Summary
In mammals, fusion of the mitochondrial outer membrane is controlled by two DRPs, MFN1 and MFN2, that function in place of a single outer membrane DRP, Fzo1 in yeast. We addressed the significance of two mammalian outer membrane fusion DRPs using an in vitro mammalian mitochondrial fusion assay. We demonstrate that heterotypic MFN1/MFN2 trans complexes possess greater efficacy in fusion as compared to homotypic MFN1 or MFN2 complexes. In addition, we show that the soluble form of the pro-apoptotic Bcl2 protein, Bax, positively regulates mitochondrial fusion exclusively through homotypic MFN2 trans complexes. Together, these data demonstrate functional and regulatory distinctions between MFN1 and MFN2 and provide insight into their unique physiological roles.
Introduction
Mitochondrial fusion regulates mitochondrial shape and distribution and also plays important roles in many aspects of mitochondrial function, including mitochondrial DNA (mtDNA) maintenance and thus, is critical for overall cell physiology (Benard and Karbowski, 2009; Chen and Chan, 2009; Hoppins et al., 2007). Two highly conserved dynamin-related protein (DRP) families are essential for fusion: the mitochondrial outer membrane DRPs MFN1/MFN2 (Fzo1, yeast) and the inner membrane DRP OPA1 (Mgm1, yeast). The critical cellular roles of fusion are underscored by the observation that loss of mitochondrial fusion results in embryonic lethality in mice (Chen et al., 2003). In addition, point mutations in the fusion DRPs, MFN2 and OPA1 cause two distinct neurodegenerative diseases, Charcot-Marie-Tooth Type 2A (CMT2A) and dominant optic atrophy (DOA), respectively (Amati-Bonneau et al., 2009; Cartoni and Martinou, 2009; Chan, 2006).
In mammalian cells there are two homologous outer membrane DRPs, MFN1 and MFN2 that function in place of a single outer membrane DRP, Fzo1, in the simpler yeast cell. While it is clear that both MFN1 and MFN2 function in mitochondrial fusion and that they form both homo and heterotypic complexes (Chen et al., 2003), several lines of evidence suggest that they are functionally distinct. Data from in vitro analyses suggest that MFN1 mediates mitochondrial tethering more efficiently than MFN2, suggesting the possibility that homotypic cis and trans MFN1 interactions may be more efficient and/or stable than the cognate MFN2 interactions (Ishihara et al., 2004). This apparent difference may be related to the role of MFN2 in the tethering of mitochondria to ER in cells (de Brito and Scorrano, 2008). In addition, mutations in MFN2 uniquely result in the neurodegenerative disease, CMT2A (Cartoni and Martinou, 2009). Mitochondrial fusion defects associated with MFN2CMT2A mutants can be complemented in cells by expression of MFN1, but not MFN2, suggesting that each MFN is functionally distinct within a hetero-oligomeric complex (Detmer and Chan, 2007). Consistently, overexpression of MFN1 rescues the neuronal axon mitochondrial transport defect associated with Mfn2CMT2A mutations (Misko et al., 2010). Finally, although MFN1 and MFN2 are both ubiquitously expressed in tissues, the relative level of MFN1 and MFN2 expression in a given tissue varies significantly. For example, MFN2 is the prevalent species in heart, skeletal muscle and brain (Eura et al., 2003; Lein et al., 2007). The fact that MFN2 is predominant in the brain raises the possibility that the neuronal-specific phenotypes associated with MFN2 mutations arise due to an accumulation of non-functional MFN2 homotypic complexes (Eura et al., 2003; Santel et al., 2003).
Mitochondrial fusion is also both positively and negatively regulated by cellular signaling pathways, including those that regulate stress responses, cell division and cell death, although the regulatory mechanisms are not understood (Cerveny et al., 2007). Stress conditions such as UV exposure or cycloheximide treatment stimulate mitochondrial fusion, resulting in the formation of a hyperfused mitochondrial structure and improved cell survival (Tondera et al., 2009). In contrast, apoptosis and mitochondrial outer membrane permeabilization (MOMP) negatively regulate mitochondrial fusion (Karbowski et al., 2004). Conversely, in healthy cells the pro-apoptotic Bcl2 protein, Bax, positively regulates mitochondrial fusion, indicating that Bcl2 proteins may also play important housekeeping roles in the regulation of mitochondrial dynamics (Karbowski et al., 2006). To explore the functional and mechanistic differences between MFN1 and MFN2 and to investigate how fusion is regulated, we recapitulated mammalian mitochondrial fusion in vitro using mitochondria derived from mouse embryonic fibroblasts (MEFs), where knockout lines of the essential fusion genes have been created.
Results
Reconstitution and energetics of mammalian mitochondrial outer and inner fusion in vitro
To dissect the mammalian mitochondrial fusion machines and fusion regulatory mechanisms, we developed a direct in vitro visual content mixing assay for outer and inner membrane fusion similar to the established yeast-based in vitro assay (Meeusen et al., 2004). We utilized mitochondria isolated from MEFs, as knockout lines are established for all essential genes encoding fusion components, thus enabling genetic analysis of mitochondrial fusion. We utilized established and newly created stable MEF lines that expressed mitochondrial matrix targeted EGFP (m-EGFP) or matrix targeted Discosoma sp. red fluorescent protein (m-dsRed). Crude preparations of labeled mitochondria were isolated by differential centrifugation, mixed in equivalent amounts, centrifuged into a pellet, and resuspended in a buffer containing an energy regeneration system (ERS), ATP and GTP (stage 2, S2 conditions). Reactions were analyzed by fluorescence microscopy and fusion events were scored by colocalization of the m-EGFP and m-dsRed signals in three dimensions in several random fields (Figure 1A). Several features of mitochondria containing co-localized fluorophores were consistent with fusion events, including possessing a greater diameter and a decreased pixel intensity of each fluorophore, likely due to dilution (Figure S1A and B). Mitochondrial fusion, which was quantified by dividing the number of fusion events by the total number of mitochondria in the field was efficient and time dependent with an average value of 20% of the total population fused by 60 minutes (Figure S1C). Although the assay is quantitative, we can only measure a fusion event between differentially labeled organelles, thus we are underestimating the overall fusion efficiency.
Figure 1. Mammalian mitochondrial outer and inner fusion in vitro.
(A) Schematic representation of content mixing in vitro fusion assay (top) and fluorescent images of a fusion reaction with wild type m-GFP and m-dsRed mitochondria in the absence of exogenous energy (bottom, left) and with the addition of an energy regeneration system and nucleotides (bottom, right). Fusion events are indicated with arrows. (B) Energetic requirements of mitochondrial fusion in vitro. Fusion efficiency is described as a percentage of the standard reaction which contains an energy regeneration system, ATP and GTP and was performed in parallel. Error bars show mean + standard error of at least three experiments and statistical significance was determine by paired t-test analysis *P<0.05 ** P=0.002 *** P=0.0002 **** P<0.0001. (C) Western blot analysis of mitochondrial proteins during in vitro fusion reactions. Mitochondrial fusion reactions without (standard) or with valinomycin 1 mM (val), 1 mM nigericin (nig) or 100 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP) were stopped at the indicated times and proteins were subject to SDS-PAGE and Western analysis with the indicated antibodies. (D) Electron micrograph images of a fusion reaction with wild type mitochondria in the absence of exogenous energy. Representative images of outer membrane fusion events are shown. Scale bar represents 5μm. (E) Energetic requirements of mitochondrial outer membrane fusion in vitro. Outer membrane fusion efficiency for each condition is described as a percentage of the standard reaction performed in parallel. Error bars show mean + standard error of at least two independent experiments and statistical significance * (P=0.0012) was determined by One way Anova analysis.
As expected, mitochondrial fusion in vitro was dependent on the addition of exogenous nucleotide, with ERS alone supporting minimal fusion activity. Specifically, addition of exogenous GTP, but not ATP, in the presence of an ERS supported fusion in vitro and addition of the non-hydrolyzable GTP analog GMP-PCP (β,γ-methyleneguanosine 5′-triphosphate) in the presence of ERS and ATP did not support mitochondrial fusion, consistent with the essential roles of the DRPs, MFN1, MFN2 and OPA1 in fusion (Figure 1B)(Chen et al., 2003; Song et al., 2009). In contrast, mitochondrial fusion efficiency was not affected by the non-hydrolyzable analog of ATP, adenosine 5′-O-(3-thiotriphosphate), consistent with our data indicating that ATP is not required (Figure 1B ATPγS). Treatment with the electron transport chain uncoupler carbonylcyanide m-chlorophenylhydrazone (CCCP), which abolished the mitochondrial membrane electrochemical potential, as assessed by a decrease in labeling with the potential-sensitive fluorophore Mitotracker, inhibited mitochondrial fusion in vitro, consistent with a requirement for membrane potential for mitochondrial fusion in intact mammalian cells (Figure 1B and Figure S2)(Malka et al., 2005). We further examined whether a requirement for the electrical gradient and/or the proton gradient was required using valinomycin, a K+ ionophore and nigericin, an electroneutral K+/H+ ionophore, respectively. Addition of either significantly attenuated the mitochondrial electrochemical potential, as assessed by a decrease in labeling with the potential-sensitive fluorophore, Mitotracker, and impaired mitochondrial fusion in vitro, indicating that the electrochemical potential across the mitochondrial inner membrane is essential for fusion (Figure 1B and Figure S2). These energetic requirements for mitochondrial fusion are consistent with recent observations made using an in vitro mammalian mitochondrial fusion assay that requires bimolecular complementation of a fluorescent marker (Schauss et al., 2010).
Mitochondrial fusion requires both long membrane-anchored and soluble short isoforms of OPA1; the short isoforms are generated during import and sorting to the inner membrane by constitutive proteolytic processing pathway, primarily via the i-AAA protease, YMEL1 (Duvezin-Caubet et al., 2007; Griparic et al., 2007; Song et al., 2007). Recently it was shown that long OPA1 isoforms are also processed in a post-biogenesis manner via an inducible proteolytic pathway that depends on the ATP-independent zinc metalloprotease protease, OMA1. This OMA1-dependent inducible proteolytic pathway is triggered by dissipation of membrane potential or depletion of mitochondrial ATP and results in the conversion of long OPA1 isoforms to soluble short OPA1 isoforms, which in turn results in impaired fusion and mitochondrial fragmentation in cells (Ehses et al., 2009; Head et al., 2009). One model proposed for the mechanism of the OMA1 inducible proteolytic pathway is that in energized mitochondria OMA1 is unstable and that the loss of membrane potential stabilizes newly synthesized OMA1, which in turn cleaves long OPA1 (Head et al., 2009). To address this model, we examined the stability of OPA1 long isoforms by fixed time point Western analysis following dissipation of the inner membrane potential in in vitro mitochondrial fusion reactions (Figure 1C). Both outer membrane fusion DRPs MFN1 and MFN2 were stable for the duration of analysis in the presence or absence of ionophore treatment (Figure 1C). In contrast, OPA1 long isoforms species specifically and rapidly decreased in ionophore treated samples in comparison to untreated mitochondria (Figure 1C). These data indicate that the OMA1-dependent inducible proteolytic processing pathway is functional under our in vitro conditions in the absence of cytosol. Thus, it is unlikely that the de novo synthesis of OMA1 and OMA1 instability play significant roles in the regulation of long OPA1 processing. In addition, our data indicate that membrane potential, at least in part, regulates mammalian mitochondrial fusion via its role in the maintenance of the OPA1 long isoforms in contrast to yeast mitochondrial fusion, where membrane potential is thought to exclusively play a direct and essential role.
We have shown previously that yeast mitochondrial outer membrane fusion events occur in the absence of exogenous energy and ERS previously in vitro, and thus, outer membrane fusion can be separately staged and analyzed (stage 1, S1)(Meeusen et al., 2004). To determine if we could stage mammalian outer membrane fusion in vitro, we subjected mammalian mitochondria to S1 conditions in which the pellet is resuspended in the absence of ATP, GTP and the ERS and examined reactions by thin section electron microscopy (EM). Under these conditions, we identified outer membrane fused structures by the characteristic features of two separate matrices surrounded by a single, continuous outer membrane (Figure 1D). The abundance of these double-matrix structures was significantly reduced by the addition of non-hydrolyzable GTP, but not ATP analogs, consistent with the interpretation that they are generated via MFN-driven outer membrane fusion (Figure 1E, GMPPCP and ATPγS, respectively). Dissipation of inner membrane potential also decreased mitochondrial outer membrane fusion efficiency, although the decrease was substantially less than observed for inner membrane fusion efficiency under the same conditions (compare Figure 1B and Figure 1E). This partial inhibition is consistent with previous reports suggesting that outer membrane fusion is not abolished by CCCP treatment (Malka et al., 2005). Based on our analysis of the energetics of outer and inner membrane fusion, it seems likely that multiple regulatory mechanisms exist to link the outer and inner membrane mitochondrial fusion to mitochondrial dysfunction.
The roles of fusion DRPs
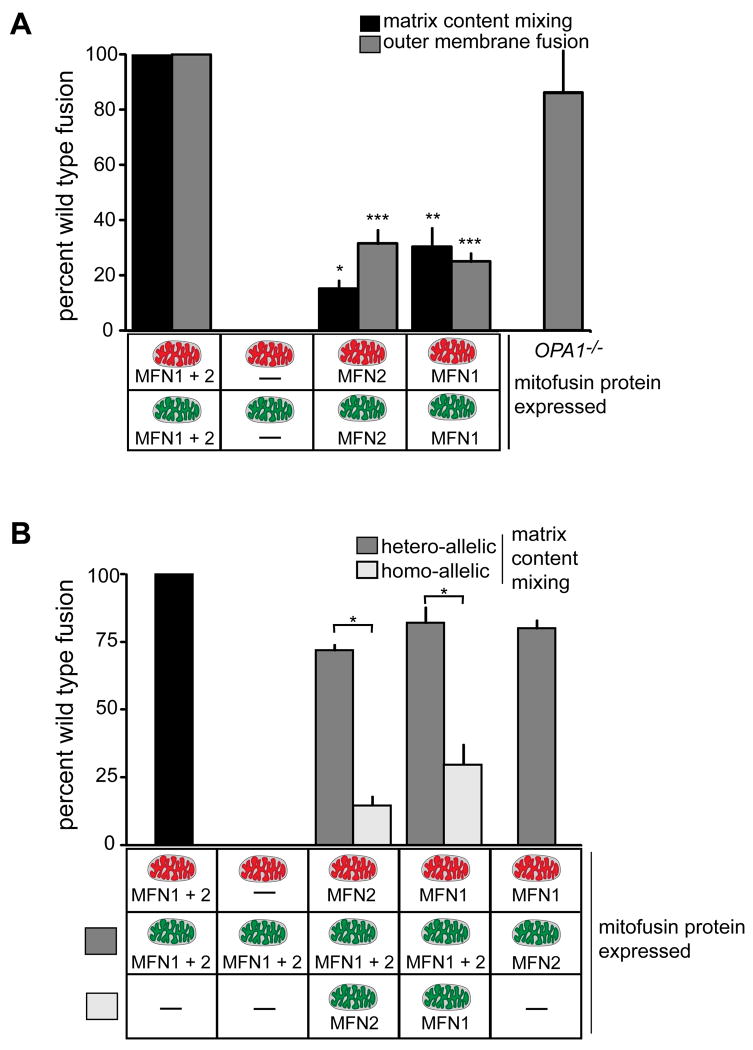
We examined the roles of the outer and inner membrane fusion DRPs by analyzing mitochondria isolated from MEF fusion DRP gene knockout lines (Chen et al., 2003; Song et al., 2009). There was no observable mitochondrial fusion activity in vitro in reactions containing mitochondria lacking both outer membrane fusion DRPs (Figure 2A). In contrast, reactions containing mitochondria expressing either MFN1 or MFN2 possessed a low level of outer and inner membrane fusion activity as compared to wild type (Figure 2A). As expected, analysis of mitochondrial fusion activity in reactions containing mitochondria isolated from OPA1 null MEF indicated that mitochondria lacking OPA1 are competent for outer membrane fusion, but are completely blocked for inner membrane fusion (Figure 2A, OPA1-/-) (Song et al., 2007). These findings demonstrate that the in vitro mammalian fusion assay faithfully recapitulates mitochondrial fusion events in cells.
Figure 2. Analysis of the role of MFN1, MFN2 and OPA1 in mitochondrial fusion.
(A) Mitochondria were isolated from wild type, Mfn1-/-Mfn2+/+, Mfn1+/+Mfn2-/-, Mfn1-/-Mfn2-/- or OPA1-/- cells and subjected to either S2 fusion conditions (black bars) or S1 fusion conditions (grey bars) to assess mitochondrial inner and outer versus mitochondrial outer membrane fusion efficiency, respectively. Data are expressed as a percent of the wild type control reactions performed in parallel and statistical significance was determined by paired t-test analysis. *P=0.0002 **P=0.0017 ***P=0.0095 (B) Mitochondria isolated from the indicated cell lines were subjected to S2 fusion conditions in hetero-allelic reactions (grey bars) to assess the requirement for each protein on mitochondria fusion partners. For comparison, results are shown with data from homotypic fusion experiments performed in parallel from (A) (light grey bars) and wild type control (black bar). For both, error bars show mean + standard error of at least three independent experiments and Paired t-test analysis was performed to determine statistical significance *P=0.0049.
To address whether functional differences exist between MFN1 and MFN2, we examined the fusion efficiency of heteroallelic reactions containing mitochondria expressing different combinations of these two outer membrane fusion DRPs. No fusion was observed in heteroallelic reactions containing wild type mitochondria and mitochondria lacking both MFN1 and MFN2, indicating that at least one MFN is required on each fusion partner, which is consistent with the model that MFN-trans interactions are essential for outer membrane fusion (Figure 2B) (Chen et al., 2005). Interestingly, in heteroalleleic reactions of wild type mitochondria with mitochondria expressing either MFN1-only or MFN2-only, the level of fusion was comparable to wild type controls and significantly higher than that observed for either homoallelic MFN1-only or MFN2-only reactions (Figure 2B, compare grey and white bars). Importantly, as assessed by Western analysis, the levels of Mfn1 and Mfn2 in isolated Mfn1+/+Mfn2-/- and Mfn1-/-Mfn2+/+ mitochondria were comparable to wildtype mitochondria, respectively (Figure S3)(Chen et al., 2003). Thus, these data support the conclusion that MFN1-MFN2 heterotypic complexes formed in trans are more efficacious for mitochondrial fusion than the MFN1 or MFN2 only trans complexes formed in homoallelic reactions. To further test this idea, we measured fusion activity in reactions containing MFN2-only mitochondria and MFN1-only mitochondria. Significantly and consistently, the fusion activity in this heteroallelic reaction was comparable to that observed in wild type reactions (Figure 2B). This observation is consistent with the observation that expression of only wild type MFN1 complements the mitochondrial fusion defect caused by MFN2CMT2A mutant proteins in MEFs (Detmer and Chan, 2007) and extends our understanding of the significance of two MFNs under conditions of normal homeostasis.
Soluble Bax positively regulates mitochondrial fusion
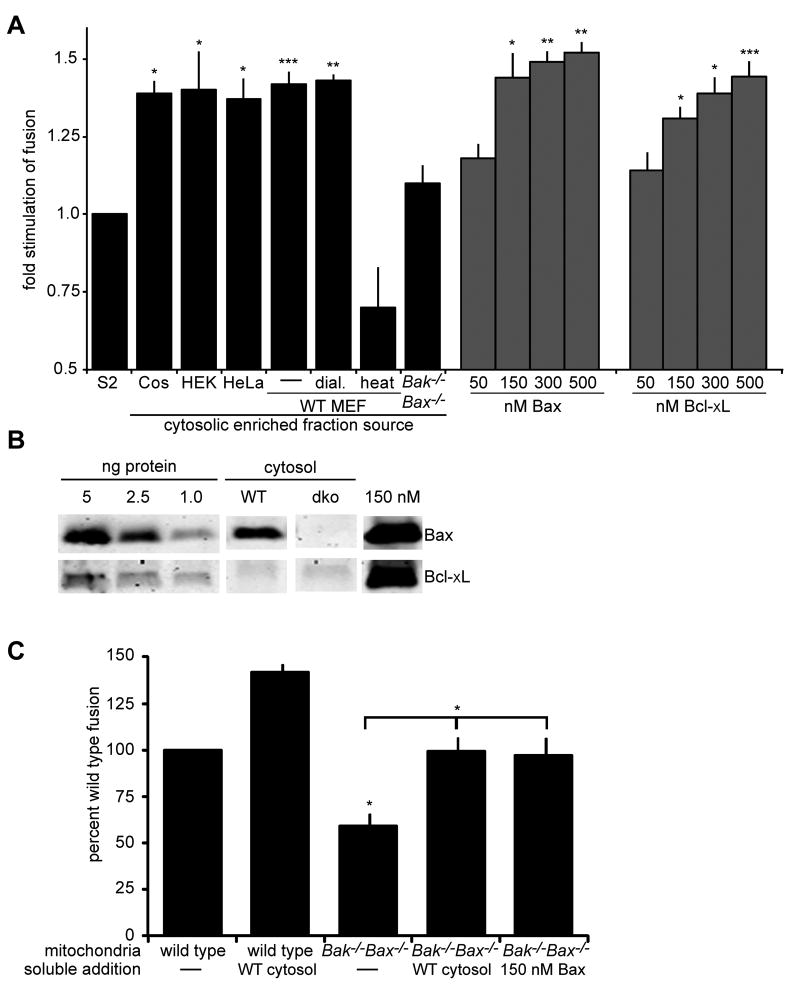
We examined the dependence of mitochondrial fusion on cytosol-enriched extracts isolated by differential centrifugation to remove nuclei and heavy intracellular membranes. Wild type MEF cytosolic-enriched extract significantly stimulated mitochondrial fusion, indicating the presence of a regulatory factor(s) (Figure 3A, compare S2 and WT MEF cytosolic enriched fraction). Cytosolic-enriched extract isolated from COS, HEK 293T and HeLa cells also stimulated mitochondrial fusion to a similar degree, suggesting that the regulatory factor(s) is ubiquitous (Figure 3A). MEF cytosolic-enriched extract maintained the ability to stimulate fusion following dialysis to remove species less than 10 kDa, but treatment of the cytosolic-enriched extract at 95°C for 15 minutes abolished the positive stimulatory effect, indicating that the regulatory factor(s) is proteinaceous in nature (Figure 3A, WT MEF dial. and heat, respectively).
Figure 3. Bax regulates mammalian mitochondrial fusion in vitro.
(A) Mitochondria were isolated from wild type cells and subjected to S2 fusion conditions in the presence of the indicated cytosolic enriched fraction (black bars) or the indicated concentration of recombinant purified Bax or Bcl-xL (grey bars). Error bars show mean + standard error of at least three independent experiments and Paired t-test analysis was performed to determine statistical significance *P≤0.05 **P≤0.004 ***P=0.008 (B) The indicated amounts of purified recombinant Bax (top) and Bcl-xL (bottom) and the equivalent amount of either cytosolic-enriched extract (wild type or Bak-/-Bax-/-) or recombinant purified Bax or Bcl-xL added to one fusion reaction were subject to SDS-PAGE and Western analysis with the indicated antibody. Some lanes of the same exposure from the same blots were rearranged to generate the figure. (C) Mitochondria isolated from either wild type or Bak-/-Bax-/- cells were subjected to S2 fusion conditions under standard conditions or with the addition of either wild type MEF cytosolic enriched fraction or 150 nM purified recombinant Bax. After 60 minutes, the fusion reactions were stopped, fusion was quantified and is expressed as a fold induction of each standard reaction, performed in parallel. Error bars show mean + standard error of at least three independent experiments and Paired t-test analysis was performed to determine statistical significance *P≤0.05
Bax is a pro-apoptotic effector of the Bcl2 protein family, which is predominantly cytosolic in healthy cells, but upon stimulation of apoptotic signaling, translocates and inserts into the outer mitochondrial membrane, where it mediates MOMP (Lovell et al., 2008). Bax has been previously implicated in the regulation of mitochondrial fusion and thus we reasoned that it may represent the regulatory factor in cytosolic-enriched extracts (Karbowski et al., 2006). Consistent with this possibility, we observed no stimulation of fusion in reactions containing cytosolic-enriched extract isolated from Bax-/-Bak-/- cells (Figure 3A compare S2 and Bax-/-Bak-/). Furthermore, addition of purified recombinant full-length untagged monomeric soluble Bax stimulated fusion of mitochondria in vitro in a dose dependent manner and to a similar extent as observed for cytosolic-enriched extract (Figure 3A, Bax, grey bars). In contrast, the addition of purified BSA or purified recombinant mitochondrial division dynamin, Drp1, had no observable effect on mitochondrial fusion efficiency (Figure S4) (Suzuki et al., 2000). Interestingly, addition of purified recombinant full-length monomeric soluble Bcl-xL also stimulated mitochondrial fusion in vitro in a dose dependent manner (Figure 3A). This finding is consistent with the observation that over expression of Bcl-xL increases mitochondrial connectivity in cells and suggests that Bcl2 proteins share the ability to positively modulate mitochondrial fusion (Berman et al., 2009; Delivani et al., 2006). In this context, Bcl-xL was undetectable by western analysis of wild type MEF cytosolic-enriched extract, consistent with the previously published work (Figure 3B) (Berman et al., 2009). In comparison, western analysis of equivalent volume of 150 nM recombinant Bcl-xL, which is a concentration in the range required to stimulate fusion, was readily detected (Figure 3B). Thus, Bcl-xL is not present in MEF cytosolic-enriched extract at a sufficient concentration to stimulate fusion in vitro under our conditions. In contrast, western analysis and comparison of equivalent volumes of wild type MEF cytosolic-enriched extract and 150 nM recombinant Bax indicated that Bax is present in cytosolic-enriched extract in the range of concentrations required for fusion stimulation. Indeed, using recombinant Bax as a standard, quantitative western analysis indicated that it is present at approximately 70 nM in cytosolic-enriched extract (Figure 3B). Together our results are consistent with the conclusion that Bax is the key mitochondrial fusion stimulatory factor in cytosolic-enriched extract.
To further test whether Bax is a positive mediator of mitochondrial fusion, we examined the in vitro fusion activity of mitochondria isolated from MEFs lacking both Bax and the mitochondrial membrane associated pro-apoptotic Bcl2 protein, Bak (Figure 3C). We observed that mitochondrial fusion efficiency of Bax-/-Bak-/- mitochondria was significantly decreased as compared to the fusion efficiency of wild type mitochondria (Figure 3C). Mitochondrial fusion efficiency of Bax-/-Bak-/- mitochondria was significantly stimulated to a similar extent by the addition of either wild type cytosolic-enriched extract or recombinant full length Bax (Figure 3C). These data are consistent with our hypothesis that Bax is the soluble cytosolic factor that positively regulates fusion in vitro. However, added recombinant Bax at levels where it maximally stimulated fusion of wild type mitochondria to reactions containing Bax-/-Bak-/- mitochondria did not fully restore fusion efficiency to the level observed for wild type mitochondria in the presence of wild type cytosolic-enriched extract, consistent with the recent report that membrane bound Bak may also play a positive regulatory role in fusion (Figure 3C)(Cleland et al., 2010).
Bax undergoes multiple conformational changes during the induction of apoptosis that coincide with its transition from the apoptotic inactive soluble protein to the apoptotic active membrane integrated oligomeric form. To gain insight into whether the soluble or membrane integrated conformations of Bax mediate the regulation of mitochondrial fusion, we utilized the characterized oligomerization deficient Bax mutants Bax63-65Ala and Bax92-94Ala (George et al., 2007). GFP-Bax63-65Ala and GFP-Bax92-94Ala were expressed in Bax-/-Bak-/- cells and cytosolic-enriched extracts were prepared by differential centrifugation and tested for their ability to modulate mitochondrial fusion in vitro (Figure 4A). Given that a small fraction of wild type Bax is present in crude preparations of mitochondria isolated from wild type MEFs (not shown), we utilized Bax-/-Bak-/- MEF mitochondria for in vitro fusion analysis. Consistent with our previous observation (Figure 3A), cytosolic-enriched extract prepared from the Bax-/-Bak-/- cells had no observable effect on mitochondrial fusion in vitro in reactions containing Bax-/-Bak-/- mitochondria as compared to cytosolic-enriched extract from wild type MEFs (Figure 4A, compare wild type and empty). Cytosolic-enriched extract prepared from cells overexpressing wild type GFP-Bax stimulated mitochondrial fusion to a greater extent than wild type cytosolic-enriched extract, consistent with our observations that Bax stimulates mitochondrial fusion in vitro in a dose-dependent manner in the 0-500 nM concentration range (Figure 4A, Bax-WT). Significantly, cytosolic-enriched extracts prepared from Bax-/-Bak-/- cells expressing GFP-Bax63-65A and GFP-Bax92-94A also stimulated mitochondrial fusion to a level similar to cytosolic-enriched extract from cells overexpressing wild type GFP-Bax extracts (Figure 4A, Bax63-65A and Bax92-94A). Together, these observations are consistent with a model in which the soluble, non-oligomerized form of Bax is the primary cytosolic regulator of mitochondrial fusion.
Figure 4. Soluble Bax stimulates mitochondrial fusion in vitro.
(A) Cytosolic enriched fractions prepared from wild type, Bak-/-Bax-/- cells or Bak-/-Bax-/- cells expressing the indicated GFP-Bax variant were separated by SDS-PAGE and subject to Western analysis with Bax-specific antibody (top panel). Mitochondria were isolated from Bak-/-Bax-/- cells and subjected to standard S2 fusion conditions in the absence (black bar) or presence of wild type MEF cytosolic enriched fraction or cytosolic enriched fractions from Bak-/-Bax-/- cells expressing the indicated GFP-Bax variant (light grey bars). Error bars show mean + standard error of at least three independent experiments and Paired t-test analysis was performed to determine statistical significance *P=0.04 **P≤0.025 (B) Mitochondria isolated from wild type MEFs were subject to S2 fusion conditions in the presence of the indicated amount of recombinant purified Bax1-2/L-6 or wild type Bax. After 60 minutes, the fusion reactions were stopped, fusion was quantified and is expressed as a fold induction of each standard reaction, all performed in parallel. Error bars show mean + standard error of at least three independent experiments and Paired t-test analysis was performed to determine statistical significance *P=0.05 **P=0.003.
To gain insight into whether conformational changes in soluble Bax are important for its ability to stimulate mitochondrial fusion, we tested full length recombinant Bax1-2/L-6, which is engineered to contain only two intermolecular disulfide bonds that tether helix 1 and 2 (F30C and L63C) and constrain the flexible loop between helix 1 and 2 to the tip of helix 6 (E44C and P130C). Structural analysis of Bax1-2/L-6 by NMR indicates that it is locked in its native soluble state and unable to undergo conformation changes associated with membrane insertion and oligomerization (Edlich et al., in preparation). Significantly and in contrast to wild type recombinant Bax, we observed no effect of added recombinant Bax1-2/L-6 on mitochondrial fusion in vitro at concentrations in which wild type pure recombinant Bax produces maximal stimulation (Figure 4B, compare light versus dark grey bars). This observation suggests a model where soluble Bax dynamically interacts with mitochondria and via conformational changes stimulates mitochondrial fusion.
Our findings raise the possibility that Bax membrane insertion and oligomerization serve to coordinately mediate mitochondrial outer membrane permeabilization (MOMP) and attenuate mitochondrial fusion during apoptosis. To test this, we examined the effect of cytosolic-enriched extract isolated from MEFs treated with the apoptotic stimulus, staurosporine (STS) for 4 hours, which, as assessed by western analysis of Bax and cytochrome c was in a time frame in which Bax translocated to mitochondria and initiated MOMP (Figure S5A). We observed no stimulation of mitochondrial fusion in vitro with this cytosolic-enriched extract from STS-treated cells, suggesting that the fusion-active confirmation of Bax is depleted and/or inactivated during apoptosis (Figure 5A, MEF + 2 μM STS). In contrast, in vitro addition of STS had no effect on the ability of wild type cytosolic-enriched extract to stimulate mitochondrial fusion (Figure 5A, 2 μM STS). Consistently, we observed that addition of pure recombinant Bax activator, tBid attenuated the ability of purified recombinant Bax, but not purified recombinant Bcl-xL to stimulate mitochondrial fusion in vitro, but purified recombinant tBid had no effect on mitochondrial fusion efficiency when added alone (Figure 5B). Western analysis of supernatant and pellet fractions produced by centrifugation of these fusion reactions with anti-cytochrome c indicates that a significant fraction of cytochrome c was observed only in the supernatant fraction of the reaction containing both t-Bid and Bax (Figure 5C). This finding is consistent with t-Bid dependent activation of Bax-mediated MOMP. Taken together, these findings indicate upon apoptosis, the soluble form of Bax that positively modulates fusion is depleted from and/or inactivated in the cytosol of cells due to apoptosis induced conformational changes and translocation to the mitochondrial outer membrane.
Figure 5. Apoptotic active Bax cannot stimulate mitochondrial fusion.
(A) Mitochondria were isolated from wild type cells and subjected to S2 fusion conditions in the presence of cytosolic enriched fractions collected from wild type MEFs treated with DMSO or 2 μM STS for 4 hours (left) or wild type MEF cytosolic enriched fraction containing either DMSO or 2 μM STS (right). Error bars show mean + standard error of at least three independent experiments and Paired t-test analysis was performed to determine statistical significance *P=0.017 **P=0.008 ***P=0.005 (B) Mitochondria were isolated from wild type cells and subjected to S2 fusion conditions (standard) in the presence of either 150 nM recombinant purified Bax or Bcl-xL in the presence (grey bars) or absence (black bars) of 20 nM recombinant purified tBid. After 60 minutes, fusion was quantified and expressed as a fold induction of each standard reaction performed in parallel. Error bars show mean + standard error of at least three independent experiments and Paired t-test analysis was performed to determine statistical significance *P=0.01 **P=0.03 ***P=0.007. (C) Samples from (B) were split and analyzed for MOMP by monitoring cytochrome c release from the mitochondrial fraction (P) to the supernatant fraction (S) by Western analysis. (D) Mitochondria were isolated from wild type cells and subjected to S2 fusion conditions in the absence (standard) and presence of 20 nM tBid. Samples were split and analyzed for either MOMP (top panel, as in C) or fusion (bottom panel). Error bars show mean + standard error of at least three independent experiments and Paired t-test analysis was performed to determine statistical significance *P=0.0086.
To examine the effect of the membrane inserted and oligomerized forms of Bax and Bak on mitochondrial fusion in vitro, we added purified recombinant tBid to an in vitro fusion reaction containing wild type mitochondria and measured both cytochrome c release, by differential centrifugation and Western analysis of the supernatant and pellet fractions, and mitochondrial fusion efficiency over time (Figure 5D, top and bottom panel respectively). In the absence of tBid, fusion efficiency increased in a time dependent manner and cytochrome c was observed predominantly in the pellet fraction for the duration of the experiment (Figure 5D). In contrast, addition of tBid caused a decrease in pellet/mitochondrial associated cytochrome c and a corresponding increase of cytochrome c in the supernatant fraction at 60 and 90 minutes (Figure 5D). Significantly, also at 60 and 90 minutes, mitochondrial fusion was inhibited as compared to the control reaction in the absence of tBid (Figure 5D). These data demonstrate that mitochondrial fusion is directly inhibited during apoptosis concomitant with Bax activation and MOMP. Together, our observations further suggest that Bax mediates fusion inhibition during apoptosis by at least two mechanisms: depletion and/or inactivation of the soluble, fusion active form of Bax from the cytosol and formation of oligomerized membrane associated forms of Bax and Bak and/or MOMP.
Bax specifically stimulates fusion mediated by MFN2 homotypic complexes
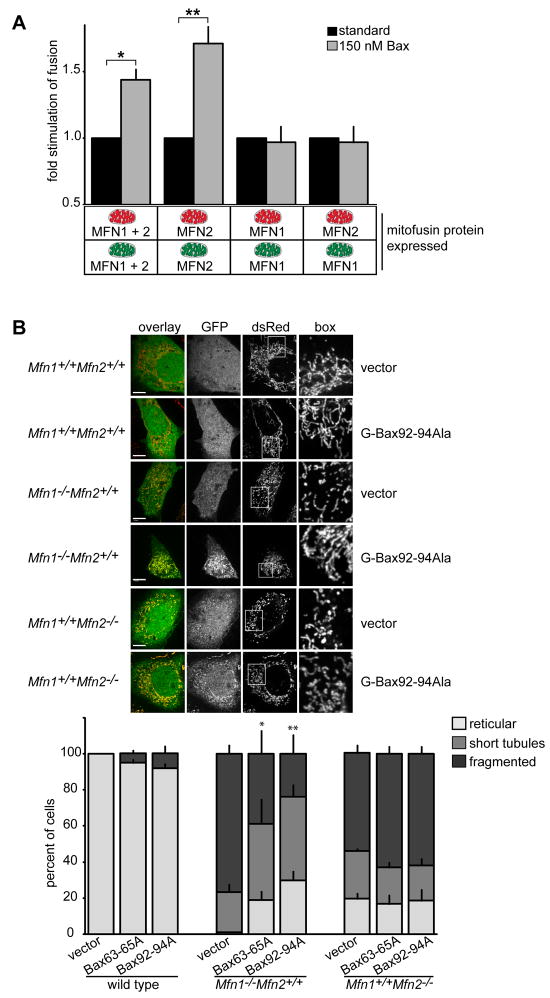
Using our in vitro fusion assay, we demonstrated that homotypic MFN1 and MFN2 complexes and MFN1/MFN2 heterotypic complexes possess different fusion efficacies and are thus functionally distinct (Figure 2). Given this, we asked if the regulation of mitochondrial fusion by Bax is specific to an MFN complex by comparing the fusion efficiencies of MFN homoallelic and heteroallelic reactions in the absence and presence of recombinant purified Bax (Figure 6A, black and grey bars respectively). In comparison to reactions containing wild type mitochondria where Bax significantly stimulated fusion, the fusion efficiency of reactions containing MFN1-only mitochondria was not affected by the addition of recombinant Bax (Figure 6A). In contrast, the fusion activity of reactions containing MFN2-only mitochondria was significantly increased by the addition of recombinant Bax (Figure 6A). Finally, we observed no significant effect of addition of purified recombinant Bax on the fusion activity in heteroalleleic reactions containing MFN1-only mitochondria and MFN2-only mitochondria, suggesting that Bax does not alter the activity of MFN1-MFN2 trans heterotypic complexes (Figure 6A). Together, these data indicate that Bax specifically alters and stimulates fusion mediated by MFN2 homotypic complexes on mitochondria.
Figure 6. Bax positively regulates MFN2 homotypic fusion activity in vitro and in cells.
(A) Mitochondria were isolated from the indicated cell lines and subjected to S2 in vitro fusion conditions in the absence (black bars) or presence (grey bars) of 150 nM recombinant purified Bax. After 60 minutes, the fusion reactions were stopped, fusion was quantified and is expressed as a fold induction of each standard reaction, performed in parallel (ie absence of Bax). Error bars show mean + standard error of at least three independent experiments and Paired t-test analysis was performed to determine statistical significance *P=0.03 **P=0.02. (B) Representative images of the indicated cell lines expressing empty vector (vector) or GFP-Bax92-94Ala (upper panel). Scale bar is 10 μm. Quantification of mitochondrial morphology of GFP-positive cells expressing the GFP-Bax variants shown (lower panel). Error bars show mean + standard error of at least three experiments and Paired t-test analysis was performed to determine statistical significance *P=0.04 **P=0.01.
Given that MFN2 is more highly expressed in specific tissues such as the heart, skeletal muscle and brain, our in vitro data suggest that the specificity in regulation for MFN2 homotypic complexes by soluble Bax may play an important physiological role in vivo. To begin to test this idea, we examined whether Bax stimulates fusion in an MFN-specific manner in cells. Specifically, we utilized Mfn1+/+Mfn2-/- and Mfn1-/-Mfn2+/+ MEF lines and assessed mitochondrial morphology in the presence and absence of expression of oligomerization-defective GFP-Bax92-94A or GFP-Bax63-65A to circumvent the effects of apoptosis. Representative images of mitochondria are shown for cells expressing GFP-Bax92-94A and morphological phenotypes were quantified for cells expressing GFP-Bax92-94A or GFP-Bax63-65A (Figure 6B). In wild type MEF cells, no significant difference in mitochondrial morphology was observed upon expression of GFP-Bax92-94A or GFP-Bax63-65A as compared to control cells (Figure 6B). As expected, in the absence of GFP-Bax92-94A or GFP-Bax63-65A expression, mitochondria were significantly fragmented in Mfn1+/+Mfn2-/- and Mfn1-/-Mfn2+/+ MEF cells as a consequence of an attenuation of fusion as compared to wild type cells (Figure 6B)(Chen et al 2005). The predominantly fragmented steady state morphology of mitochondria observed in of Mfn1+/+Mfn2-/- cells was also observed in Mfn1+/+Mfn2-/- cells expressing GFP-Bax92-94A or GFP-Bax63-65A (Figure 6B). In contrast, expression of GFP-Bax92-94A or GFP-Bax63-65A in Mfn1-/-Mfn2+/+caused mitochondria in cells to become significantly less fragmented and more tubular and reticular as compared to the fragmented morphology observed in Mfn1-/-Mfn2+/+ cells expressing the empty vector control (Figure 6B). These findings indicate that in cells soluble Bax stimulates mitochondrial fusion specifically mediated by MFN2 homotypic complexes.
Discussion
Our data suggest a mechanistic model for the regulation of mitochondrial fusion by Bax in which soluble Bcl2 family proteins dynamically and selectively interact with MFN2 on the surface of mitochondria and through conformational changes facilitate the ability of MFN2 complexes to mediate mitochondrial fusion. This model is consistent with cytological data indicating that Bax modifies the behavior of MFN2 on mitochondria in cells and immunoprecipitation data suggesting that Bax and MFN2 interact (Brooks et al., 2007; Delivani et al., 2006; Karbowski et al., 2006). In addition, yeast two-hybrid analysis indicates that Bax selectively interacts with MFN2 and not MFN1, suggesting that Bax directly modifies the ability of MFN2 to promote membrane fusion (not shown).
Our data indicate that soluble, monomeric Bax is the “active” form for fusion and that a mutually exclusive relationship exists between the role of Bax in the positive regulation of mitochondrial fusion and its role in apoptosis, where the “active” form is membrane integrated, oligomerized Bax. In this context, our findings suggest a model for how Bcl2 family proteins centrally regulate mitochondrial dynamics in healthy and apoptotic cells. Specifically, during apoptosis, our data indicate that mitochondrial fusion is attenuated by the loss of the soluble fusion active form of Bax from the cytosol and also inhibited by the accumulation of membrane inserted oligomerized Bax and/or MOMP. The concerted action of these inhibitory pathways may function as a switch to shift the balance of mitochondrial dynamics from mitochondrial fusion to mitochondrial division and potentiate apoptosis.
Our findings also provide insight into the physiological relevance of two outer membrane DRPs in mammalian mitochondria in light of the fact that MFN1 and MFN2 have unique tissue specific expression profiles (Eura et al., 2003; Lein et al., 2007). Our data indicate that MFN1 and MFN2 heterotypic trans complexes mediate fusion at a significantly greater efficiency that homotypic MFN1 and MFN2 complexes. This observation raises the possibility that a simple regulatory fusion mechanism is provided by the relative expression levels of MFN1 and MFN2 in different cell types. Specifically, a tissue that expresses both MFN1 and MFN2 in equivalent amounts will have higher levels of cellular basal fusion in comparison to cells in a tissue that express significantly more of either MFN1 or MFN2. Thus, altering the normal relative expression levels of MFN1 and MFN2 likely also contributes to the pathophysiology of diseases, such as cardiac hypertrophy, where it has been demonstrated that the expression of MFN2 is down regulated (Fang et al., 2007). In the context of our data indicating that the soluble apoptotic inactive form of Bax interacts with and regulates MFN2-dependent fusion, it is also interesting to speculate that MFN2 may act in part as an anti-apoptotic effector in cells. Indeed, overexpression of MFN2 protects from apoptosis and inhibition of MFN2-mediated fusion renders cell more responsive to apoptotic stimuli (Neuspiel et al., 2005; Sugioka et al., 2004). In addition, the specificity of Bax regulation to MFN2 raises the possibility that certain MFN2CMT2A mutant proteins may be pathogenic as a result of an altered response to Bcl2 protein-dependent regulation.
Experimental Procedures
Preparation of mitochondria or cytosolic-enriched extract
For each experiment, 10-15cm plates each of mouse embryonic fibroblasts stably expressing either mitochondrial-targeted ds-Red or EGFP were grown to ∼90% confluency. Cells were harvested by trypsinization, pelleted and washed in mitochondrial isolation buffer (MIB) (0.25 M sucrose, 10 mM Tris-MOPS pH 7.4, 1 mM EGTA). The cell pellet was resuspended in 1 cell pellet volume of cold MIB and cells were homogenized by 20 strokes with a Kontes Potter-Elvehjem tissue grinder on ice. The homogenate was centrifuged (600 × g, 10 minutes, 4°C) to remove nuclei and unbroken cells and homogenization of the pellet fraction was repeated one time followed by centrifugation at 600 × g, 10 minutes, 4°C. The supernatant fractions were combined and centrifuged (7400 × g, 10 minutes, 4°C) to pellet a crude mitochondrial fraction. The supernatant fraction was the cytosolic-enriched fraction. Crude mitochondrial pellet was resusupended in a small volume of 0.3 M trelahose, 10 mM Tris-MOPS pH 7.4, 1 mM EGTA. Protein concentration of fractions was determined by Bradford assay (Biorad).
In vitro mitochondrial fusion
An equivalent mass (15 μg) of ds-Red and EGFP mitochondria were mixed and concentrated by centrifugation (7400 × g for 10 minutes at 4°C). Following a 10 minute incubation on ice, the supernatant was removed and the mitochondrial pellet was resuspended in 10 μl of either S2 fusion buffer (20 mM PIPES-KOH pH 6.8, 150 mM KOAc, 5 mM Mg(OAc)2, 0.2 M sorbitol, 0.12 mg/ml creatine phosphokinase, 40 mM creatine phosphate, 0.75 mM ATP, 0.75 mM GTP) or S1 fusion buffer (20 mM PIPES-KOH pH 6.8, 150 mM KOAc, 5 mM Mg(OAc)2, 0.6 M sorbitol). Fusion reactions were incubated at 37°C for 60 minutes and are defined as standard, except where noted in the figure and figure legends.
Analysis of mitochondrial fusion
For quantification, fusion reactions were fixed by resuspension in 4% paraformaldehyde in PBS pH 7.4 for 30 minutes on ice. Aliquots were immobilized on microscope slides by mixing with an equal volume of 3% low melt agarose in S1 fusion buffer. Mitochondria were viewed with an Olympus IX70 Deltavision Microscope using a 60× 1.4 N.A. objective (Olympus) and a 100 watt mercury lamp (Applied Precision Inc). Light microscopy images were collected using an integrated, cooled CCD-based Photometrics CoolsnapHQ equipped with a Sony Interline Chip. For each condition tested, a Z-stack of 10 sections were collected from at least 5 different, random fields on the slide. Datasets were processed using Deltavision's iterative, constrained three dimensional deconvolution method to remove out-of-focus light. Mitochondrial fusion was assessed by counting ≥350 total mitochondria per condition (50-100 per image collected) and fusion was scored by co-localization of the red and green fluorophores in three dimensions. Stage 1 fusion reactions were analyzed using conventional EM as described (Meeusen et al., 2004). Specifically, mitochondria were fixed in suspension by the addition of fixative (3% paraformaldehyde, 1.5% gluteraldehyde, 2.5% sucrose contained in 100 mM cacodylate pH 7.4) at 4°C. Mitochondria were washed in 100 mM cacodylate, centrifuged and post-fixed in OsO4 for 1 hr at 4°C and subsequently en bloc stained in Kellenberger's uranyl acetate over night. The pellets were dehydrated through a graded series of ethanol, infiltrated with EPON and allowed to polymerize 24-48 hours at 60°C. 80 nm sections were cut, collected onto 400 mesh high transmission grids, post-stained with lead citrate and uranyl acetate and analyzed on a Philips EM 410 TEM. Images were analyzed in Photoshop using linear adjustments of brightness and contrast. For each experiment, ≥250 mitochondria total were counted and outer membrane fusion was scored when mitochondria contained two separate matrixes surrounded by a single, continuous outer membrane.
Cell Culture
Mouse embryonic fibroblasts (MEFs) MFN1, MFN2, OPA1 knock out lines that were SV-40 immortalized and stably expressing mito-Red/GFP expressing genotypes were obtained from David Chan (California Institute of Technology). Bak-/-Bax-/- lines stably expressing mito-Red or mito-GFP were generated by retroviral transduction. Specifically, the mitochondrial targeting sequence from subunit VIII of human cytochrome oxidase and the Discosoma sp. red protein coding sequence were cloned into pBABE-puro using BamHI and EcoRI and retroviral transduction was performed as described below. HeLa, COS, HEK 293 cells were obtained from ATCC. Cells were maintained in Advanced DMEM containing 4500 mg/L glucose, non-essential amino acids, 110 mg/L sodium pyruvate and supplemented with 1× Glutamax (Invitrogen) and 1× penicillin/streptomycin and either 2% FBS (HeLa, COS, HEK 293, wild type MEF, MFN1 null, MFN2 null, Bak-/-Bax-/-) or 10% FBS (MNF1-MFN2 double null).
Transfection and Immunofluorescence
MEFs were plated at 5×104 cells per well in a 12 well dish containing washed coverslips 12-18 hours prior to transfection. Cells were transfected with Nanofectin (PAA) according to manufacturer's instructions and 12-16 hours following transfection, cells were fixed in 3.7% paraformaldehyde in PBS for 15 minutes at room temperature. Cells were permeablized with 0.25% Triton-X-100 in PBS, blocked with 5% normal goat serum (Jackson Labs) in PBS + 0.1% Triton-X-100 and incubated with anti-GFP-Alexa488 (Invitrogen) in blocking buffer. Cells were washed with PBS+0.1% Triton-X-100 and imaged with an Intelligent Imaging Innovations hybrid Spinning Disk Confocal-TIRF-Widefield microscope with a Yokogawa spinning disk head. Light microscopy data were collected using Cascade, back-thinned EM cameras and images were manipulated in Adobe Photoshop making linear adjustments to brightness or contrast.
Retroviral Production and Infection
Plasmids encoding GFP-Bax variants were obtained from Xu Luo (Nebraska Medical Center) and the coding region was subcloned into pBABE-puro. Retroviral constructs were transfected into Platinum-E cells (Cell Biolabs Inc) using FuGene 6 (Roche) according to manufacturer's instructions and viral supernatant was collected at 48 and 72 hours post-transfection and used to infect Bak-/-Bax-/- MEFs in the presence of 8 mg/mL polybrene. Cells were subject to selection with 1μg/ml puromycin for 7-10 days and cytosol-enriched fractions were collected as described above.
Purification of Recombinant Bax and Bcl-xL
Soluble, monomeric full length Bax, Bcl-xL and Bax1-2/L-6 were purified as described (Suzuki et al., 2000).
Highlights.
An MFN1-MFN2 trans complex is the most efficacious for mitochondrial fusion.
Soluble Bax is the “active form” for the positive regulation of mitochondrial fusion.
Oligomeric Bax and/or MOMP inhibits mitochondrial fusion in vitro.
Bax specifically regulates fusion via MFN2 homotypic trans complexes.
Supplementary Material
Acknowledgments
We are grateful to members of the Nunnari lab for discussions and comments on the manuscript. This work was supported by an NIH 2R01GM062942 grant to JN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amati-Bonneau P, Milea D, Bonneau D, Chevrollier A, Ferre M, Guillet V, Gueguen N, Loiseau D, de Crescenzo MA, Verny C, et al. OPA1-associated disorders: phenotypes and pathophysiology. Int J Biochem Cell Biol. 2009;41:1855–1865. doi: 10.1016/j.biocel.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Benard G, Karbowski M. Mitochondrial fusion and division: Regulation and role in cell viability. Semin Cell Dev Biol. 2009;20:365–374. doi: 10.1016/j.semcdb.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SB, Chen YB, Qi B, McCaffery JM, Rucker EB, 3rd, Goebbels S, Nave KA, Arnold BA, Jonas EA, Pineda FJ, Hardwick JM. Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J Cell Biol. 2009;184:707–719. doi: 10.1083/jcb.200809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni R, Martinou JC. Role of mitofusin 2 mutations in the physiopathology of Charcot-Marie-Tooth disease type 2A. Exp Neurol. 2009;218:268–273. doi: 10.1016/j.expneurol.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Cerveny KL, Tamura Y, Zhang Z, Jensen RE, Sesaki H. Regulation of mitochondrial fusion and division. Trends Cell Biol. 2007;17:563–569. doi: 10.1016/j.tcb.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland MM, Norris KL, Karbowski M, Wang C, Suen DF, Jiao S, George NM, Luo X, Li Z, Youle RJ. Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ. 2010 doi: 10.1038/cdd.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Delivani P, Adrain C, Taylor RC, Duriez PJ, Martin SJ. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol Cell. 2006;21:761–773. doi: 10.1016/j.molcel.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J Cell Biol. 2007;176:405–414. doi: 10.1083/jcb.200611080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvezin-Caubet S, Koppen M, Wagener J, Zick M, Israel L, Bernacchia A, Jagasia R, Rugarli EI, Imhof A, Neupert W, et al. OPA1 processing reconstituted in yeast depends on the subunit composition of the m-AAA protease in mitochondria. Mol Biol Cell. 2007;18:3582–3590. doi: 10.1091/mbc.E07-02-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187:1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eura Y, Ishihara N, Yokota S, Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem (Tokyo) 2003;134:333–344. doi: 10.1093/jb/mvg150. [DOI] [PubMed] [Google Scholar]
- Fang L, Moore XL, Gao XM, Dart AM, Lim YL, Du XJ. Down-regulation of mitofusin-2 expression in cardiac hypertrophy in vitro and in vivo. Life Sci. 2007;80:2154–2160. doi: 10.1016/j.lfs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- George NM, Evans JJ, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;21:1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178:757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187:959–966. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Arnoult D, Chen H, Chan DC, Smith CL, Youle RJ. Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol. 2004;164:493–499. doi: 10.1083/jcb.200309082. Epub 2004 Feb 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Malka F, Guillery O, Cifuentes-Diaz C, Guillou E, Belenguer P, Lombes A, Rojo M. Separate fusion of outer and inner mitochondrial membranes. EMBO Rep. 2005;6:853–859. doi: 10.1038/sj.embor.7400488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen S, McCaffery JM, Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Science. 2004;305:1747–1752. doi: 10.1126/science.1100612. [DOI] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci. 2010;30:4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem. 2005;280:25060–25070. doi: 10.1074/jbc.M501599200. [DOI] [PubMed] [Google Scholar]
- Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci. 2003;116:2763–2774. doi: 10.1242/jcs.00479. [DOI] [PubMed] [Google Scholar]
- Schauss AC, Huang H, Choi SY, Xu L, Soubeyrand S, Bilodeau P, Zunino R, Rippstein P, Frohman MA, McBride HM. A novel cell-free mitochondrial fusion assay amenable for high-throughput screenings of fusion modulators. BMC Biol. 2010;8:100. doi: 10.1186/1741-7007-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20:3525–3532. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004;279:52726–52734. doi: 10.1074/jbc.M408910200. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009;28:1589–1600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.