Abstract
Objective:
Cancer patients receiving chemotherapy are at increased risk of anemia. We conducted a population-based historical cohort study in newly diagnosed cancer patients with chemotherapy-associated anemia in order to characterize red blood cell transfusion (RBCT) use.
Design:
This study evaluated cancer patients diagnosed between January 1, 1998 and December 31, 2003 using Danish National Patient Registry data. Patients were receiving chemotherapy and had a hemoglobin level ≤10.9 g/dL during the 4 months following cancer diagnosis. We characterized patterns of RBCT use and inpatient and outpatient hospitalization for transfusion. Adjusted Poisson regression models were used to evaluate the likelihood of RBCT, estimated by relative risk (RR), based on demographic and clinical factors.
Results:
Women constituted 58% of 1782 patients studied; the median age was 58 years. Two-thirds (67%) had solid tumors; 67% had stage III or IV disease at diagnosis. Overall, 713 (40%) patients received an RBCT within 120 days of cancer diagnosis, of which 94% were administered in the inpatient setting; 84% of these patients required subsequent transfusions. The median (Q1, Q3) pretransfusion hemoglobin level was 9.0 (8.4, 9.8) g/dL. Patients aged <20 years were more likely to receive an RBCT than older patients (RR 1.89; 95% confidence interval [CI] 1.44–2.49). Compared with stage IV disease, those with stage II or III disease had a lower likelihood of RBCT (stage II: RR 0.52, 95% CI: 0.37–0.72; stage III: RR 0.68, 95% CI: 0.55–0.83). Patients diagnosed with breast cancer were less likely to receive an RBCT than patients with hematologic cancers (RR 0.34, 95% CI: 0.21–0.55).
Conclusion:
In this study, 40% of cancer patients with chemotherapy-associated anemia in Western Denmark received an RBCT, usually in the inpatient setting; of these, most required subsequent transfusions. Younger age increased the likelihood of receiving an RBCT, and earlier stage or breast cancer decreased RBCT likelihood.
Keywords: red blood cell transfusions, epidemiology, anemia
Introduction
Patients with cancer can develop anemia, which is associated with decreased functional capacity and quality of life and shorter survival; anemia in cancer develops most often in response to chemotherapy.1,2 In the European Cancer Anemia Survey (ECAS), anemia (defined as a hemoglobin level <12 g/dL) was reported in 62.7% of patients who received their first chemotherapy treatment during the study and who were not anemic at enrollment.3 Rates were higher for patients with some tumor types, including lung cancer (77%) and gynecologic cancers (81%). Among patients who were anemic at least once during the ECAS, those receiving chemotherapy were more likely to experience anemia than were untreated patients (75% vs 40%).3
Until the 1990s, red blood cell transfusion (RBCT) was the only option for treating chemotherapy-associated anemia.4,5 Emerging concerns about the availability of sufficient quantities of safe blood products and transfusion-related risks led to the increasing use of erythropoiesis-stimulating agents (ESAs) in place of RBCTs.6–8 Recently, however, guidelines for the use of ESAs have been changed,9–13 stimulating renewed interest in RBCTs.
The use of blood products in Denmark has previously been reported to be the highest in Europe because of the extensive availability of donated blood and easier access to inexpensive blood products.14 Blood donation is considered a civic responsibility in Denmark, and voluntary nonremunerated blood donations provide for the highest level of transfusions per capita in the world. In this setting, the current study was undertaken to characterize the use of RBCT among newly diagnosed cancer patients receiving chemotherapy. This study examined the proportion of patients with chemotherapy-associated anemia who received their first and subsequent RBCTs, the hemoglobin levels before and after the first RBCT, the use of health care resource utilization related to RBCT, and the predictors for RBCT.
Materials and methods
Study design and population
This was a population-based historical cohort study. Residents of the counties of Aarhus and North Jutland in Western Denmark constituted the source population for the study (approximately 1.2 million people). The Danish National Health Service provides tax-supported free health care for the entire Danish population, and each citizen of Denmark has a unique civil personal registration (CPR) number. The CPR number is used to link various health care databases under the auspices of the Danish Government. The Danish National Registry of Patients (DNRP) collects data from all patients admitted to hospitals, emergency rooms, and outpatient clinics. Admissions to hospitals have been registered since 1977, and contacts to emergency units and outpatient clinics have been registered since 1995. More than 99% of all discharges from Danish nonpsychiatric hospital departments are recorded in the DNRP.15 The Danish Cancer Registry (DCR), which has recorded incident cases of cancer throughout the country since 1943, provides accurate and almost complete identification of cancer cases.16 Reporting of new cases of cancer has been required by Danish law since 1987.16 The Danish Transfusion Data Base (DTDB) records all patients receiving blood transfusions and describes the use of blood components in Denmark, including the number of blood components transfused and the concentration of hemoglobin at each visit. The DTDB obtains the transfusion data directly from the blood bank systems in which registration of all blood products is mandatory according to Danish law. The number of transfusions registered in the DTDB is in accordance with the official statistics on use of blood products in Denmark reported by the Danish Medicines Agency.17
In this study, the DCR was searched to identify eligible patients newly diagnosed with cancer by type between January 1, 1998 and December 31, 2003 (Table 1). The DCR was then linked to the DTDB to identify chemotherapy-treated patients with anemia, defined as a hemoglobin level ≤10.9 g/dL. To be eligible for the study, patients had to have received chemotherapy within the first 4 months after their cancer diagnosis. Patients with renal disease, pre-existing anemia, or those receiving radiation therapy either alone or in combination with another therapy were excluded.
Table 1.
Cancer codes in the International Classification of Diseases, 7th Revision (ICD-7)24
| Malignancy type | ICD-7 codesa | |
|---|---|---|
| Hematologic | Non-Hodgkin’s lymphoma | 64, 68 |
| Hodgkin’s lymphoma | 65 | |
| Multiple myeloma | 66 | |
| Leukemia | 67 | |
| Gastrointestinal | Esophagus | 19 |
| Stomach | 20 | |
| Small intestine | 21 | |
| Liver | 24, 26 | |
| Gallbladder | 25 | |
| Pancreas | 27 | |
| Urinary tract | Kidney | 48 |
| Urinary bladder | 49 | |
| Prostate | Prostate | 44 |
| Lung | Lung | 32, 33, 34 |
| Mediastinum | 35 | |
| Gynecologic | Cervix uteri | 38 |
| Corpus uteri | 39, 40 | |
| Ovary | 41 | |
| Other and unspecified female genital organs | 42 | |
| Head and neck | Lip | 13 |
| Tongue | 14 | |
| Salivary glands | 15 | |
| Mouth | 16 | |
| Pharynx | 17 | |
| Nasal cavities and sinuses | 30 | |
| Larynx | 31 | |
| Breast | Breast | 36 |
| Colorectal | Colon | 22 |
| Rectum | 23 | |
| Other | Other and unspecified sites | 62 |
| Endocrinal glands | 57 | |
| Bone | 58 | |
| Melanoma of skin | 51 | |
| Connective tissue | 59 | |
| Other and unspecified male genital organs | 46 | |
| Testis | 45 | |
| Eye | 54 | |
| Brain and nervous system | 55 | |
| Thyroid | 56 | |
| Metastases | 61 | |
| Nonmelanoma of skin | 52 | |
| Peritoneum and unspecified connective tissue | 28 |
Note:
Only the first two digits of the ICD-7 codes are listed.
We examined patient demographic and clinical characteristics including tumor site, tumor stage at diagnosis, age at diagnosis, and hemoglobin level before and after transfusion. We evaluated the proportion of patients with chemotherapy-associated anemia receiving an RBCT and subsequent RBCT by tumor type, by solid tumors versus hematologic cancers, and by cancer stage. We also assessed the number of RBC units administered in the first and subsequent transfusions. Using the DTDB and the DNRP, transfusions were linked to hospital visits. We assessed health care resource utilization related to RBCT (ie, inpatient hospitalizations, outpatient physician visits, and emergency room visits).
Statistical analyses
Descriptive statistics, including medians with first and third quartiles (Q1, Q3) or minimum and maximum values, were calculated for continuous variables. Counts and proportions were calculated for categorical variables.
Because of the high prevalence of RBCTs observed in our study, which may lead to convergence problems, Poisson regression rather than logistic regression was used to estimate the relative risk (RR) of RBCT with 95% confidence intervals (CI),18,19 adjusting for cancer type (hematologic cancers or solid tumors) or cancer site (hematologic; colorectal and gastrointestinal; gynecologic, urinary tract, and prostate; lung; breast; and other, which included endocrinal glands, bone, melanoma of skin, connective tissue, and unspecified male genital organs, testis, eye, brain and nervous system, thyroid, metastases, nonmelanoma of skin, peritoneum, and unspecified connective tissue). All analyses were carried out using the statistical software SAS®, Version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
We identified 28,272 incident cancer patients in the DCR. Of these, 3878 received chemotherapy within the 4 months after diagnosis; 298 were excluded because they were diagnosed with renal disease or pre-existing anemia or were receiving radiation therapy either alone or in combination with another therapy. Of the 3580 patients remaining, 1782 had anemia (hemoglobin ≤10.9 g/dL).
Demographics and clinical characteristics of patients with chemotherapy-associated anemia
The majority of patients with chemotherapy-associated anemia in the study (N = 1782) were female (58%) with a median age (minimum, maximum) of 58 (0.1, 90) years (Table 2). Ninety-eight patients (5.5%) were less than 20 years of age. Approximately two-thirds of patients had solid tumors, with the remainder diagnosed with hematologic cancers. The majority of patients were diagnosed with stage III or IV disease, with differences by tumor type shown in Table 2.
Table 2.
Demographics and clinical characteristics of chemotherapy-induced anemia patients for selected cancers: Aarhus and North Jutland, Denmark (1998–2003)
| All cancers | Hematologic | Solid tumors (all) | Lung | Colorectal | Breast | Gynecologic | Urinary tract | Gastrointestinal | Prostate | Othera | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number and proportion of patients, n (%) | 1782 (100) | 590 (33) | 1192 (67) | 324 (18) | 256 (14) | 214 (12) | 204 (11) | 50 (3) | 44 (2) | 5 (0.3) | 95 (5) |
| Median age (years) | 58 | 57 | 59 | 62 | 63 | 51 | 58 | 57 | 64 | 61 | 36 |
| Minimum, maximum | (0.1, 90) | (0.4, 90) | (0.1, 88) | (31, 81) | (24, 85) | (28, 82) | (1, 86) | (1, 84) | (24, 88) | (54, 73) | (0.1, 86) |
| Male sex, n (%) | 754 (42) | 334 (57) | 420 (35) | 183 (56) | 127 (50) | 1 (0.5) | 0 (0) | 25 (50) | 24 (54) | 5 (100) | 55 (58) |
| Female sex, n (%) | 1028 (58) | 256 (43) | 772 (65) | 141 (44) | 129 (50) | 213 (99.5) | 204 (100) | 25 (50) | 20 (46) | 0 (0) | 40 (42) |
| Treatment, n (%) | |||||||||||
| Chemotherapy | 1048 (59) | 568 (96) | 480 (40) | 301(93) | 21 (8) | 32 (15) | 23 (11) | 24 (48) | 37 (84) | 4 (80) | 38 (40) |
| Chemotherapy and surgery | 734 (41) | 22 (4) | 712 (60) | 23 (7) | 235 (92) | 182 (85) | 181 (89) | 26 (52) | 7 (16) | 1 (20) | 57 (60) |
| Cancer stage, n (%) | |||||||||||
| I | 20 (1) | 0 (0) | 20 (2) | 0 (0) | 0 (0) | 0 (0) | 20 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| II | 215 (12) | 50 (8) | 165 (14) | 23 (7) | 4 (2) | 79 (37) | 15 (7) | 11 (22) | 3 (7) | 0 (0) | 30 (32) |
| III | 562 (32) | 51 (9) | 511 (43) | 121 (37) | 135 (53) | 98 (46) | 108 (53) | 10 (20) | 14 (32) | 2 (40) | 23 (24) |
| IV | 618 (35) | 163 (28) | 455 (38) | 170 (52) | 114 (44) | 29 (14) | 58 (28) | 23 (46) | 23 (52) | 2 (40) | 36 (38) |
| Unknown | 367 (21) | 326 (55) | 41 (3) | 10 (3) | 3 (1) | 8 (4) | 3 (2) | 6 (12) | 4 (9) | 1 (20) | 6 (6) |
Note:
Includes endocrinal glands, bone, melanoma of skin, connective tissue, other and unspecified male genital organs, testis, eye, brain and nervous system, thyroid, metastases, nonmelanoma of skin, and peritoneum and unspecified connective tissue.
Red blood cell transfusions in patients with chemotherapy-associated anemia
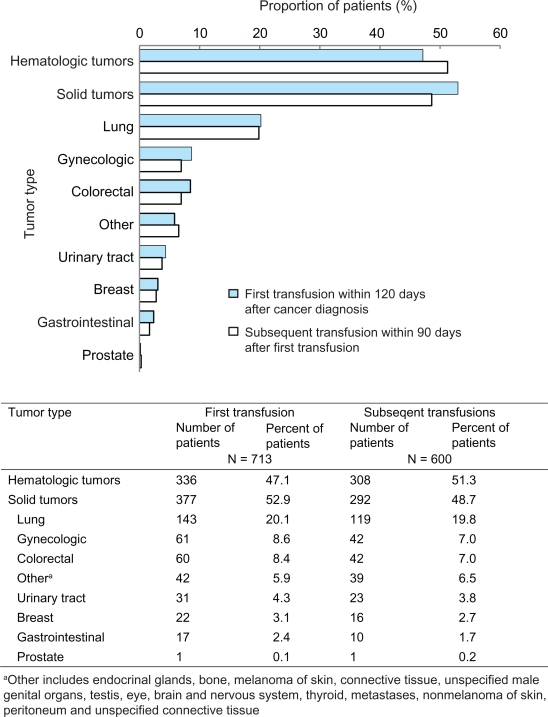
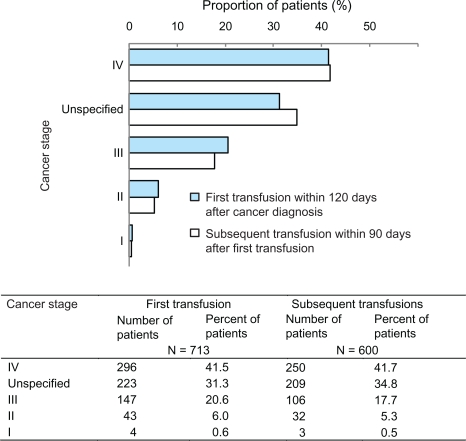
Overall, a total of 713 of 1782 (40%) patients received an RBCT within 120 days of cancer diagnosis (Figure 1). Among these patients, approximately 23% underwent surgery, including 159 patients (22%) with solid tumors and eight patients (1%) with hematologic malignancies (data not shown). RBCTs were administered most frequently to patients with hematologic cancers (47%) and lung cancer (20%) (Figure 1). In contrast, only 2% of breast cancer patients and 3% of gastrointestinal cancer patients received an RBCT. Among patients whose cancer stage was known, RBCTs were administered most frequently to patients with stage IV disease (42%).
Figure 1.
Proportion of patients receiving red blood cell transfusions by tumor type: Aarhus and North Jutland, Denmark (1998–2003).
Similar patterns were observed for subsequent RBCTs within 90 days after the first transfusion (Figure 1). Among those who had a first transfusion, 84% (600 of 713) received a subsequent transfusion; most were patients with hematologic cancers (51%) or lung cancer (20%). Only 3% of breast cancer patients required subsequent transfusions. As was oberved in patients receiving a first transfusion, the highest proportion of those receiving subsequent RBCTs were patients with stage IV disease (42%, Figure 2).
Figure 2.
Proportion of patients receiving red blood cell transfusions by cancer stage: Aarhus and North Jutland, Denmark (1998–2003).
Overall, patients received a median (Q1, Q3) of 2 (1, 2) RBC units in their first transfusion, a pattern that was similar among patients with all tumor types (data not shown). A median (Q1, Q3) of 5 (3, 10) RBC units were administered to patients who received a subsequent transfusion. Patients with hematologic cancers or with stage II disease required more RBC units in subsequent transfusions; the median (Q1, Q3) was 8 (4, 17) RBC units for hematologic cancers and 7 (2, 8) RBC units for stage II disease.
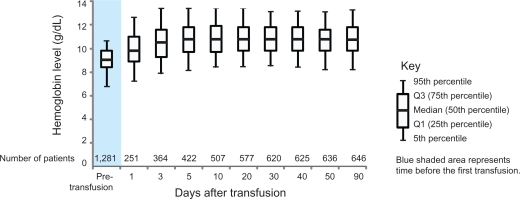
Among patients with chemotherapy-associated anemia who received a transfusion, the median (Q1, Q3) hemoglobin level before the first transfusion was 9.0 g/dL (8.4, 9.8); 5% of patients had hemoglobin levels of 6.8 g/dL or lower (Figure 3). The median (Q1, Q3) hemoglobin value reached 10.8 g/dL (9.7, 11.8) within 5 days after transfusion. The median (Q1, Q3) time from the first hemoglobin measurement to transfusion was 1 (1.0, 3.0) day.
Figure 3.
Pre- and post-distribution of hemoglobin levels for the first red blood cell transfusion: Aarhus and North Jutland, Denmark (1998 2003), n = 501.
Predictors of red blood cell transfusion in chemotherapy-associated anemia patients
Age, tumor stage, and tumor site were significant predictors of RBCT after adjusting for cancer type or tumor site (Table 3). An approximately two-fold increase in the likelihood of RBCT was found for chemotherapy-associated anemia patients younger than 20 years (RR 2.08, 95% CI: 1.58–2.73 adjusted for tumor type; RR 1.89, 95% CI: 1.44–2.49 adjusted for tumor site). Stage II or stage III disease was a predictor of decreased likelihood of RBCT compared with stage IV disease (stage II, RR 0.47, 95% CI: 0.34–0.65 adjusted for tumor type; RR 0.52, 95% CI: 0.37–0.72 adjusted for tumor site; stage III, RR 0.66, 95% CI: 0.54–0.81 adjusted for tumor type; RR 0.68, 95% CI: 0.55–0.83 adjusted for tumor site). The likelihood of transfusion was reduced for patients with breast cancer compared with those with hematologic cancers (RR 0.34, 95% CI: 0.21–0.55) (Table 3).
Table 3.
Likelihood of red blood cell transfusion in chemotherapy-associated anemia patients: Aarhus and North Jutland, Denmark (1998–2003)
| Crude likelihood (RR) for transfusion | Likelihood (RR) for transfusion adjusted for tumor typea | Likelihood (RR) for transfusion adjusted for tumor siteb | |
|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Gender | |||
| Female | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Male | 1.21 (1.04–1.40) | 0.94 (0.81–1.09) | 0.90 (0.77–1.05) |
| Age (years) | |||
| 0–19 | 2.61 (2.00–3.40) | 2.08 (1.58–2.73) | 1.89 (1.44–2.49) |
| 20–49 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 50–59 | 1.02 (0.81–1.27) | 0.99 (0.79–1.25) | 0.98 (0.78–1.23) |
| 60–69 | 1.11 (0.89–1.38) | 1.04 (0.83–1.30) | 1.01 (0.81–1.27) |
| 70–79 | 1.21 (0.95–1.54) | 1.06 (0.83–1.35) | 1.02 (0.80–1.31) |
| 80+ | 1.23 (0.81–1.85) | 0.91 (0.60–1.38) | 0.87 (0.57–1.31) |
| Tumor spread | |||
| Stage I | 0.42 (0.16–1.12) | 0.64 (0.23–1.74) | 0.48 (0.17–1.33) |
| Stage II | 0.42 (0.30–0.57) | 0.47 (0.34–0.65) | 0.52 (0.37–0.72) |
| Stage III | 0.55 (0.45–0.67) | 0.66 (0.54–0.81) | 0.68 (0.55–0.83) |
| Stage IV | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Unspecified | 1.27 (1.07–1.51) | 0.93 (0.76–1.15) | 0.97 (0.78–1.19) |
| Cancer type | |||
| Hematologic cancers | 1.00 (reference) | 1.00 (reference) | |
| Solid tumors | 0.56 (0.48–0.64) | 0.87 (0.71–1.07) | |
| Cancer site | |||
| Hematologic cancers | 1.00 (reference) | 1.00 (reference) | |
| Colorectal and gastrointestinal | 0.45 (0.35–0.58) | 0.72 (0.52–1.00) | |
| Gynecologic, urinary tract, and prostate | 0.63 (0.50–0.79) | 1.01 (0.74–1.37) | |
| Lung | 0.78 (0.64–0.94) | 0.93 (0.73–1.17) | |
| Breast | 0.18 (0.12–0.28) | 0.34 (0.21–0.55) | |
| Other | 0.78 (0.56–1.07) | 1.04 (0.73–1.50) | |
Notes:
Adjusting for tumor type in two groups: hematologic cancers and solid tumors;
Adjusting for tumor site: hematologic cancers; colorectal and gastrointestinal; gynecologic, urinary tract, and prostate; lung; breast; and other, which included endocrinal glands, bone, melanoma of skin, connective tissue and unspecified male genital organs, testis, eye, brain and nervous system, thyroid, metastases, nonmelanoma of skin, and peritoneum and unspecified connective tissue.
Abbreviations: CI, confidence interval; RR, relative risk.
Health care resource utilization in patients with chemotherapy-associated anemia
Of the 713 patients with chemotherapy-associated anemia who received an RBCT within 120 days of cancer diagnosis, 667 transfusions (94%) were administered in the inpatient setting, 36 (5%) in the outpatient setting, and none in the emergency room setting; we could not link 10 transfusions (1%) to a specific hospital visit. The diagnoses of the 667 patients who received transfusions in the inpatient setting were approximately equally divided between hematologic cancers (48.6%) and solid tumors (51.4%) (Table 4). Inpatient transfusion recipients included 276 patients (41%) with stage IV disease and 133 patients (20%) with stage III disease. Stage at diagnosis was unspecified for 215 patients (32%) who received inpatient transfusions.
Table 4.
Healthcare resource utilization for patients with chemotherapy-associated anemia receiving a red blood cell transfusion: Aarhus and North Jutland, Denmark (1998–2003)
|
Inpatient visit (n = 667) |
Outpatient visit (n = 36) |
Othera (n = 10) |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Tumor type | ||||||
| Hematologic cancers | 324 | 48.6 | 10 | 27.8 | 2 | 20.0 |
| Solid tumors | 343 | 51.4 | 26 | 72.2 | 8 | 80.0 |
| Tumor site | ||||||
| Gastrointestinal | 15 | 2.2 | 2 | 5.6 | 0 | 0 |
| Urinary tract | 30 | 4.5 | 0 | 0 | 1 | 10.0 |
| Prostate | 1 | 0.1 | 0 | 0 | 0 | 0 |
| Lung | 127 | 19.0 | 13 | 36.1 | 3 | 30.0 |
| Gynecologic | 59 | 8.8 | 0 | 0 | 2 | 20.0 |
| Breast | 20 | 3.0 | 1 | 2.8 | 1 | 10.0 |
| Colorectal | 51 | 7.6 | 8 | 22.2 | 1 | 10.0 |
| Otherb | 40 | 6.0 | 2 | 5.6 | 0 | 0 |
| Stage at diagnosis | ||||||
| I | 4 | 0.6 | 0 | 0 | 0 | 0 |
| II | 39 | 5.8 | 3 | 8.3 | 1 | 10.0 |
| III | 133 | 19.9 | 11 | 30.6 | 3 | 30.0 |
| IV | 276 | 41.4 | 16 | 44.4 | 4 | 40.0 |
| Unspecified | 215 | 32.2 | 6 | 16.7 | 2 | 20.0 |
Notes:
Transfusions that could not be linked to a specific hospital visit;
Includes endocrinal glands, bone, melanoma of skin, connective tissue, unspecified male genital organs, testis, eye, brain and nervous system, thyroid, metastases, nonmelanoma of skin, peritoneum, and unspecified connective tissue.
The 36 outpatient transfusion recipients included 26 patients (72%) with solid tumors and 10 (28%) with hematologic malignancies. Sixteen (44%) of these patients had stage IV disease and 11 (31%) had stage III disease.
Hematologic cancers were the most frequent diagnosis among patients who received transfusions in both the inpatient (49%) and outpatient (28%) settings (Table 4). Among solid tumor patients, lung cancer was the most frequent diagnosis for patients who received transfusions as inpatients (19%) or outpatients (36%) (Table 4). In the inpatient setting, gynecologic cancer (9%) and colorectal cancer (7%) were the next most frequent diagnoses. For patients who received transfusions in the outpatient setting, colorectal cancer (22%) and gastrointestinal cancer and other cancer sites (6% each) followed lung cancer in frequency of diagnosis (Table 4).
The median number (Q1, Q3) of transfusion-related inpatient visits was 2 (0, 6) for the study population overall, 5 (0, 12) for patients with hematologic cancers, and 1 (0, 4) for patients with solid tumors. The median number (Q1, Q3) of outpatient visits within 90 days after transfusion in our study population was 0 (0, 0) overall (data not shown).
Discussion
In this study of patients with chemotherapy-associated anemia in Western Denmark, 40% of the patient population received at least one RBCT, with a median total of two RBC units within 4 months after their cancer diagnosis. This finding is consistent with ECAS data, which showed that 39% of anemic patients received anemia treatment (RBCT, ESAs, or iron) at any time during the survey period.3 Among patients with chemotherapy-associated anemia who received a transfusion, a large proportion received a subsequent transfusion, with a median total of five RBC units within 90 days after the first transfusion. Although we did not have information on the chemotherapy cycle in which transfusions were administered, this need for additional transfusion is consistent with the study by Groopman and Itri,1 which reported that mean hemoglobin concentration decreased progressively with treatment cycle for cancer patients. Predictors of RBCT include age, cancer stage, and tumor site.
Among patients with chemotherapy-associated anemia who received a transfusion, the median hemoglobin level before the transfusion was 9.0 g/dL. This finding is similar to that of the ECAS study, in which the mean hemoglobin level for initiation of anemia treatment was 9.7 g/dL.3 This practice is consistent with the 2008 guidelines of the European Organization for Research and Treatment of Cancer (EORTC), which recommend treatment of symptomatic anemia at a hemoglobin level from 9 g/dL to 11 g/dL and evaluation for transfusion if the hemoglobin level is less than 9 g/dL.10
Most transfusions in our study were administered in the inpatient setting, regardless of tumor type or cancer stage. We did not have information regarding the primary reason for hospital admission of these patients. However, patients in this study had a primary diagnosis of cancer and may have had multiple comorbid conditions in addition to chemotherapy-associated anemia; any of these might have led to hospitalization. We speculate that the large proportion of patients who received transfusions in the inpatient setting may in fact have been hospitalized for other conditions. Further study of these factors is warranted to develop a comprehensive overview of health care resource utilization in cancer patients with chemotherapy-associated anemia.
Our study confirms previous research on predictors of transfusion use in chemotherapy-associated anemia. In our study, age was a predictor of RBCT in chemotherapy-associated anemia patients. This result is consistent with findings from a large European survey of 18 major pediatric oncology centers in seven countries that quantified the incidence of anemia or treatment in the pediatric patient population. That survey showed that anemia is a very common complication in children with cancer; approximately 80% of all patients met the World Health Organization and EORTC criteria for anemia, and most patients were treated with RBCT.20 In our study, there was a two-fold increased likelihood for RBCT in patients aged younger than 20 years.
In published reports, the highest incidence of RBCTs are said to occur in anemic patients with lymphomas, lung tumors, and gynecologic (ovarian) or genitourinary tumors.3,21–23 Furthermore, the ECAS showed that anemic patients with breast cancer were least likely to receive anemia treatment. With the exception of gynecologic tumors, these findings are consistent with our results, even though the definitions of anemia differed among the studies. The observation of low transfusion rates for breast cancer could be related to the fact that 70% of patients with breast cancer had stage II or III disease (Table 2), a predictor of decreased likelihood of RBCT (Table 3), whereas only 17% of patients with hematologic cancers had stage II or III disease (Table 2). The low proportions of transfused patients with chemotherapy-associated anemia diagnosed with hematologic and lung cancers who underwent surgery in our study (2% and 6%, respectively) suggest that RBCTs in these patients were not administered as a consequence of a surgical procedure.
Several study limitations should be noted. Because of the historical cohort design of our study, the specific reasons for the underlying anemia of the patients in this study are unknown. Also unknown is the date on which chemotherapy was initiated, which may have affected the timing of the requirement for RBCTs. Anemia could be a direct effect of the malignancy on the bone marrow, a result of high tumor burden or of biologic effects mediated by inflammatory cytokines, the result of treatment, or a combination of these effects. Patterns of transfusion usage in Denmark differ from those in other countries because of the ready availability of donated blood, which may limit the generalizability of these results. In addition, populations that are racially or ethnically more heterogeneous may exhibit different patterns than were observed in the predominantly Caucasian Danish population. We were not able to assess other anemia treatments. Although ESAs were available throughout the study period, their use was not common in Western Denmark during the time period of this study. Examination of ESA use in the study population is beyond the scope of this analysis.
Strengths of this study include the population-based lifelong follow-up of patients in Denmark providing longitudinal capture of medical information. The CPR number system allowed unambiguous individual level record linkage between the different registries, which have been shown to be accurate and virtually complete. Detailed medical data were available, including information on all transfusions and hemoglobin values before and after transfusions. This descriptive study adds to the body of evidence about chemotherapy-associated anemia patients and patterns of RBCT in Denmark, a country with an extensively available blood supply, and provides a context for these measures in countries with different transfusion practices.
Acknowledgments
We thank Dr Henrik Sørensen for his valuable feedback and helpful discussions during development of the manuscript, and Dr Marieke Schoonen for her contributions to the development of the study. We also thank Sue Hudson, whose services were funded by Amgen Inc., for medical writing assistance.
Footnotes
Disclosure
Mellissa Yong is currently employed at Amgen Inc. Jon P Fryzek was an employee of Amgen Inc., at the time this study was conducted. The other authors report no conflicts of interest in this work.
References
- 1.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91:1616–1634. doi: 10.1093/jnci/91.19.1616. [DOI] [PubMed] [Google Scholar]
- 2.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 3.Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40:2293–2306. doi: 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo JD, Lichtin AE, Woolf SH, et al. Use of epoetin in patients with cancer: evidence-based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of Hematology. J Clin Oncol. 2002;20:4083–4107. doi: 10.1200/JCO.2002.07.177. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo JD, Lichtin AE, Woolf SH, et al. Use of epoetin in patients with cancer: evidence-based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of Hematology. Blood. 2002;100:2303–2320. doi: 10.1182/blood-2002-06-1767. [DOI] [PubMed] [Google Scholar]
- 6.Engert E. Recombinant human erythropoietin as an alternative to blood transfusion in cancer-related anemia. Dis Manage Health Outcomes. 2000;8:259–272. [Google Scholar]
- 7.Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. First of two parts–blood transfusion. N Engl J Med. 1999;340:438–447. doi: 10.1056/NEJM199902113400606. [DOI] [PubMed] [Google Scholar]
- 8.Williamson LM, Lowe S, Love EM, et al. Serious hazards of transfusion (SHOT) initiative: analysis of the first two annual reports. BMJ. 1999;319:16–19. doi: 10.1136/bmj.319.7201.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrijvers D, De Samblanx H, Roila F. Erythropoiesis-stimulating agents in the treatment of anaemia in cancer patients: ESMO Clinical Practice Guidelines for use. Ann Oncol. 2010;21(Suppl 5):v244–v247. doi: 10.1093/annonc/mdq202. [DOI] [PubMed] [Google Scholar]
- 10.Aapro MS, Link H. September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. Oncologist. 2008;13(Suppl 3):33–36. doi: 10.1634/theoncologist.13-S3-33. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo JD, Brouwers M, Hurley P, et al. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28:4996–5010. doi: 10.1200/JCO.2010.29.2201. [DOI] [PubMed] [Google Scholar]
- 12.Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 2010;116:4045–4059. doi: 10.1182/blood-2010-08-300541. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network (NCCN) Cancer- and Chemotherapy-induced Anemia, Version 2.2011. 2011. http://www.nccn.org/professionals/physician_gls/PDF/anemia.pdf. Accessed November 3, 2010.
- 14.Van der Poel C, Janssen M. The collection, testing, and use of blood and blood products in Europe in 2003. http://www.coe.int/t/dg3/health/Source/2003reportfinal_en.pdf. Accessed August 26, 2010.
- 15.Andersen TF, Madsen M, Jorgensen J, et al. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–268. [PubMed] [Google Scholar]
- 16.Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish Cancer Registry: history, content, quality and use. Dan Med Bull. 1997;44:535–539. [PubMed] [Google Scholar]
- 17.Danish Medicines Agency Redegørelse for blodproduktområdet 2007. 2008. Copenhagen;
- 18.Cummings P. Re: “Estimating the relative risk in cohort studies and clinical trials of common outcomes”. Am J Epidemiol. 2004;159:213. 214–215. doi: 10.1093/aje/kwh021. author reply. [DOI] [PubMed] [Google Scholar]
- 19.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 20.Michon J. Incidence of anemia in pediatric cancer patients in Europe: results of a large, international survey. Med Pediatr Oncol. 2002;39:448–450. doi: 10.1002/mpo.10183. [DOI] [PubMed] [Google Scholar]
- 21.Skillings JR, Sridhar FG, Wong C, Paddock L. The frequency of red cell transfusion for anemia in patients receiving chemotherapy. A retrospective cohort study. Am J Clin Oncol. 1993;16:22–25. doi: 10.1097/00000421-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Heddens D, Alberts D, Garcia D, et al. Factors associated with platinum-induced anemia in ovarian cancer (ovca) patients (pts) in Southwest Oncology Group(s) studies [abstract 1387] Proc ASCO. 1998;17:359a. [Google Scholar]
- 23.Dalton J, Bailey N, Barrett-Lee P, O’Brien M. Multicenter UK audit of anemia in patients receiving cytotoxic chemotherapy. Paper presented at: American Society of Clinical Oncology; 1998. [Google Scholar]
- 24.World Health Organization (WHO) Manual of the International Statistical Classification of Diseases, Injuries and Causes of Death: Seventh Revision. Geneva: WHO; 1957. [Google Scholar]