Abstract
The extraocular muscles (EOM) are spared from pathology in aging and many forms of muscular dystrophy. Despite many studies, this sparing remains an enigma. The EOM have a distinct embryonic lineage compared to somite-derived muscles, and we have shown that they continuously remodel throughout life, maintaining a population of activated satellite cells even in aging. This data suggested the hypothesis that there is a population of myogenic precursor cells (mpcs) in EOM that is different from those in limb, with either elevated numbers of stem cells and/or mpcs with superior proliferative capacity compared to mpcs in limb. Using flow cytometry, EOM and limb muscle mononuclear cells were compared, and a number of differences were seen. Using two different cell isolation methods, EOM have significantly more mpcs per mg muscle than limb skeletal muscle. One specific subpopulation significantly increased in EOM compared to limb was positive for CD34 and negative for Sca-1, M-cadherin, CD31, and CD45. We named these the EOMCD34 cells. Similar percentages of EOMCD34 cells were present in both newborn EOM and limb muscle. In addition, they were retained in aged EOM, whereas the population decreased significantly in adult limb muscle and were extremely scarce in aged limb muscle. Most importantly, the percentage of EOMCD34 cells were elevated in the EOM from both the mdx and the mdx/utrophin−/− (DKO) mouse models of DMD and extremely scarce in the limb muscles of these mice. In vitro, the EOMCD34 cells had myogenic potential, forming myotubes in differentiation media. After determining a media better able to induce proliferation in these cells, a fusion index was calculated. The cells isolated from EOM had a 40% higher fusion index compared to the same cells isolated from limb muscle. The EOMCD34 cells were resistant to both oxidative stress and mechanical injury. These data support our hypothesis that the EOM may be spared in aging and in muscular dystrophies due to a subpopulation of mpcs, the EOMCD34 cells, that are retained in significantly higher percentages in normal, mdx and DKO mice EOM, appear to be resistant to elevated levels of oxidative stress and toxins, and actively proliferate throughout life. Current studies are focused on further defining the EOMCD34 cell subtype molecularly, with the hopes that this may shed light on a cell type with potential therapeutic use in patients with sarcopenia, cachexia, or muscular dystrophy.
Keywords: extraocular muscles, muscular dystrophy, satellite cells, myogenic precursor cells, flow cytometry, CD34, aging
Introduction
Duchenne (DMD) and Becker muscular dystrophy, sarcoglycan deficiency, and merosin-deficient muscular dystrophy are all muscle degenerative diseases for which there is no cure [1–3]. Sarcopenia, an age-related loss of both muscle mass and function with associated progressive fibrosis and adipogenic transformation, [4,5] is a common problem and is increasing with the aging of the world population. One approach for therapeutic intervention in these patient groups is to replace the skeletal muscle regenerative cell population with muscle precursor cells (mpcs) that would fuse with existing myofibers and restore regenerative cells to the satellite cell niche. Early attempts at “myoblast transfer” were only minimally successful. Unfortunately, the vast majority of transplanted cells died [6,7]. Additionally, when the goal was replacement of dystrophin, it was viewed as a foreign protein, making it subject to immune surveillance [8,9]. This necessitates pharmacologic immune suppression, which can result in significant and severe side effects [10–12]. It became apparent in cell therapy studies that surviving cells arise from a very small number of the transplanted precursor cells [13]. This observation supports the view that transplantation of cells with myogenic potential would be more effective if a specific cell type could be identified with greater capacity to engraft, survive, and self-renew.
There are two major obstacles in identifying an appropriate mpc for transplantation. First, a large number of cell markers are used by different laboratories for identification of these cells (i.e. Pax7 [14]; CD34 and myf5 [15]; syndecan-4[16]; cMet [17]; SM-C/2.6 [18]). Second, it is becoming increasingly clear that the mononuclear cells with myogenic potential that reside in adult skeletal muscle are extremely heterogeneous [19,20]. The potential for co-expression of multiple cell markers makes it increasingly unclear how many different subpopulations might exist in normal adult muscle simultaneously [21], complicated even more when different species are compared [22].
The extraocular muscles (EOM) are craniofacial muscles that are both morphologically [23] and functionally [24] spared in DMD patients. This sparing is striking in that all the parts of the dystrophin-dystroglycan complex are the same in limb muscles and EOM [25]. EOM also are spared in other forms of muscular dystrophy, including Becker muscular dystrophy [24], merosin-deficient congenital muscular dystrophy [26], sarcoglycan deficiency [27], and congenital muscular dystrophy [28]. Myofibers within EOM are also spared from age-related sarcopenia and associated fibrotic changes, maintaining normal physiologic properties even in aged individuals [29,30]. Although many physiologic and biochemical differences between EOM and non-cranial skeletal muscle have been studied as potential mechanisms for EOM sparing in aged and dystrophic skeletal muscle, none of these proved mechanistic [26,31–35]. It appears that constitutive differences between the EOM and non-cranial skeletal muscles are responsible for this sparing [32].
Our recent studies in EOM suggest two hypotheses that might explain EOM sparing in aged and dystrophic skeletal muscle: 1) Normal adult EOM maintain ongoing, continuous satellite cell activation, division, and fusion into apparently normal myofibers, resulting in myofiber remodeling throughout life [29,36–38]. This process appears to be sufficient in maintaining EOM integrity in diseased and aged EOM. It follows that 2) mpcs in EOM may be constitutively different. These hypotheses are based on several additional lines of evidence. First, EOM and limb skeletal muscle are developmentally different. Early genes that turn on myogenic pathways significantly differ in somitic and cranial mesoderm. Transgenic mice that do not express Pax3 do not develop body or limb musculature, but the EOM develop completely normally [39]. Instead, the formation of the EOM depends on the presence and gene dose of Pitx2 [40]. Second, the EOM are able to maintain the process of continuous myonuclear addition and myofiber remodeling throughout life, as activated satellite cells were present in EOM from 74 and 82 year old human donors [29]. In addition, the reactivity of EOM mpcs to injury and denervation is extremely vigorous compared to limb muscle. For example, functional denervation of the EOM results in large increases in satellite cell division while in limb muscle there is only an abortive response [41]. If intrinsic differences in the EOM mpcs are responsible for EOM sparing in aged and dystrophic muscle, EOM mpcs may provide a potential source of autologous cells for myoblast transfer therapy with enhanced survival or replicative properties. If this mpc can be characterized molecularly, it might allow it to be purified from another source or constructed using inducible stem cell technology.
We have identified a candidate mononuclear cell population from normal adult mice EOM which display a number of significant differences compared to mononuclear cells derived from limb skeletal muscles. In particular, this cell population isolated from EOM is more resistant to apoptosis than similar cells from limb muscles. We propose that the EOM contains a subpopulation of mpcs predicted to show enhanced survival and proliferative capacity. As such, these cells also are predicted to have the ability to self-renew and enter the satellite cell niche. If mpcs with increased proliferative capacity can be engrafted into aged and diseased skeletal muscle, affected muscles might be able to continuously repair themselves without stem cell exhaustion.
Methods
Mice
Mice were maintained by Research Animal Resources at the University of Minnesota. All experiments were approved by the Institutional Animal Care and Usage Committee at the University of Minnesota and adhered to the NIH guidelines for use of animals in research. BALB/c (NCI), C57/Bl.6 (NCI) or C57/Bl.10 mice (Taconic or Harlan) were used as wild type (WT) animals for experiments comparing EOM progenitor populations to limb progenitor populations and were sacrificed using CO2 asphyxiation. Neonates were sacrificed at age 2–4 days, adult mice at 2–6 months, and aged mice at 20–26 months. Dystrophic animals were maintained as a colony at the University of Minnesota through mdx:utr+/− breeding pairs originating from Washington University [42]. Dystrophin-negative animals that were homozygous for wild type utrophin were used as mdx mice, and those homozygous for the mutant utrophin were used as “double knockout (DKO)” mice. Experiments comparing WT, mdx, and DKO mice were performed on young adult (9–12 week) animals that were age-matched. All FACS analysis is based on pooled extraocular muscles from 12 mice, unless otherwise noted. The number of replications is within the appropriate text section.
Isolation of Mononuclear Cells
Tibialis anterior (limb) and superior, inferior, lateral and medial rectus extraocular muscles (EOM) were isolated immediately after sacrifice into cold Dulbecco’s modified eagle’s medium (DMEM) (Invitrogen, Carlsbad CA). After dissection, muscles were removed from DMEM momentarily for weighing. Mononuclear cells were prepared from the muscles using one of two methods. The first approach used the Hoechst dye exclusion assay (43, 44). Leg muscle was minced into small pieces using surgical scissors; EOM did not require mincing, as muscles were very small. All tissue was incubated in DMEM containing collagenase type B and dispase type II (Roche Diagnostics, Indianapolis, IN) [43] at 37°C for 15 minutes, triturated, and incubated for another 15 minutes until virtually no visible chunks of tissue remained. After digestion, all cell suspensions were filtered over 70µm nylon cell strainers to remove debris. Labeling was based on our modification of the standard method for muscle-derived cells [43,44]. Briefly, cells were diluted to 1 million cells/ml in warm DMEM + and incubated with 8µg/ml Hoechst 33342 dye (Sigma Aldrich) with or without 20µM verapamil (Sigma Aldrich) at 37°C for 30 or 60 minutes. Cells were washed with cold DMEM+, centrifuged at 1400rpm and resuspended in DMEM+ to a final concentration of 1–2×106 cells/ml for flow cytometry (FACS). Hoechst dye analysis and cell sorting were performed on a FACSDiva or LSR flow cytometer (Becton Dickinson [BD] Biosciences, San Jose, CA) at the University of Minnesota Cancer Center Flow Core Facility. The side population (SP) Hoechst-sorted cells were immediately fixed in 2% formaldehyde and subsequently stained with fluorescent antibodies. Analysis of fluorescent antibody staining was performed on a FACSCalibur cytometer. Freshly isolated or Hoechst-sorted cells were incubated with Fc Block (CD16/CD32, BD Pharmingen, San Diego, CA) and as necessary, avidin and biotin blocking agents (Vector Laboratories, Burlingame, CA). Cells were stained using antibodies against CD34, Sca-1, M-cadherin (BD Biosciences), and Pax-7 (R&D Systems, Minneapolis, MN). CD34 and Sca-1, were directly conjugated to either phycoerythrin (PE), fluorescein isothiocyanate (FITC), phycoerythrin-Cy5 (PE-Cy5) or allophycocyanin (APC). Additionally a biotinylated Sca-1 was used and detected with streptavidin APC. M-Cadherin and Pax-7 were detected with a biotinylated anti-mouse IgG (Vector Laboratories) followed by streptavidin APC. All antibody staining controls included appropriate isotype antibodies for primary and secondary reagents, as well as samples stained with secondary only or secondary and tertiary only reagents. For intracellular staining, after fixation in 2% formaldehyde for 20 minutes, samples were incubated with an FCS- and saponin-containing PBS. Antibodies for the intracellular antigens were added at 3–9µg/1× 106 cells. Analysis of flow cytometric data was performed using CellQuest Pro Version 5.2 (BD). Gates were drawn based on fluorescence above isotype control staining.
The second method directly stained all mononuclear cells for FACS, obtained similarly to the method previously outlined up to the Hoechst dye step. Samples that were directly stained for FACS were washed with either cold Hanks balanced salt solution (HBSS) (Invitrogen) supplemented with 2% fetal calf serum (FCS) (Atlas Biologicals, Fort Collins, CO) and 0.001% sodium azide (Sigma Aldrich, St. Louis, MO) as the sorter buffer or phosphate buffered saline containing 25mM HEPES, 2mM EDTA and 1% FCS (Figures 3 and 8). Cells that were permeabilized for intracellular staining were washed twice with cold phosphate buffered saline (PBS) (Invitrogen), resuspended in PBS, and mixed with an equal volume of 4% formaldehyde. Cells were fixed for 20 minutes at room temperature, rinsed, and incubated in saponin-containing PBS supplemented with 25% FCS (permeabilization buffer). All antibody-labeling steps were performed using permeabilization buffer, and washing steps were performed with a diluted permeabilization buffer containing only 10% FCS. Cells for culture were dissected in a laminar flow hood with sterilized instruments, and all reagents were filter-sterilized over a 2µm filter. Culture cells were washed with cold DMEM at pH 7.2–7.4 and supplemented with 10mM HEPES buffer (Invitrogen) and 2% FCS (DMEM+). All cells were centrifuged at 1400rpm for 5 minutes and resuspended in sorter buffer (for direct labeling), PBS (for permeabilization) or DMEM+ (for cell culture).
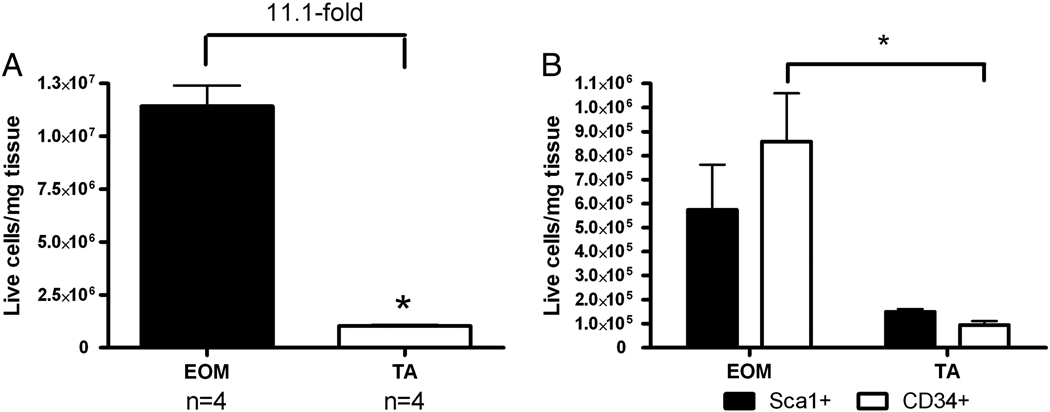
Figure 3.
A. Percentage of all freshly isolated live mononuclear cells from wild type EOM and tibialis anterior muscles (N=4). B. Percentage of all freshly isolated live mononuclear cells from wild type EOM and tibialis anterior muscles positive for Sca-1 or CD34 (N=4).
Figure 8.
Day 5 CD34+/Sca1−/CD45−/CD31− cells from (A) EOM or (B) tibialis anterior (TA) and immunostained for desmin (brown) and nuclei (purple). C. Quantification of the fusion index calculated as the number of nuclei present in myotubes divided by the total number of nuclei (N=3).
Antibody Labeling for FACS
Cells were diluted to 1–10 × 106 cells/ml and incubated at 4°C with 1:10 purified anti-mouse CD16/32 in sorter buffer, permeabilization buffer, or DMEM+ for 10 minutes to block non-specific Fc binding. Cells were stained with one or more of the following anti-mouse antibodies: Sca-1, CD34, M-cadherin, CD31, CD45 (BD Biosciences, San Jose, CA), CD34 (eBiosciences, San Diego, CA), Pax-7 (R&D Systems, Minneapolis, MN or Developmental Studies Hybridoma Bank, University of Iowa, Ames, Iowa), and SM-C/2.6 (generously provided by Hiroshi Yamamoto) [18]. Biotinylated antibody labeling was visualized with streptavidin-fluorescein isothiocyanate (FITC), phycoerythrin (PE) or allophycocyanin (APC) (BD Biosciences). Purified antibody labeling was detected with appropriate biotinylated secondary antibodies and fluorochrome-labeled streptavidin. Cells were incubated with antibodies at 4°C for 30 minutes and washed with sorter buffer, permeabilization buffer, or DMEM+. In order to exclude dead and dying cells from analysis, 7-aminoactinomycin D (7AAD) (BD Biosciences) was added at a 1:20 dilution ten minutes prior to flow cytometric analysis, except for permeabilized cells, which were incubated with 7AAD immediately pre-fixation.
BrdU labeling
Age-matched wild type or mdx animals were injected intraperitoneally with BrdU in saline (50mg/kg) daily for 28 days. Animals were sacrificed two weeks after the final BrdU dose. Myoblasts cells were prepared as above, and BrdU was detected using a BrdU for Flow Cytometry Kit (BD Biosciences).
Immunohistochemistry
Cultured cells were fixed in 4% paraformaldehyde for 10 minutes, blocked with 10% normal horse serum and the Avidin/Biotin Blocking kit (Vector Laboratories, Burlingame, CA), and incubated with anti-mouse desmin antibody (1:100, abcam, Cambridge, MA or 1:200, Dako, Carpinteria, CA). Desmin was visualized using the Vectastain ABC kit (Vector Laboratories) followed by incubation with diaminobenzidine (DAB). Sections of normal rabbit rectus muscles were double-stained for dystrophin and CD34. The sections were incubated with an antibody to dystrophin (Vector) and reaction with reagents from the Elite mouse IgG kit (Vector) and DAB. Second, the sections were incubated with an antibody to CD34 (1:500, eBiosciences, San Diego CA), followed by incubation with reagents from the blue alkaline phosphatase substrate kit (Vector).
In Vitro Differentiation Assay
Live CD34+/ Sca-1−/CD31−/CD45− cells were sorted from wild type mouse EOM by flow cytometry and cultured on collagen-coated plates in myoblast growth medium containing 100U/ml penicillin, 100ug/ml streptomycin (Invitrogen) [43]. Differentiation into myotubes was induced with DMEM supplemented with 5% horse serum.
Fusion Index
Live CD34+/Sca1−/CD45−/CD31− cells from EOM or TA from 10 wild-type mice were sorted directly into cold proliferation media (DMEM, 10% fetal bovine serum, 10% horse serum, 1% penicillin/streptomycin, 0.5% chick embryo extract) [45]. Cells from EOM and TA were plated at the same starting density into separate wells of an 8-well chamber slide coated with Matrigel (BD Biosciences). Proliferation media was replaced every other day until cells were ~70% confluent at which time the media was switched to differentiation media (DMEM, 5% horse serum, 1% penicillin/streptomycin). Cells were allowed to differentiate for 5 days, replacing media every other day. On Day 5 of differentiation, cells were fixed and stained for Desmin using diaminobenzidine and counterstained with hematoxylin. Cells were visualized at 20X magnification. The fusion index was calculated as the number of nuclei present in myotubes divided by the total number of nuclei. A minimum of four fields from at least three wells were counted for three independent experiments.
Apoptosis Assay
Mononuclear cells were isolated, pre-plated on collagen for 30 minutes to remove fibroblasts, and plated onto plastic tissue culture dishes at 20,000 cells per well and incubated overnight in myoblast growth media, consisting of Ham’s F-10 medium containing 20% fetal calf serum (FCS) and 2.5ng/ml basic fibroblast growth factor (bFGF) at 37°C. H2O2 was added to a final concentration of 150 µM, and plates were incubated at 37°C for 90 minutes, at which time H2O2 was washed out. Cells were analyzed by flow cytometry for 7AAD and Annexin V 24 hours after the oxidative stress using a FITC-Annexin V Apoptosis Detection Kit (BD Biosciences).
Statistical Analysis
All data was analyzed for statistical significance using Student’s t-tests for paired data. All other data was assessed for significance using analysis of variance (ANOVA) and either Dunn’s multiple comparison tests or post-hoc Tukey’s comparison using the Prism and Statmate software for Macintosh (Graphpad, San Diego, CA) or SigmaStat 2.03 (SPSS Science, Chicago, IL). For data collected as percentages, angular transformation of the data and ANOVA tests were performed using Microsoft Excel for Macintosh. Errors were calculated based on the propagation of errors associated with each measurement. An F-test was used to verify that the variances were not significantly different. Data was considered significantly different if p≤0.05.
Results
EOM contains more precursor cells than limb muscle
Isolated mononuclear SP cells from EOM and limb muscle were examined by flow cytometry for expression of five markers of progenitor cells: CD34, Sca-1, Pax-7, M-cadherin, and SM-C/2.6 (N = 8 (CD34), N = 6 (Sca-1), N = 4 (Pax-7, M-cadherin, and SM-C/2.6) independent sorts, 12 mice per replication) (Fig 1, 2). FACS analysis of the percentage of live cells positive for the differentiated satellite cell marker SM-C/2.6 showed a significantly greater percentage of positive mononuclear cells in limb muscle than that obtained from EOM (Figure 1). When the percentage of SP cells positive for the remaining 4 cell markers was examined, there were no differences in the percentage of cells positive for CD34, Sca-1, M-cadherin or Pax-7 (Figure 1, 2A). When the total number of SP cells was calculated per mg of muscle significant differences were evident between EOM and limb skeletal muscle (Figure 2B) for these 4 precursor cell markers. Fold increases in EOM compared to limb skeletal muscle levels were calculated as follows: Sca-1+ cells, 5.74; CD34+, 7.18; M-cadherin, 8.62; and Pax-7, 8.69.
Figure 1.
A. Percentage of all freshly isolated live mononuclear cells from wild type EOM (left) and hindlimb muscles examined for expression of M-cadherin. No significant difference in the percentage that were positive was seen. B. Percentage of all freshly isolated live mononuclear cells from wild type EOM (left) and hindlimb muscles (right) examined for expression of Pax-7. No significant difference in the percentage that were positive was seen. C. Percentage of all freshly isolated live mononuclear cells from wild type EOM and hindlimb muscles examined for expression of SM-C/2.6 (N=4) [18]. The percentage of mononuclear cells positive for this differentiated satellite cell marker was significantly greater in hindlimb than in EOM.
Figure 2.
A. Percentage of all freshly isolated live mononuclear cells from wild type EOM and hindlimb muscles positive for Sca-1 (N=6), CD34 (N=8), M-cadherin (N=4), and Pax-7 (N=4). B. Live cells per mg of wild type EOM and hindlimb muscles positive for Sca1, CD34, M-cadherin, and Pax-7. * indicates significantly different from EOM muscle for the identical marker. C. Identification of a population of CD34+/Sca-1− significantly increased in wild type EOM compared with limb skeletal muscle. Top dot plots represent isotype control staining. Lower plots show gates for CD34+/Sca-1− and CD34+/Sca-1+ cells. D. Histograms show M-cadherin staining of each gated population; black histograms are CD34+/Sca-1− cells, white histograms are CD34+/Sca-1+ cells. E. Isotype control (white) and CD34 (black) staining of wild type EOM or hindlimb mononuclear cells, and the line indicates CD34+ gate for bottom dot plots. F. Dot plots show the Sca-1 and CD31 expression for the CD34+ cells.
The EOM mpcs contained a significantly greater percentage of cells that were CD34+/Sca-1- compared to limb skeletal muscle (Figure 2C). In EOM 14.7 ± 1.8% of the mononuclear cells were CD34+/Sca-1−, while this subpopulation represented only 3.3 ± 0.9% in adult limb skeletal muscle. The CD34+/Sca-1− cells were examined for expression of the myogenic marker M-cadherin (Figure 2D). In both EOM and limb muscle the CD34+/Sca-1− cells did not express M-cadherin (Figure 2D, black histograms), while CD34+ cells co-expressing Sca-1 mostly stained positive for M-cadherin (Figure 2D, grey histograms). Further analysis of the CD34+/Sca-1− cells demonstrated that 37.55% of this population in EOM did not co-express the endothelial lineage marker CD31 compared with 12.2% in limb (Figure 2E). Note that the CD34+/Sca-1− cells were rare in limb, demonstrated by the M-cadherin data (Figure 2D). CD34+/Sca-1− cells also did not express Pax-7 (data not shown). Based on the analysis of the SP cells, these mononuclear cells isolated from EOM that are CD34+ /Sca-1− /CD45−/CD31−/M-cadherin− will be referred to as the EOMCD34 cells. Since the EOMCD34 cells were homogenously negative for Pax-7 and M-cadherin, antibodies against these markers were not used in each experiment.
Based on the amount of cell death caused by the Hoechst dye exclusion assay [44], all additional analyses were performed on all live mononuclear cells (Figure 3). The same differential numbers of cells were seen, with over 11-fold more mononuclear cells per mg tissue in EOM compared to tibialis anterior muscle (Figure 3A), and 8–9-fold more Sca1+ cells (Figure 3B) and CD34+ cells per mg tissue (Figure 3B) in EOM compared to tibialis anterior (N=4 replicates, 2 mice per replication).
EOMCD34 cells in neonatal and aging
The normal adult EOM express a number of molecules normally down-regulated in normal adult body and limb skeletal muscles. These include expression of developmental and neonatal myosin heavy chain isoforms [44], the immature form of the acetylcholine receptor [45], N-CAM uniformly on myofiber surfaces [46], and insulin growth factor receptor [47]. Due to the retention of multiple characteristics considered “immature” in adult and aged EOM, the percentage of EOMCD34 cells was assessed in neonatal, adult, and aged mice. The EOMCD34 cells comprised a larger percentage of the mononuclear cells from both EOM (32.9% ±0.57) and limb (27.7%±0.48) in neonatal mice compared with adult animals (N= 4 replicates, 12 mice per replication) (Figure 4). The population virtually disappeared in limb skeletal muscle of adult (2.12%±0.11) and aged (0.65%±0.30) mice, and at 20–26 months these mice were extremely old. However, EOM maintained this population in adult (12.44%±0.18) and even the very aged (8.59%±2.14) mice (Figure 4), supporting the hypothesis that the EOMCD34 cells may play a role in the sparing of EOM in aged and dystrophic mice and human patients.
Figure 4.
Quantification of percentage of EOMCD34 cells from EOM and hindlimb muscles of neonatal, adult and aged wild type mice (N=4). Cells were first gated on live CD31−/CD45− /Sca1− FACS dot plots. * indicates significantly different from EOM percentages. # indicates significantly different from controls based on age of animals.
EOMCD34 cells in dystrophic muscles
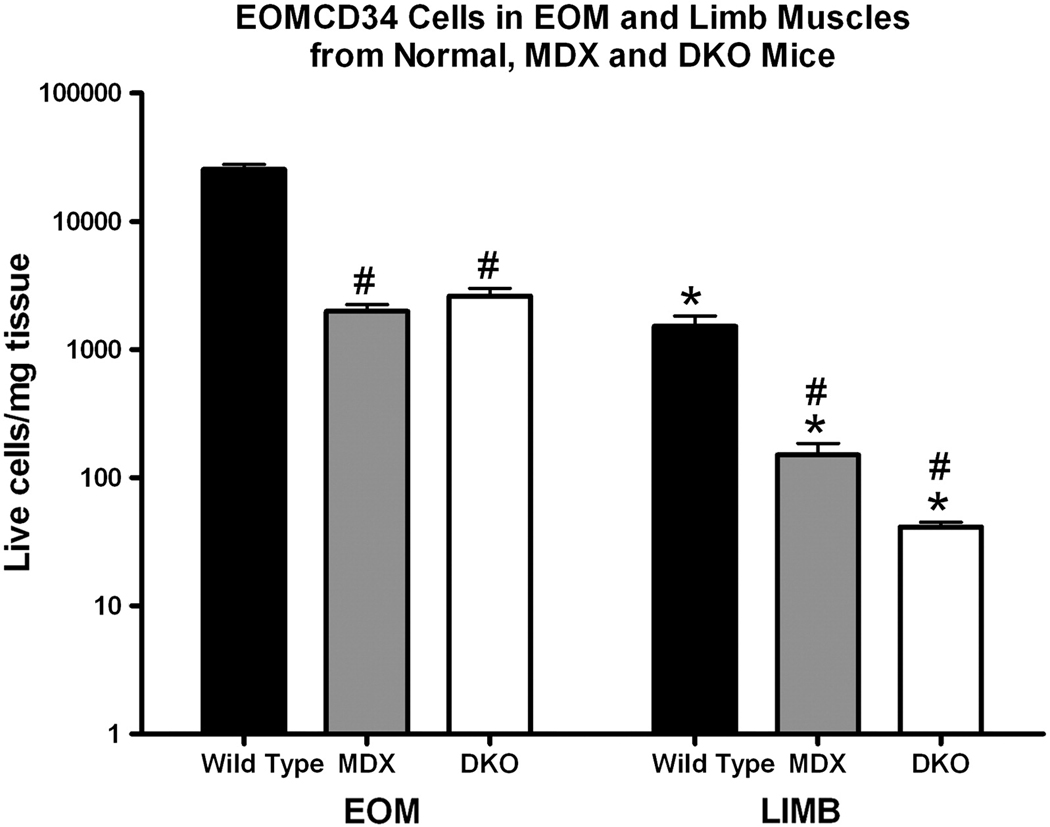
If the EOMCD34 cells are important for the sparing of the EOM in muscular dystrophy, then they should be retained in the EOM from the mdx and DKO mouse models of the disease. Although there was a decrease of EOMCD34 cells in the EOM of the mdx and DKO mice compared to wild type controls, the percentage of these cells was still significantly elevated compared to limb muscles from the mdx and DKO mice (N = 6 replicates, 12 mice per replication) (Figure 5). The limb muscles from the mdx and DKO mice showed an extremely significant decrease in EOMCD34 cells that correlated with disease severity, with DKO limb muscles having negligible levels of these cells (Figure 5). Control EOM levels had a 16.7-fold, mdx EOM a 13.3-fold, and DKO EOM a 64.0-fold larger population of EOMCD34 cells per mg of muscle than levels in limb skeletal muscles from the same three strains. Statistical analysis revealed an interaction between strain and muscle source, indicating that EOMCD34 cells from limb muscle were more sensitive to the disease than cells with the same cellular markers isolated from EOM.
Figure 5.
Quantification of EOMCD34 cells per mg of muscle from EOM and hindlimb of wild type, mdx, and DKO mice (N=4). Cells were first gated on live, CD31−/CD45−/ Sca1− FACS dot plots.
EOMCD34 cells can differentiate into myotubes
If the EOMCD34 cells are important for muscle repair and remodeling, they must have myogenic potential. Thus, the EOMCD34 cells were sorted using flow cytometry and placed in vitro. Cultured on tissue culture plastic in standard myoblast proliferation medium, the EOMCD34 cells replicated slowly over the course of 6 weeks, and under these conditions, they did not reach confluence in this time period. As a result of their relatively loose adherence to tissue culture plastic, they remained extremely small, round, and phase-bright (Figure 6A). After 6 weeks, differentiation medium was added to the cultures, and the cells readily formed multinucleated myotubes (Figure 6B). When the EOMCD34 cells were grown on glass coverslips, where they have increased adherence, addition of differentiation medium resulted in cell fusion resulting in desmin-positive multinucleated myotubes (Figure 6C). Thus, the EOMCD34 cells have myogenic potential in vitro.
Figure 6.
A. EOMCD34 cells cultured on plastic culture plates in myoblast growth media for six weeks. B. Differentiation on plastic culture plates of EOMCD34 cells cultured for six weeks in proliferation media and one week in differentiation medium. C. Differentiated myotubes from EOMCD34 cells grown on glass coverslips and immunostained for the presence of desmin.
EOMCD34 cells proliferate in vivo
Since proliferation in culture is not necessarily reflective of in vivo behavior, we investigated the capacity of these cells to proliferate in vivo. Prior investigations revealed that mpcs proliferate without injury in normal adult EOM [36,38]. This ability is unlike mpcs of limb skeletal muscles, which remain quiescent until activated by muscle injury or disease. To assess the short-term proliferation of EOMCD34 cells in vivo, wild type and mdx adult animals were injected with BrdU intraperitoneally daily for 28 days, followed by a BrdU-free period of 14 days (N = 4 replicates,12 mice per replication). Examination of CD34+/Sca-1+ co-expressing cells showed that many of these were BrdU+ in both mdx (61.27 ±0.14%) and wild type mice (69.78 ±0.04%) (Figure 7). The EOMCD34 cells showed less proliferation over this 28 day period, with only 19.7% and 15.0% positive for BrdU in the wild type and mdx EOM, respectively (Figure 7). In these short time periods, it appears that the CD34+/Sca-1+ progenitor cells were more proliferative. Long-term studies are in progress to examine the turnover rate of the EOMCD34 cells.
Figure 7.
Wild type and mdx mice were treated with brdU for 28 days. After a brdU-free period of 14 days, cells were analyzed by flow cytometry for expression of CD34 and Sca-1 and subsequently for brdU. Cells were first gated on live CD31−/CD45− FACS dot plots (N=3). ANOVA was performed following angular transformation, and * indicate significant difference due to mouse phenotype and # indicates significant difference due to cell type.
In vitro fusion index of EOMCD34 cells derived from EOM and leg muscle
In order to determine if there were myogenicity differences between EOMCD34 cells derived from EOM and leg skeletal muscle, a fusion index assay was performed (Figure 8). Qualitatively, the cultures derived from EOM tissue had a greater number of myotubes, and each myotube appeared to have greater numbers of nuclei within it (Figure 8A) compared to the same cell population isolated from limb skeletal muscle (Figure 8B). The fusion index was determined, and it is approximately 40% greater in EOM-derived cells compared to the “same” population from limb skeletal muscle (Figure 8C).
CD34-positive cells in EOM and limb muscle in vivo
CD34-positive cells were visualized in adult mice EOM and limb muscle by immunochemistry (Figure 9). Two distinct groups of CD34-positive cells were visible in EOM, although all were external to the dystrophin-positive sarcolemma (Figure 9A). In EOM, one subtype of CD34-positive cell was small and tended to be in the perimysial connective tissue (Figure 9A, vertical arrow), while the second group was larger and tended to be in the satellite cell position (Figure 9A, horizontal arrow). While they tended to be randomly localized, with some large areas of muscle devoid of these cells, other regions of the muscle had clusters of CD34-positive cells (Figure 9B,C). The outer layer of the EOM, referred to as the orbital layer, had a greater density of these cells within it (Figure 9B). In the sections thus far examined, there were no CD34-positive cells in the EOM associated with the vasculature (Figure 9C, green arrow), or nerve (Figure 9D, green asterisks). In limb skeletal muscle, CD34-positive cells were only very rarely associated with muscle fibers. Those that were seen were associated with the vasculature (Figure 9E).
Figure 9.
Photomicrographs of limb muscle and EOM immunostained for the presence of CD34-positive cells (blue/purple) and dystrophin (brown). A. The CD34-positive cells (black arrows) were small and irregularly located within EOM cross-sections. Two types of CD34-positive cells were present, one small and found within the perimysium (vertical arrow) and one larger, found adjacent to the dystrophin-positive sarcolemma (horizontal arrow). Bar equals 20 microns. B. The density of CD34+ cells was greater in the orbital layers of the EOM (orb). Within the global layer (glob), large areas were often relatively devoid of cells. C. Blood vessel in the EOM devoid of CD34-positive cells (green arrow). Note positive cell cluster around a single myofiber (black arrow). D. The nerve was also devoid of CD34-positive cells (green asterisks). Black arrow indicates a CD34-positive cell in the satellite cell position. E. Section through a leg muscle cutting a blood vessel tangentially and immunostained for CD34. CD34+ cells in the satellite cell position were rare in leg muscle. They were usually associated with blood vessels. Inset is a higher power version of positive cells found associated with the blood vessel (black arrow). Magnification bars equal 10 microns.
Cell death decreased in EOM progenitor cells
Initial comparison of wild type EOM and limb skeletal muscles revealed a surprising difference in the percentage of cells taking up 7AAD, a marker of dead and dying cells, after cell isolation only (Figure 10A). When the percentage of cells positive for 7AAD was determined for EOM and limb muscles of wild type, mdx, and DKO mice, cell isolation alone resulted in significantly fewer 7AAD positive mononuclear cells from EOM compared to those derived from limb skeletal muscle in all animals (P= 0.0012, N = 4 replicates, 12 mice per replication) (Figure 10B).
Figure 10.
A. FACS dot plots show the forward-side scatter (top) and CD34/Sca-1 expression (bottom) gating strategies of mononuclear cells from muscle. Histograms show the percentage of cells that are 7AAD+ based on FACS gating that included CD34+/Sca-1+ cells (top 3), or CD34+/Sca-1− (bottom 3). B. Percentages of dead or dying cells from EOM and limb skeletal muscle of wild type, mdx, and DKO animals are indicated (N= 3). C. Percentage of apoptotic cells defined by Annexin-V and CD34+staining of cultured wild type mononuclear cells 24 hours after treatment with H2O2 (N=5).
This observation suggested a second potential mechanism for EOM sparing in DMD: mpcs from EOM might be more resistant to injury than mpcs derived from limb skeletal muscle. This hypothesis is supported by studies that demonstrate that in the EOM, as well as other craniofacial musculature such as the larynx, proliferative cells react to denervation and injury in vivo with a significant up-regulation of activity, while the same treatment of tibialis anterior muscle results in only a small and abortive response [41,50]. Mononuclear cells from EOM and hindlimb muscles of wild type mice were cultured with H2O2 for 3 hours, followed by a 24-hour post-treatment recovery period, and examined for expression of CD34 and Annexin-V, a marker of early apoptosis. Significantly greater numbers of mononuclear cells isolated from limb muscle became apoptotic even without treatment (P< 0.0001, N = 3 replicates, 12 mice per replication) (Figure 10C). In addition, EOM-derived CD34+ cells were significantly less sensitive to oxidative stress caused by the H2O2 insult, as there were greater numbers of apoptotic cells in the treated limb skeletal muscle derived cells than in the control limb cells (P= 0.0011, N = 3) (Figure 10C).
Discussion
The hypothesis that a subpopulation of myogenic precursor cells in EOM may be responsible for EOM sparing in aging and in the mdx and DKO mouse models of muscular dystrophy is supported by the following findings. First, compared to normal adult limb skeletal muscle, whether isolated from all live mononuclear cells or when isolated as the side population cells using the Hoechst dye exclusion assay, the EOM contain more mononuclear cells per mg tissue that express myogenic and stem cell markers. Second, compared to adult limb skeletal muscle, the EOM contain a subpopulation of cells that is significantly increased. These cells, the EOMDC34 cells, express CD34 but are negative for CD31, CD45, Sca-1, and M-cadherin. Third, while neonatal EOM and limb skeletal muscle had relatively similar percentages of the EOMCD34 cells, these are significantly reduced in adult and aged limb skeletal muscle while retained in adult and aged EOM. The EOMCD34 cells can form myotubes in vitro, but those cells derived from EOM showed an enhanced ability to fuse into myotubes when compared to similarly isolated cells derived from limb muscles of the same mice. Fifth, and most supportive of our working hypothesis, the EOM retain the EOMCD34 cells in two mouse models of DMD, the mdx and DKO mice, while mdx and DKO limb skeletal muscle contained very few of these cells.
Different muscles contain varying numbers of satellite cells [15,51–53]. However, the density of mpcs in EOM is significantly greater than in any muscles thus far examined, and this is true for all the species thus far examined. Elevated numbers of mpcs could, in itself, account for maintenance of EOM in aging, and in DMD and related disorders in humans. In a seminal study specifically using human muscle, the proliferative life spans of mpcs derived from young and old skeletal muscle were compared, and these were compared to cells isolated from a 9-year old boy with DMD [54]. While the proliferative capacity of the normal muscle satellite cells isolated from the normal 9-year-old boy was approximately 30 divisions before replicative senescence, the cells derived from the 9-year-old DMD patient had only 13 divisions in vitro. This finding suggests that the many cycles of degeneration and regeneration essentially exhausted their proliferative potential, despite the patient’s young age. This hypothesis was supported by the demonstration of significantly shorter telomeres in the myoblasts from dystrophic patients compared to age matched controls [55,56]. Increased numbers of mpcs may contribute to EOM sparing in aged and dystrophic muscle, but it is unlikely to be the single explanation.
Identification of a specific mpc responsible for EOM sparing is complicated by the large number of cellular markers that have been correlated with self-renewal and differentiation: e.g. Pax7 [14], c-met [17], and sprouty1 [57] with quiescence; HGF [17] and MyoD [58] with activation. The mpcs in muscle are also functionally heterogeneous [19,20], such that despite specific isolation techniques, the progeny of only a very small number of cells survive after myoblast transplantation, while the majority of the transplanted cells die [13]. Heterogeneity of the mononuclear cell pool in skeletal muscle is even more sensitively demonstrated by the use of single muscle stem cell transplantation, where extensive proliferation, as well as localization of progeny into the satellite cell niche, is seen [59]. These results suggest that there is a rare population of mononuclear cells in limb skeletal muscle with superior abilities to replicate and self-renew, and these characteristics are critical for their potential therapeutic use. The EOMCD34 cells, defined as CD34+ /Sca-1− /CD45−/CD31−//M-cadherin−, have myogenic potential in vitro, and are significantly increased per mg tissue in the EOM compared to limb skeletal muscle in adult mice. It is possible that the large numbers of EOMCD34 cells in eye muscles compared to limb muscles may be due to different signaling molecules in the stem cell niche within the EOM [60, 61], and in the absence of this niche they will not survive. In enhanced proliferation media, the EOMCD34 cells derived from EOM displayed a superior ability to fuse into myotubes compared to the ostensibly similar cells derived from limb skeletal muscle of the same mice. Future studies are directed at further dissecting the differences between these cells from EOM and limb skeletal muscle and how the niche they occupy might play a role in their numbers and functionality.
Support that the EOMCD34 cells, or a subpopulation of them, may be responsible for EOM sparing in aging and muscular dystrophy is provided by their presence in the EOM of both aged EOM and in the EOM of the mdx and DKO mouse models of DMD. In both aged limb and in the DKO limb muscle, the EOMCD34 cells are essentially gone. This report is the first demonstration of a significantly elevated subpopulation of mpcs that is differentially present in EOM muscle from aging and in the muscles of the mdx and DKO mouse models of Duchenne muscular dystrophy.
In the most commonly used proliferative media, EOMCD34 cells in vitro had a lower proliferative rate with the short-term BrdU-labeling protocol than the same cells derived from limb skeletal muscle. A number of laboratories have performed proliferation assays on mpcs derived from limb skeletal muscle, and subpopulations with different proliferative rates have been described [19]. One subpopulation proliferates minimally and differentiates completely. A second subpopulation constitutes the majority; these mpcs proliferate rapidly and differentiate but also produce cells that reenter the satellite cell niche and thus are self-renewing. A third population appears to divide very infrequently yet also has the ability to both self-renew and produce progeny that can form differentiated muscle fibers. Based on their in vitro properties, the EOMCD34 cells appear to be in one of the latter two groups.
Expression of CD34 without expression of known satellite cell-specific markers in EOM-derived mpcs does not imply that the cells are necessarily myogenic, but their ability to differentiate into myotubes was confirmed in vitro. The molecules that control early myogenic cell fate determination are not completely understood. For example, in the complete absence of MyoD, a molecule expressed early during mpc differentiation, mpcs from MyoD knock-out mice divide, differentiate, and self-renew after myoblast transfer into regenerating muscle [62]. Additionally, these cells showed increased survival compared with mpcs derived from normal mice. The retention of a large population of EOMCD34 cells in both aging and muscular dystrophy, including EOM from DKO mice lacking both dystrophin and utrophin, supports the hypothesis that the EOM may contain a true stem cell reservoir [63]. However, preliminary analyses suggest that the EOMCD34 cells as currently defined are still a heterogeneous population. This is supported by the enhanced ability of the EOMCD34 cells derived from EOM to form myotubes compared to the “same” cells derived from limb skeletal muscle from the same mice, when placed in an enhanced proliferative medium. There were many cells that did not form myotubes in these cultures. While further work is needed to define the molecular properties of the EOMCD34 population with more specificity, their extremely elevated numbers in EOM should improve the odds of their successful identification.
The demonstration that mpcs from EOM are less sensitive to oxidative stress in their local environment when compared to mpcs from limb skeletal muscle suggests a second hypothesis to explain EOM sparing in aging and muscular dystrophies. This confirms our earlier study demonstrating that mpcs derived from EOM are less sensitive to injury from toxins such as Hoechst dye [61]. In man as well as the other species that have been examined thus far, the EOM respond to denervation and drug-induced injury in vivo with robust mpc activation and replication [41,50,64,65]. It is well known that the tissue milieu in dystrophic muscle is a hostile environment, containing high levels of inflammatory cell mediators including transforming growth factor beta 1(TGFb1) [66,67] as well as high levels of reactive oxygen species [68]. This preferential ability of EOM mpcs to survive in hostile environments, as well as their ability to maintain normal muscle mass and function after chemical or physical injury, may explain EOM sparing in both aging and muscular dystrophies.
In summary, the normal adult EOM contain a significantly increased subpopulation of mpcs, and specifically EOMCD34 cells, compared to normal adult limb skeletal muscle. These cells are present in similar numbers in neonatal EOM and limb skeletal muscle. The EOMCD34 cells are CD34+ but negative for all other markers examined thus far, including CD31, an endothelial lineage marker; CD45, a hematopoeitic lineage marker; Sca-1, a general precursor cell marker; and in the SP cells, two markers for satellite cells, Pax-7 and M-cadherin. The increased percentages of EOMCD34 cells are retained in the EOM both in aging and in muscular dystrophy, and are essentially absent in DKO and aging limb skeletal muscle. The EOMCD34 cells have a significantly increased ability to resist injury, including oxidative stress. These data support the hypothesis that the EOMCD34 cells, or a subset within them, are responsible for the sparing of EOM from the pathologic changes associated with DMD and sarcopenic changes in aging. Current studies are attempting to more clearly dissect the molecular identity of these cells. It is hoped that EOMCD34 cells may provide insight into a potential candidate for myoblast therapy; this is based on their resistance to apoptosis and their retention in aging and muscular dystrophies. EOMCD34 cells may allow for completely autologous myoblast transplantation, which would greatly reduce the complications associated with long-term immunosuppression. Additionally, and maybe more importantly, these cells may allow us to define more specifically the molecular characteristics of a candidate population of cells with greater proliferative and survival potential that could then be isolated from another source or generated using induced pluripotent stem cell technology [69].
Acknowledgments
Support: EY55137 and EY11375 from the National Eye Institute, T32DE007288 from NIDCR (SLH) and T32EY007133 (AAM), Minnesota Medical Foundation, Fight for Sight, Nash Avery Fund, Minnesota Lions and Lionesses, and an unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu. Rev. Physiol. 2009;71:37–57. doi: 10.1146/annurev.physiol.010908.163216. [DOI] [PubMed] [Google Scholar]
- 2.Rando TA. The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve. 2001;24:1575–1594. doi: 10.1002/mus.1192. [DOI] [PubMed] [Google Scholar]
- 3.Manzur AY, Muntoni F. Diagnosis and new treatments in muscular dystrophies. J. Neurol. Neurosurg. Psychiatry. 2009;80:706–714. doi: 10.1136/jnnp.2008.158329. [DOI] [PubMed] [Google Scholar]
- 4.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 5.Taylor-Jones JM, McGehee RE, Rando TA, Lecka-Czernik B, Lipschitz DA, Peterson CA. Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev. 2002;123:649–661. doi: 10.1016/s0047-6374(01)00411-0. [DOI] [PubMed] [Google Scholar]
- 6.Gussoni E, Pavlath GK, Lanctot AM, Sharma KR, Miller RG, Steinman L, Blau HM. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992;356:435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- 7.Miller RG, Sharma KR, Pavlath GK, Gussoni E, Mynhier M, Lanctot AM, Greco CM, Steinman L, Blau HM. Myoblast implantation in Duchenne muscular dystrophy: the San Francisco study. Muscle Nerve. 1997;20:469–478. doi: 10.1002/(sici)1097-4598(199704)20:4<469::aid-mus10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Pavlath GK, Rando TA, Blau HM. Transient immunosuppressive treatment leads to long-term retention of allogeneic myoblasts in hybrid myofibers. J. Cell Biol. 1994;127:1923–1932. doi: 10.1083/jcb.127.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremblay JP, Bouchard JP, Malouin F, Theau D, Cottrell F, Collin H, Rouche A, Gilgenkrantz S, Abbadi N, Tremblay M, Tome F, Fardeau M. Myoblast transplantation between monozygotic twin girl carriers of Duchenne muscular dystrophy. Neuromuscul. Disord. 1993;3:583–592. doi: 10.1016/0960-8966(93)90121-y. [DOI] [PubMed] [Google Scholar]
- 10.Hong F, Lee J, Song JW, Lee SJ, Ahn H, Cho JJ, Ha J, Kim SS. Cyclosporin A blocks muscle differentiation by inducing oxidative stress and inhibiting the peptidyl-prolylcis-trans isomerase activity of cyclophilin A: cyclophilin A protects myoblasts from cyclosporin A-induced cytotoxicity. FASEB J. 2002;16:1633–1635. doi: 10.1096/fj.02-0060fje. [DOI] [PubMed] [Google Scholar]
- 11.Karpati G, Ajdukovic D, Arnold D, Gledhill RB, Guttmann R, Holland P, Koch PA, Shoubridge E, Spence D, Vanasse M, Walters GV, Abrahamowicz M, Duff C, Worton RG. Myoblast transfer in Duchenne muscular dystrophy. Ann. Neurol. 1993;34:8–17. doi: 10.1002/ana.410340105. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita I, Vilquin JT, Guerette B, Asselin I, Roy R, Tremblay JP. Very efficient myoblast allotransplantation in mice under FK506 immunosuppression. Muscle Nerve. 1994;17:1407–1415. doi: 10.1002/mus.880171210. [DOI] [PubMed] [Google Scholar]
- 13.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 15.Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev. Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- 17.Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J. Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- 18.Fukada S, Higuchi S, Segawa M, Koda K, Yamamoto Y, Tsujikawa K, Kohama Y, Uezumi A, Imamura M, Miyagoe-Suzuki Y, Takeda S, Yamamoto H. Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp. Cell Res. 2004;296:245–255. doi: 10.1016/j.yexcr.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Ono Y, Boldrin L, Knopp P, Morgan JE, Zammit PE. Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev. Biol. 2010;337:29–41. doi: 10.1016/j.ydbio.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi CA, Pozzobon M, Ditadi A, Archacka K, Gastaldello A, Sanna M, Franzin C, Malerba A, Milan G, Cananzi M, Schiaffino S, Campella M, Vettor R, De Coppi P. Clonal characterization of rat muscle satellite cells: proliferation, metabolism and differentiation define an intrinci heterogeneity. PloS One. 2010;5:e8523. doi: 10.1371/journal.pone.0008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 22.Lindström M, Thornell LE. New multiple labelling method for improved satellite cell identification in human muscle: application to a cohort of power-liefters and sedentary men. Histochem Cell Biol. 2009;132:141–157. doi: 10.1007/s00418-009-0606-0. [DOI] [PubMed] [Google Scholar]
- 23.Karpati G, Carpenter S. Small caliber skeletal muscle fibers do not suffer deleterious consequences of dystrophic gene expression. Am. J. Med. Genet. 1986;25:653–658. doi: 10.1002/ajmg.1320250407. [DOI] [PubMed] [Google Scholar]
- 24.Kaminski HJ, al-Hakim M, Leigh RJ, Katirji MB, Ruff RL. Extraocular muscles are spared in advanced Duchenne dystrophy. Ann. Neurol. 1992;32:586–588. doi: 10.1002/ana.410320418. [DOI] [PubMed] [Google Scholar]
- 25.Andrade FH, Porter JD, Kaminski HJ. Eye muscle sparing by the muscular dystrophies: lessons to be learned? Microsc. Res. Techn. 2000;48:192–203. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<192::AID-JEMT7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 26.Porter JD, Karathanasis P. Extraocular muscle in merosin-deficient muscular dystrophy: cation homeostasis is maintained but is not mechanistic in muscle sparing. Cell Tissue Res. 1998;292:495–501. doi: 10.1007/s004410051078. [DOI] [PubMed] [Google Scholar]
- 27.Porter JD, Merriam AP, Hack AA, Andrade FH, McNally EM. Extraocular muscle is spared despite the absence of an intact sarcoglycan complex in gamma- or delta-sarcoglycan-deficient mice. Neuromuscul. Disord. 2001;11:197–207. doi: 10.1016/s0960-8966(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 28.Mendell JR, Sahenk Z, Prior TW. The childhood muscular dystrophies: diseases sharing a common pathogenesis of membrane stability. J. Child Neurol. 1995;10:150–159. doi: 10.1177/088307389501000219. [DOI] [PubMed] [Google Scholar]
- 29.McLoon LK, Wirtschafter J. Activated satellite cells in extraocular muscles of normal adult monkeys and humans. Invest. Ophthalmol. Vis. Sci. 2003;44:1927–1932. doi: 10.1167/iovs.02-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMullen CA, Ferry AL, Gamboa JL, Andrade FH, Dupont-Versteegden EE. Age-related changes of cell death pathways in rat extraocular muscle. Exp. Gerontol. 2009;44:420–425. doi: 10.1016/j.exger.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khurana TS, Prendergast RA, Alameddine HS, Tome FM, Fardeau M, Arahata J, Sugita H, Kunkel LM. Absence of extraocular muscle pathology in Duchenne's muscular dystrophy: role for calcium homeostasis in extraocular muscle sparing. J. Exp. Med. 1995;182:467–475. doi: 10.1084/jem.182.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porter JD, Merriam AP, Khanna S, Andrade AH, Richmonds CR, Leahy P, Cheng G, Karathanasis P, Zhou X, Kusner LL, Adams ME, Willem M, Mayer U, Kaminski HJ. Constitutive properties, not molecular adaptations, mediate extraocular muscle sparing in dystrophic mdx mice. FASEB J. 2003;17:893–895. doi: 10.1096/fj.02-0810fje. [DOI] [PubMed] [Google Scholar]
- 33.Ragusa RJ, Chow CK, Porter JD. Oxidative stress as a potential pathogenic mechanism in an animal model of Duchenne muscular dystrophy. Neuromuscul. Disord. 1997;7:379–386. doi: 10.1016/s0960-8966(97)00096-5. [DOI] [PubMed] [Google Scholar]
- 34.Wehling M, Stull JT, McCabe TJ, Tidball JG. Sparing of mdx extraocular muscles from dystrophic pathology is not attributable to normalized concentration or distribution of neuronal nitric oxide synthase. Neuromuscul. Disord. 1998;8:22–29. doi: 10.1016/s0960-8966(97)00136-3. [DOI] [PubMed] [Google Scholar]
- 35.Zhou L, Porter JD, Cheng G, Gong B, Hatala DA, Merriam AP, Zhou X, Rafael JA, Kaminski HJ. Temporal and spatial mRNA expression patterns of TGF-beta1, 2, 3 and TbetaRI, II, III in skeletal muscles of mdx mice. Neuromuscul. Disord. 2006;16:32–38. doi: 10.1016/j.nmd.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 36.McLoon LK, Wirtschafter JD. Continuous myonuclear addition to single extraocular myofibers in uninjured adult rabbits. Muscle Nerve. 2002;25:348–358. doi: 10.1002/mus.10056. [DOI] [PubMed] [Google Scholar]
- 37.McLoon LK, Wirtschafter JD. Activated satellite cells are present in uninjured extraocular muscles of mature mice. Trans. Am. Ophthalmol. Soc. 2002;100:119–123. [PMC free article] [PubMed] [Google Scholar]
- 38.McLoon LK, Rowe J, Wirtschafter J, McCormick KM. Continuous myofiber remodeling in uninjured extraocular myofibers: myonuclear turnover and evidence for apoptosis. Muscle Nerve. 2004;29:707–715. doi: 10.1002/mus.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 ac upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- 40.Diehl AG, Zareparsi S, Qian M, Khanna R, Angeles R, Gage PJ. Extraocular muscle morphogenesis and gene expression are regulated by Pitx2 gene dose. Invest Ophthalmol Vis Sci. 2006;47:1785–1793. doi: 10.1167/iovs.05-1424. [DOI] [PubMed] [Google Scholar]
- 41.Ugalde I, Christiansen SP, McLoon LK. Botulinum toxin treatment of extraocular muscles in rabbits results in increased myofiber remodeling. Invest. Ophthalmol. Vis. Sci. 2005;46:4114–4120. doi: 10.1167/iovs.05-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90:729–738. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 43.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 44.Kallestad KM, McLoon LK. Defining the heterogeneity of skeletal muscle-derived side and main population cells isolated immediately ex vivo. J. Cell. Physiol. 2010;222:676–684. doi: 10.1002/jcp.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, Peault B, Cummins J, Huard J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat. Protoc. 2008;3:1501–1509. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 46.Wieczorek DF, Periasamy M, Butler-Browne GS, Whalen RG, Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985;101:618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horton RM, Manfredi AA, Conti-Tronconi BM. The embryonic gamma subunit of the nicotinic acetylcholine receptor is expressed in adult extraocular muscle. Neurology. 1993;43:983–986. doi: 10.1212/wnl.43.5.983. [DOI] [PubMed] [Google Scholar]
- 48.McLoon LK, Wirtschafter JD. N-CAM is expressed in mature extraocular muscles in a pattern conserved among three species. Invest. Ophthalmol. Vis. Sci. 1996;37:318–327. [PubMed] [Google Scholar]
- 49.Anderson BC, Christiansen SP, Grandt S, Grange RW, McLoon LK. Increased extraocular muscle strength with direct injection of insulin-like growth factor-1. Invest. Ophthalmol. Vis. Sci. 2006;47:2461–2467. doi: 10.1167/iovs.05-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinners MJ, Goding GS, McLoon LK. Effect of recurrent laryngeal nerve section on the laryngeal muscles of adult rabbits. Otolaryngol. Head Neck Surg. 2006;134:413–418. doi: 10.1016/j.otohns.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 51.McLoon LK, Thorstenson KM, Solomon A, Lewis MP. Myogenic precursor cells in craniofacial muscles. Oral Dis. 2007;13:134–140. doi: 10.1111/j.1601-0825.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 52.Wozniak AC, Pilipowicz O, Yablonka-Reuveni Z, Greenway S, Craven S, Scott E, Anderson JE. C-Met expression and mechanical activation of satellite cells on cultured muscle fibers. J. Histochem. Cytochem. 2003;51:1437–1445. doi: 10.1177/002215540305101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zammit PS, Heslop L, Hudon V, Rosenblatt JD, Tajbakhsh S, Buckingham ME, Beauchamp JR, Partridge TA. Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp. Cell Res. 2002;281:39–49. doi: 10.1006/excr.2002.5653. [DOI] [PubMed] [Google Scholar]
- 54.Renault V, Piron-Hamelin G, Forestier C, DiDonna S, Decary S, Hentati F, Saillant G, Butler-Browne GS, Mouly V. Skeletal muscle regeneration and the mitotic clock. Exp. Gerontol. 2000;35:711–719. doi: 10.1016/s0531-5565(00)00151-0. [DOI] [PubMed] [Google Scholar]
- 55.Decary S, Hamida CB, Mouly V, Barbet JP, Hentati F, Butler-Browne GS. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul. Disord. 2000;10:113–120. doi: 10.1016/s0960-8966(99)00093-0. [DOI] [PubMed] [Google Scholar]
- 56.Mouly V, Aamiri A, Bigot A, Cooper RN, Di Donna S, Furling D, Gidaro T, Jacquemin V, Manchaoui K, Negroni E, Perie S, Renault V, Silva-Barbosa SS, Butler-Browne GS. The mitotic clock in skeletal muscle regeneration, disease and cell medicated gene therapy. Acta Physiol. Scand. 2005;184:3–15. doi: 10.1111/j.1365-201X.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- 57.Shea KL, Xiang W, LaPorta VS, Licht JD, Keller C, Basson MA, Brack AS. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell. 2010;6:117–129. doi: 10.1016/j.stem.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith CK, Janney MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J. Cell Physiol. 1994;159:379–385. doi: 10.1002/jcp.1041590222. [DOI] [PubMed] [Google Scholar]
- 59.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cellul Physiol. 2010;224:7–16. doi: 10.1002/jcp.22127. [DOI] [PubMed] [Google Scholar]
- 62.Asakura A, Hirai H, Kablar B, Morita S, Ishibashi J, Piras BA, Christ AJ, Verma M, Vineretsky KA, Rudnicki MA. Increased survival of muscle stem cells lacking the MyoD gene after transplantation into regeneration skeletal muscle. Proc. Natl. Acad. Sci. USA. 2007;104:16552–16557. doi: 10.1073/pnas.0708145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudnicki MA, Le Grand F, McKinnell I, Kuang S. The molecular regulation of muscle stem cell function. Cold Spring Harb. Symp. Quant. Biol. 2008;73:323–331. doi: 10.1101/sqb.2008.73.064. [DOI] [PubMed] [Google Scholar]
- 64.Spencer RF, McNeer KW. Botulinum toxin paralysis of adult monkey extraocular muscle. Structural alterations in orbital, singly innervated muscle fibers. Arch. Ophthalmol. 1987;105:1703–1711. doi: 10.1001/archopht.1987.01060120101035. [DOI] [PubMed] [Google Scholar]
- 65.Porter JD, Edney DP, McMahon EJ, Burns LA. Extraocular myotoxicity of the retrobulbar anesthetic bupivacaine hydrochloride. Invest. Ophthalmol. Vis. Sci. 1988;29:163–174. [PubMed] [Google Scholar]
- 66.Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, Hoffman EP. Early onset of inflammation and later involvement of TGFb in Duchenne muscular dystrophy. Neurology. 2005;65:826–834. doi: 10.1212/01.wnl.0000173836.09176.c4. [DOI] [PubMed] [Google Scholar]
- 67.Demoule A, Divangahi M, Danialou G, Gvozdic D, Larkin G, Bao W, Petrof BJ. Expression and regulation of CC class chemokines in the dystrophic (mdx) diaphragm. Am. J. Respir. Cell Mol. Biol. 2005;33:178–185. doi: 10.1165/rcmb.2004-0347OC. [DOI] [PubMed] [Google Scholar]
- 68.Tidball JG, Wehling-Henricks M. The role of free radicals in the pathophysiology of muscular dystrophy. J. Appl. Physiol. 2007;102:1677–1686. doi: 10.1152/japplphysiol.01145.2006. [DOI] [PubMed] [Google Scholar]
- 69.Darabi R, Perlingeiro RC. Lineage-specific reprogramming as a strategy for cell therapy. Cell Cycle. 2008;7:1732–1737. doi: 10.4161/cc.7.12.6159. [DOI] [PubMed] [Google Scholar]