent information in clinical depression and anxiety. Behav Res Ther. 1995 Sep;33(7):755–770. doi: 10.1016/0005-7967(95)00029-w. [DOI] [PubMed] [Google Scholar]
- 21.Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998 Jul;11(3):111–119. [PubMed] [Google Scholar]
- 22.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003 Apr;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 23.Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Dev. 1999 May-Jun;70(3):660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- 24.Bishop SJ, Dalgleish T, Yule W. Memory for emotional stories in high and low depressed children. Memory. 2004 Mar;12(2):214–230. doi: 10.1080/09658210244000667. [DOI] [PubMed] [Google Scholar]
- 25.Hammen C. Self-cognitions, stressful events, and the prediction of depression in children of depressed mothers. J Abnorm Child Psychol. 1988 Jun;16(3):347–360. doi: 10.1007/BF00913805. [DOI] [PubMed] [Google Scholar]
- 26.Hughes J, Worchel F, Stanton S, Stanton H, Hall B. Selective memory for positive and negative story content in children with high self- and peer-ratings of symptoms of depression. School Psychology Quarterly. 1990;5:265–279. [Google Scholar]
- 27.Hipwell AE, Klostermann S, Sapotichne B, Battista D, Keenan K. Autobiographical memory as a marker of vulnerability for depressed mood in young girls. Journal of Clinical Child and Adolescent Psychology. 2010 doi: 10.1080/15374416.2011.546037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuyken W, Howell R, Dalgleish T. Overgeneral autobiographical memory in depressed adolescents with, versus without, a reported history of trauma. J Abnorm Psychol. 2006 Aug;115(3):387–396. doi: 10.1037/0021-843X.115.3.387. [DOI] [PubMed] [Google Scholar]
- 29.Park RJ, Goodyer IM, Teasdale JD. Categoric overgeneral autobiographical memory in adolescents with major depressive disorder. Psychol Med. 2002 Feb;32(2):267–276. doi: 10.1017/s0033291701005189. [DOI] [PubMed] [Google Scholar]
- 30.Cole DA, Jordan AE. Competence and memory: integrating psychosocial and cognitive correlates of child depression. Child Dev. 1995 Apr;66(2):459–473. doi: 10.1111/j.1467-8624.1995.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 31.Nelson CA, Bloom FE, Cameron JL, Amaral D, Dahl RE, Pine D. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Dev Psychopathol. 2002 Summer;14(3):499–520. doi: 10.1017/s0954579402003061. [DOI] [PubMed] [Google Scholar]
- 32.Phelps EA, Anderson A. Emotional Memory: What does the amygdala do? Curr Biol. 1997;7:223–233. doi: 10.1016/s0960-9822(06)00146-1. [DOI] [PubMed] [Google Scholar]
- 33.LeDoux JE. Emotion, memory and the brain. Sci Am. 1994 Jun;270(6):50–57. doi: 10.1038/scientificamerican0694-50. [DOI] [PubMed] [Google Scholar]
- 34.Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- 35.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pine DS, Lissek S, Klein RG, et al. Face-memory and emotion: associations with major depression in children and adolescents. J Child Psychol Psychiatry. 2004 Oct;45(7):1199–1208. doi: 10.1111/j.1469-7610.2004.00311.x. [DOI] [PubMed] [Google Scholar]
- 37.Roberson-Nay R, McClure EB, Monk CS, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An FMRI study. Biol Psychiatry. 2006 Nov 1;60(9):966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Cohen P, Cohen J. The clinician’s illusion. Arch Gen Psychiatry. 1984 Dec;41(12):1178–1182. doi: 10.1001/archpsyc.1984.01790230064010. [DOI] [PubMed] [Google Scholar]
- 39.Keenan K, Hipwell A, Feng X, et al. Subthreshold symptoms of depression in preadolescent girls are stable and predictive of depressive disorders. J Am Acad Child Adolesc Psychiatry. 2008 Dec;47(12):1433–1442. doi: 10.1097/CHI.0b013e3181886eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keenan K, Hipwell AE, Hinze AE, Babinski DE. The association of pain and depression in preadolescent girls: moderation by race and pubertal stage. J Pediatr Psychol. 2009 Aug;34(7):727–737. doi: 10.1093/jpepsy/jsn111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angold A, Costello EJ, Messer SC, Pickles A. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International Journal of Methods in Psychiatric Research. 1995;5:237–249. [Google Scholar]
- 42.Gadow KD, Sprafkin J. Child Symptom Inventory. State University of New York at Stony Brook; Stony Brook, NY: 1996. [Google Scholar]
- 43.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997 Jul;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 44.Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning, I: methodology and validation in healthy people. Neuropsychopharmacology. 2001;25(5):766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 45.Tottenham N, Borscheid A, Ellertsen K, Marcus DJ, Nelson CA. Categorization of facial expressions in children and adults: establishing a larger stimulus set; Paper presented at: Cognitive Neuroscience Society Annual Meeting; San Francisco, CA. April 15, 2002. [Google Scholar]
- 46.Ekman P, Friesen WV. Pictures of Facial Affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- 47.Wechsler D. WISC-III: Wechsler Intelligence Scale for Children-3rd Edition Manual. 3rd ed. Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- 48.Donders J, Strom D. The effect of traumatic brain injury on children with learning disability. Pediatr Rehabil. 1997 Jul-Sep;1(3):179–184. doi: 10.3109/17518429709167356. [DOI] [PubMed] [Google Scholar]
- 49.Kaufman AS, Kaufman JC, Balgopal R, McLean JE. Comparison ofthree WISC-III short forms: Weighing psychometric, clinical, and practicalfactors. J Clin Child Psych. 1996;25:97–105. [Google Scholar]
- 50.Petersen AC, Crockett LJ, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 51.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 52.Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developental Science. 2006;10:30–56. [Google Scholar]
- 53.Tanner JM. Growth at Adolescence. Blackwell Scientific Publications; Oxford: 1962. [Google Scholar]
- 54.Robertson E, Skinner M, Love M, Elder G, Conger R, Dubas J, et al. The Pubertal Development Scale: A rural and suburban comparison. Journal of Early Adolescence. 1992;12:174–186. [Google Scholar]
- 55.Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child Dev. 1987 Jun;58(3):829–841. [PubMed] [Google Scholar]
- 56.Pine DS, Kentgen LM, Bruder GE, et al. Cerebral laterality in adolescent major depression. Psychiatry Res. 2000 Mar 6;93(2):135–144. doi: 10.1016/s0165-1781(00)00101-3. [DOI] [PubMed] [Google Scholar]
- 57.Stattin H. Developmental trends in the appraisal of anxiety-provoking situations. J Pers. 1984 Mar;52(1):46–57. doi: 10.1111/j.1467-6494.1984.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 58.Guyer AE, McClure EB, Adler AD, et al. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. 2007 Sep;48(9):863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 59.Hipwell AE, Klostermann S, Sapotichne B, Battista D, Keenan K. Autobiographical memory as a marker of vulnerability for depressed mood in young girls. Journal of Clinical Child and Adolescent Psychology. doi: 10.1080/15374416.2011.546037. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009 Jan;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988 Mar;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 62.Keenan K, Feng X, Hipwell A, Klostermann S. Depression begets depression: comparing the predictive utility of depression and anxiety symptoms to later depression. J Child Psychol Psychiatry. 2009 Sep;50(9):1167–1175. doi: 10.1111/j.1469-7610.2009.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]