Recommended definition of MGD: Meibomian gland dysfunction (MGD) is a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. This may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease.
Previous definitions and criteria of MGD: There is no firmly established definition of MGD published in the literature. Most researchers have used a criterion-based approach to describe the condition, with combinations of objective findings and measurements. Anatomic changes of the lid margin, expressibility of meibomian lipids, gland dropout by meibography, evaporimetry, and meibometry are most commonly used (Table 1).
Table 1.
Criteria of Meibomian Gland Dysfunction Used in Previous Works
| Mathers et al.1,2 |
| Meibomian gland dropout and normal tear secretion |
| Thickened secretions following meibomian gland expression in most cases |
| Shimazaki et al.3 |
| Obstructive MGD characterized by: |
| The presence of gland dropout at the central two thirds of the lower tarsus |
| A lack of meibum secretion after application of moderate digital pressure |
| Lee and Tseng4 |
| Abnormal meibomian gland function characterized by: |
| Lack of active inflammation |
| Poor meibum expression |
| Orifice squamous metaplasia or acinar atrophy |
| Yokoi et al.5 |
| Abnormal findings on the lid margin (three positive findings) |
| Reduced oil expression (a negative score) |
| An Oxford staining grade of ≥2 but no abnormalities in the Schirmer and cotton-thread test results |
| Goto et al.6 |
| Noninflamed, obstructive MGD characterized by: |
| Presence of meibomian gland dropout by meibography |
| No or poor meibum expression by digital compression |
| No or negligible inflammation in the lid margin |
| Foulks and Bron7* |
| MGD defined as a symptomatic or asymptomatic condition with typical morphologic lid features and the following additional features: |
| Tear flow: normal or increased |
| Ocular surface damage: present |
| Dropout >1 (central two thirds of the lid) |
| Oil volume: <0.5 mm |
| Oil viscosity: criterion not stated |
| Meibometry reading: normal (<800 meibometer units) |
| Evaporation rate: increased |
| Seborrheic MGD can be defined as a symptomatic or asymptomatic condition with typical morphological lid features and the following additional features: |
| Tear flow: normal |
| Ocular surface damage: variable |
| Dropout: nil |
| Oil volume: >0.8 mm |
| Oil viscosity: normal |
| Meibometry reading |
| Evaporation rate: normal |
| Matsumoto et al.8,9 |
| Occluded MG orifices |
| Cloudy or inspissated glandular secretion with lack of clear meibum secretion after the application of moderate digital pressure on the tarsus of the upper and lower eye lid |
| Presence of keratinization or displacement of the mucocutaneous junction |
| Absence of inflammatory lid disease such as blepharitis as well as inflammatory skin disorders such as atopic dermatitis, seborrhea sicca, and acne rosacea |
| Absence of a history of cicatricial eyelid and conjunctival diseases, such as trachoma, erythema multiforme, ocular cicatricial pemphigoid, and chemical, thermal, or radiation injury |
| Absence of excessive meibomian lipid secretion (seborrheic MGD) |
| Arita et al.10 |
| Obstructive MGD should be suspected when any two of the three scores are abnormal |
| Amano et al.11 |
Obstructive MGD is considered to be present when all of the following three signs/findings are present
|
Symptom score: based on the questionnaire about 14 ocular symptoms: ocular fatigue, discharge, foreign body sensation, dryness, uncomfortable sensation, sticky sensation, pain, epiphora, itching, redness, heavy sensation, glare, excessive blinking, and a history of chalazion or hordeolum. Symptoms were scored from 0 through 14, according to the number of symptoms present.
Lid margin abnormality score: irregular lid margin, vascular engorgement, plugged meibomian gland orifices, and anterior or posterior replacement of the mucocutaneous junction scored from 0 through 4 according to the number of these abnormalities present in each eye.
Meibo-score is a semiquantitative score of the results of noncontact meibography:
Grade 0, no loss of meibomian glands;
Grade 1, area loss less than one third of the total meibomian gland area;
Grade 2, area loss between one third and two thirds of the total meibomian gland area;
Grade 3, area loss more than two thirds of the total meibomian gland area;
Meibo-scores summed for the upper and lower eyelids to obtain a score of 0–6 for each eye.
Terminology
The terminology associated with MGD is described in Table 2. The term blepharitis is a general one, describing inflammation of the lid as a whole. Marginal blepharitis is applied to inflammation of the lid margin and includes both anterior and posterior blepharitis.
Table 2.
Terminology of Blepharitis
| Condition | Definition |
|---|---|
| Blepharitis | Blepharitis is a general term describing inflammation of the lid as a whole; marginal blepharitis is inflammation of the lid margin and includes both anterior and posterior blepharitis. |
| Anterior blepharitis | Anterior blepharitis describes an inflammation of the lid margin anterior to the gray line and concentrated around the lashes. It may be accompanied by squamous debris or collarettes around the lashes, and inflammation may spill onto the posterior lid margin. |
| Posterior blepharitis | Posterior blepharitis describes an inflammation of the posterior lid margin, which may have different causes, including MGD, conjunctival inflammation (allergic or infective), and/or other conditions, such as acne rosacea.7,12–15 |
| MGD | MGD describes a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease. |
Anterior blepharitis describes inflammation of the lid margin anterior to the gray line and centered around the lashes.16 The gray line represents the location of the marginal region of the orbicularis muscle (the muscle of Riolan) seen through the lid skin.12 It divides the lid into an anterior lamella (skin and muscle) and a posterior lamella (tarsus and conjunctiva).13 Anterior blepharitis may be accompanied by squamous debris or collarettes around the base of the lashes and vascular changes of the lid skin.
Posterior blepharitis is used to describe inflammatory conditions of the posterior lid margin,7 including MGD. Indeed, recent literature has used the terms posterior blepharitis and meibomian gland dysfunction or MGD as if they were synonymous,14–18 but these terms are not interchangeable.19 Distinct from the portion of lid margin anterior to the gray line, which includes the skin and eyelashes, the posterior lid margin contains the marginal mucosa, the mucocutaneous junction, the meibomian gland orifices and associated terminal ductules, and the neighboring keratinized skin. Posterior blepharitis is a term used to describe inflammatory conditions of the posterior lid margin,20 of which MGD7 is only one cause. Other causes include infectious20 or allergic20 conjunctivitis and systemic conditions such as acne rosacea.13,20
Clinically, a diagnosis of posterior blepharitis implies the presence of inflammation affecting the posterior lid margin. However, in its earliest stages, MGD may not be associated with the biomicroscopic lid margin signs characteristic of posterior blepharitis.21,22 At this stage, affected individuals may be symptomatic, but alternatively they may be asymptomatic, and the condition may be regarded as subclinical.22,23 In either case, MGD may be diagnosed by meibomian gland expression alone, with demonstration of an altered quality of expressed secretions, and/or by a loss of gland functionality (decreased or absent expressibility). As it progresses, symptoms develop and lid margin signs may become visible with biomicroscopy. At this point, an MGD-related posterior blepharitis is said to be present.
The term meibomian gland dysfunction (MGD), first used in the early 1980s by Korb and Henriquez22 and then by others,14,23,24 is considered to be appropriate for describing the functional abnormalities of the meibomian glands. The general term meibomian gland disease is used to describe a broader range of meibomian gland disorders, including neoplasia and congenital disease.7,25 The more specific term MGD also emphasizes the important role that the meibomian glands play in the tear film and ocular surface.
Other terms such as meibomitis or meibomianitis describe a subset of disorders of MGD associated with inflammation of the meibomian glands. Although inflammation may be important in the classification and in the treatment of MGD, these terms are not sufficiently general, as inflammation is not always present in MGD.
When MGD occurs with increased secretion of meibomian lipids, the terms hypersecretory MGD and seborrheic MGD have been used. Confusion arises with the term seborrheic dermatitis, which is a chronic, relapsing inflammatory skin condition occurring in areas rich in sebaceous glands.26 This condition is not regularly associated with excessive secretion of sebum, nor are the sebaceous glands primarily involved. The actual underlying etiology may be related to fungal infection (genus Malassezia).26 Therefore the more appropriate and clinically understandable term is hypersecretory MGD. Similarly, the term hyposecretory MGD is used instead of obstructive MGD. Obliteration of meibomian gland ducts and orifice obstruction due to hyperkeratinization is an important finding in hyposecretory MGD.27 However, decreased lipid secretion can occur due to abnormalities in meibomian glands without concurrent remarkable obstruction. Therefore, hyposecretory MGD is used, as it covers a wider range of manifestations of the disorder.
MGD Definition Background
The term dysfunction is used because the function of meibomian glands is disturbed (Table 3). Alteration of these functions leads to decreased tear film stability (evidenced by increased evaporation, increased surface tension, contamination with sebum, unsealed lid during sleep) and/or symptoms. The dysfunction is a result of anatomic abnormalities or abnormalities in meibomian gland secretion.
Table 3.
The Functions of Healthy Meibomian Lipids7
|
The term diffuse is used in the definition of MGD, because the disorder involves most of the meibomian glands. Localized involvement of meibomian glands, such as in chalazia, does not tend to cause abnormalities in the tear film or ocular surface epithelia, and therefore is not considered to be within the context of MGD. Obstruction of the meibomian gland orifice and terminal duct is identified as the most prominent aspect of MGD (see the Report on Anatomy, Physiology, and Pathophysiology).
Subjective symptoms of eye irritation are included in the definition of MGD, as symptoms are of greatest concern to the patient, and often to the clinician.28 Improving patient symptoms is the major goal in the treatment of MGD. The enigma still exists, as in dry eye, that signs and symptoms frequently show disparity.
The role of inflammation in the etiology of MGD is controversial and uncertain. Although the cause-and-effect relationship is unclear, associations between meibomian gland dropout and ocular surface inflammatory diseases, such as chronic blepharitis,29 giant papillary conjunctivitis,30 and Sjögren syndrome,31 have been reported. Histopathologic observation in meibomian glands obtained at autopsy have revealed lipogranulomatous inflammation around the gland lobules in 18.6% of cases.32
Clinically, increased vascularization of the posterior lid margin is one of the major signs of inflammation and of MGD,25 and its importance in diagnosis and treatment is widely accepted.27 It should be noted, however, that the finding has been shown to be related to aging.33
McCulley and Sciallis18 described a condition called meibomian keratoconjunctivitis (MKC), often associated with anterior blepharitis, with the most prominent changes centered on the meibomian glands. It is usually associated with some form of skin disease and is characterized by tear film instability, ocular surface inflammation, and ocular surface damage. MKC is an important cause of symptoms in severe chronic blepharitis.27
Classification of MGD
Overall Consideration
MGD may be classified according to anatomic changes, pathophysiological changes, or the severity of disease. Any classification system must meet the needs of the clinician and researcher alike; therefore, a classification based on pathophysiology is deemed to best meet these needs and is presented here.
Previous Works on Classification of MGD
At least five different classifications have been published previously (Table 4). The first was proposed in 1921 by Gifford,34 who classified meibomian gland changes in chronic blepharoconjunctivitis. His classification emphasized the involvement of adjacent tissues (e.g., conjunctival and tarsal concretions).
Table 4.
Classification Systems for Meibomian Gland Dysfunction
Second, in the 1980s McCulley et al.16 classified chronic blepharitis into four primary categories, including both anterior and posterior blepharitis: (1) staphylococcal, (2) seborrheic, (3) primary meibomitis, and (4) other (including atopy, psoriatic, and fungal). The seborrheic category was further divided into four subcategories: (2a) seborrheic alone, (2b) mixed seborrheic/staphylococcal, (2c) seborrheic with meibomian gland seborrhea, and (2d) seborrheic with secondary meibomitis. Categories 2c, 2d, and 3 were associated with the posterior lid margin and the meibomian glands. The classification was observational and based on appearance, including meibomian gland orifice obstruction and inflammation around the glands.
Mathers et al.29 classified chronic blepharitis into four groups in 1991: (1) seborrheic MGD, (2) obstructive MGD, (3) obstructive MGD with sicca, and (4) sicca. Three parameters were used to classify MGD: (1) meibomian gland morphology using meibography, (2) tear osmolarity, and (3) Schirmer's test. This classification system was oriented more toward tear film changes rather than the changes in function or anatomy of the meibomian glands.
The 1991 classification by Bron et al.25 was based on detailed observation of the lid margins. This observational classification described the lid changes observed on slit lamp biomicroscopy and classified meibomian gland diseases into five main subcategories: (1) absence/deficiency, (2) replacement, (3) meibomian seborrhea, (4) meibomitis, and (5) meibomian neoplasia. Changes in the meibomian glands were described in terms of mucocutaneous changes, ducts, acini, and secretory performance of the gland. Each factor was graded in a semiquantitative fashion.
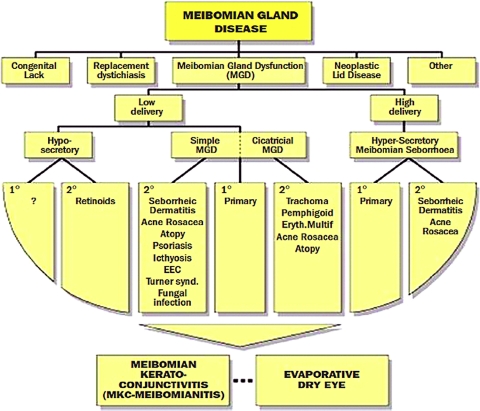
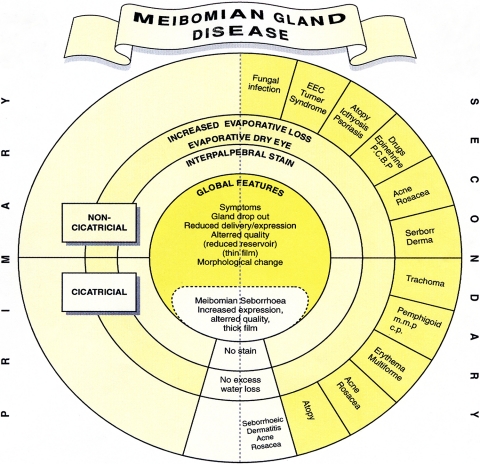
A more recent classification of MGD was published by Foulks and Bron7 in 2003. This system integrated the observation of anatomic changes and gland expressibility with biochemical alteration of meibomian gland lipids and the underlying etiology (Fig. 1). Bron and Tiffany27 presented a unique circular diagram breaking down the etiologies of meibomian gland disease into primary cicatricial and noncicatricial, secondary, and hypersecretory causes (Fig. 2).
Figure 1.
An early classification scheme of meibomian gland disease from Foulks and Bron7 included presentations other than MGD, and separated MGD into high- and low-delivery states. Reprinted with permission from Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1:107–126. © Ethis Communications, Inc.
Figure 2.
The classification system described by Bron and Tiffany27 segregated the etiologies of meibomian gland disease into primary, secondary, and hypersecretory causes. Theirs was a unique presentation in its circular configuration. Reprinted with permission from Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149–164. © Ethis Communications, Inc.
Recommended Classification of MGD by the Subcommittee
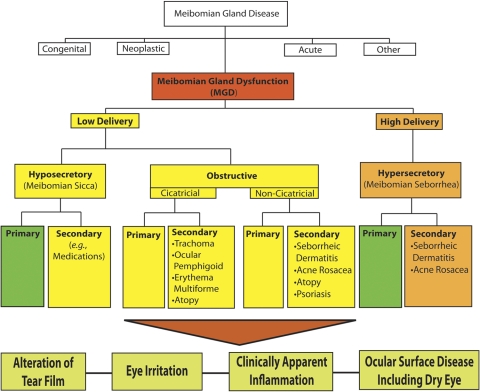
Under the broad category term, meibomian gland dysfunction, MGD is further classified into two major categories based on meibomian gland secretion (Fig. 3): low-delivery and high-delivery states. Low-delivery states are further classified as hyposecretory (meibomian sicca) and obstructive, with cicatricial and noncicatricial subcategories. Primary causes are listed under each category and refer to conditions for which there is no discernible underlying cause or etiology.
Figure 3.
The new classification system proposed by the International Workshop on MGD distinguishes among the subgroups of MGD on the basis of the level of secretions and further subdivides those categories by potential consequences and manifestations. On the basis of these proposed classifications, obstructive MGD is the most pervasive.
Low-Delivery States.
Low delivery of meibomian gland secretions is further classified into two major categories: hyposecretion and obstructive conditions. Meibomian gland hyposecretion is characterized by decreased meibomian lipid secretion without gland obstruction. Although there is no published and verified evidence of primary hyposecretion, this disorder is associated clinically with gland atrophy. A decrease in the number of functional meibomian glands is associated with contact lens wear, and this decrease appears to be proportional to the duration of contact lens wear.35
The other category under low-delivery states is meibomian gland obstruction. This is probably the most common form of MGD.3,7,32 Histopathologic changes include hypertrophy of the duct epithelium and keratinization of the orifice epithelium. Low delivery is caused by glandular obstruction due to either terminal duct obstruction or altered secretion. The disorder is seen in older subjects or after the use of retinoids for acne treatment.36 Androgen insufficiency or lack of androgen receptors is also associated with keratinization, obstruction, and alteration of meibomian gland secretions (see the Anatomy, Physiology, and Pathophysiology report).37 Obstructive causes may be further classified as cicatricial and noncicatricial. In noncicatricial obstructive MGD the ducts and orifices remain in their normal anatomic position; in cicatricial obstructive MGD the ducts and orifices are dragged posteriorly into the mucosa. Causes of cicatricial obstructive MGD include trachoma, ocular cicatricial pemphigoid, erythema multiforme, and atopic eye disease. Noncicatricial obstructive MGD may be seen in Sjögren's syndrome, seborrheic dermatitis, acne rosacea, atopy, and psoriasis. Inflammation in adjacent tissues is commonly seen in conjunctivitis and anterior blepharitis, for example. Although inflammation is frequently associated with meibomian gland obstruction (the term meibomitis has been used as a synonym), whether the inflammation is a cause or a result of meibomian gland obstruction remains unclear.
High-Delivery States.
Hypersecretory MGD is characterized by the release of a large volume of meibomian lipid at the lid margin in response to pressure on the tarsus. Although it has been reported that hypersecretory MGD is associated with seborrheic dermatitis in 100% of cases,16 this eyelid disorder is believed to occur in other diseases as well, including atopic disease and acne rosacea (secondary hypersecretory MGD). There also have been cases without the association of other diseases (primary/idiopathic hypersecretory MGD). It is not certain whether increased lipid is a result of true hypersecretion of the meibomian glands, or a result of damming back of secretions in the presence of mild obstruction. The disorder is not associated with active inflammation, and no remarkable changes in gland structure are noted by meibography. There is a recognized association between hypersecretory MGD and acne, and the evidence of the potential for increased lipid secretion by meibomian glands comes from the finding of increased sebum excretion as a major factor in the pathophysiology of acne.38 An end-organ hyperresponse of the glands to androgens is the most likely explanation for the seborrhea.39 In women with acne, the total sebum excretion rate is higher than normal. Although sebum production is influenced both by the number of active follicles and their individual capacity to excrete sebum, the severity of seborrhea most probably depends on an increased excretion of sebum by a few glands rather than on an increased number of active sebaceous follicles.40
Relationship to Ocular Surface Disease and the Tear Film
MGD can lead to alterations in the normal lipid composition in meibomian gland secretions.41–44 Lipid abnormalities can lead to abnormalities of tear film composition and function resulting in evaporative dry eye.45
Footnotes
Supported by the Tear Film and Ocular Surface Society (TFOS; http://www.tearfilm.org); individual author support is listed in the Appendix of the Introduction.
Disclosure: Each Workshop Participant's disclosure data can be found in the Appendix of the Introduction.
References
- 1. Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100:347–351 [DOI] [PubMed] [Google Scholar]
- 2. Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998;438:349–360 [DOI] [PubMed] [Google Scholar]
- 3. Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–1270 [DOI] [PubMed] [Google Scholar]
- 4. Lee SH, Tseng SC. Rose bengal staining and cytologic characteristics associated with lipid tear deficiency. Am J Ophthalmol. 1997;124:736–750 [DOI] [PubMed] [Google Scholar]
- 5. Yokoi N, Mossa F, Tiffany JM, Bron AJ. Assessment of meibomian gland function in dry eye using meibometry. Arch Ophthalmol. 1999;117:723–729 [DOI] [PubMed] [Google Scholar]
- 6. Goto E, Monden Y, Takano Y, et al. Treatment of non-inflamed obstructive meibomian gland dysfunction by an infrared warm compression device. Br J Ophthalmol. 2002;86:1403–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1:107–126 [DOI] [PubMed] [Google Scholar]
- 8. Matsumoto Y, Dogru M, Goto E, et al. Efficacy of a new warm moist air device on tear functions of patients with simple meibomian gland dysfunction. Cornea. 2006;25:644–650 [DOI] [PubMed] [Google Scholar]
- 9. Matsumoto Y, Sato EA, Ibrahim OM, Dogru M, Tsubota K. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. 2008;14:1263–1271 [PMC free article] [PubMed] [Google Scholar]
- 10. Arita R, Itoh K, Maeda S, et al. Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology. 2009;116:2058–2063 [DOI] [PubMed] [Google Scholar]
- 11. Amano S. MGD Working Group: Definition and diagnostic criteria for meibomian gland dysfunction. J Eye (Atarashii Ganka). 2010;27:627–631 [Google Scholar]
- 12. Wulc A, Dryden R, Khatchaturian T. Where is the gray line? Arch Ophthalmol. 1987;105:1092–1098 [DOI] [PubMed] [Google Scholar]
- 13. Rolando M, Papadia M. Diagnosis and management of the lid and ocular surface disorders. In: Asbell P, Lemp M. eds. Dry Eye Disease: The Clinician's Guide to Diagnosis and Treatment. New York: Thieme Medical Publishers; 2006:71 [Google Scholar]
- 14. Gutgesell VJ, Stern GA, Hood CI. Histopathology of meibomian gland dysfunction. Am J Ophthalmol. 1982;94:383–387 [DOI] [PubMed] [Google Scholar]
- 15. Keith C. Seborrheic blepharo-kerato-conjunctivitis. Trans Ophthalmol Soc UK. 1967;87:85–103 [PubMed] [Google Scholar]
- 16. McCulley JP, Dougherty JM, Deneau DG. Classification of chronic blepharitis. Ophthalmology. 1982;89:1173–1180 [DOI] [PubMed] [Google Scholar]
- 17. Luchs J. Efficacy of topical azithromycin ophthalmic solution 1% in the treatment of posterior blepharitis. Adv Ther. 2008;25:858–870 [DOI] [PubMed] [Google Scholar]
- 18. McCulley JP, Sciallis GF. Meibomian keratoconjunctivitis. Am J Ophthalmol. 1977;84:788–793 [DOI] [PubMed] [Google Scholar]
- 19. Mathers W. Meibomian gland disease. In: Pflugfelder S, Beuerman R, Stern M. eds. Dry Eye and Ocular Surface Disorders. New York: Marcel Dekker, Inc.; 2004:255 [Google Scholar]
- 20. Duke-Elder W, MacFaul P. The ocular adnexa, Part 1: inflammations of the lid margins. In: Duke-Elder W, MacFaul P. eds. System of Ophthalmology. London: H. Kimpron; 1974:205–250 [Google Scholar]
- 21. Goto E, Shimazaki J, Monden Y, et al. Low-concentration homogenized castor oil eye drops for noninflamed obstructive meibomian gland dysfunction. Ophthalmology. 2002;109:2030–2035 [DOI] [PubMed] [Google Scholar]
- 22. Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 1980;51:243–251 [PubMed] [Google Scholar]
- 23. Jester J, Nicolaides N, Smith RE. Meibomian gland studies: histologic and ultrastructural investigations. Invest Ophthalmol Vis Sci. 1981;20:537–547 [PubMed] [Google Scholar]
- 24. Jester JV, Rife L, Nii D, Luttrull JK, Wilson L, Smith RE. In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 1982;22:660–667 [PubMed] [Google Scholar]
- 25. Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease: classification and grading of lid changes. Eye. 1991;5:395–411 [DOI] [PubMed] [Google Scholar]
- 26. Naldi L, Rebora A. Seborrheic dermatitis. N Engl J Med. 2009;360:387–396 [DOI] [PubMed] [Google Scholar]
- 27. Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149–164 [DOI] [PubMed] [Google Scholar]
- 28. Begley C, Chalmers R, Abetz L, et al. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Invest Ophthalmol Vis Sci. 2003;44:4753–4761 [DOI] [PubMed] [Google Scholar]
- 29. Mathers WD, Shields WJ, Sachdev MS, Petroll WM, Jester JV. Meibomian gland dysfunction in chronic blepharitis. Cornea. 1991;10:277–285 [DOI] [PubMed] [Google Scholar]
- 30. Mathers WD, Billborough M. Meibomian gland function and giant papillary conjunctivitis. Am J Ophthalmol. 1992;114:188–192 [DOI] [PubMed] [Google Scholar]
- 31. Shimazaki J, Goto E, Ono M, Shimmura S, Tsubota K. Meibomian gland dysfunction in patients with Sjogren syndrome. Ophthalmology. 1998;105:1485–1488 [DOI] [PubMed] [Google Scholar]
- 32. Obata H, Horiuchi H, Miyata K, Tsuru T, Machinami R. Histopathological study of the meibomian glands in 72 autopsy cases (in Japanese). Nippon Ganka Gakkai Zasshi. 1994;98:765–771 [PubMed] [Google Scholar]
- 33. Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992;11:334–342 [DOI] [PubMed] [Google Scholar]
- 34. Gifford S. Meibomian glands in chronic blepharoconjunctivitis. Am J Ophthalmol. 1921;4:489–494 [Google Scholar]
- 35. Arita R, Itoh K, Inoue K, Kuchiba A, Yamaguchi T, Amano S. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009;116:379–384 [DOI] [PubMed] [Google Scholar]
- 36. Landthaler M, Kummermehr J, Wagner A, Plewig G. Inhibitory effects of 13-cis-retinoic acid in human sebaceous glands. Arch Dermatol Res. 1980;269:297–309 [DOI] [PubMed] [Google Scholar]
- 37. Sullivan B, Evans J, Cermak J, Krenzer K, Dana M, Sullivan D. Complete androgen insensitivity syndrome. Arch Ophthalmol. 2002;120:1689–1699 [DOI] [PubMed] [Google Scholar]
- 38. Cunliffe W. Acne. London: Martin Dunitz; 1989 [Google Scholar]
- 39. Cunliffe W. The sebaceous gland and acne: 40 years on. Dermatology. 1998;196:9–15 [DOI] [PubMed] [Google Scholar]
- 40. Pierard-Franchimont C, Pierard G, Saint-leger D, Leveque J, Kligman A. Comparison of the kinetics of sebum secretion in young women with and without acne. Dermatologica. 1991;183:120–122 [DOI] [PubMed] [Google Scholar]
- 41. Shine WE, McCulley JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol. 1998;116:849–852 [DOI] [PubMed] [Google Scholar]
- 42. Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:2272–2280 [PubMed] [Google Scholar]
- 43. Shine WE, McCulley JP. Role of wax ester fatty alcohols in chronic blepharitis. Invest Ophthalmol Vis Sci. 1993;34:3515–3521 [PubMed] [Google Scholar]
- 44. Shine WE, McCulley JP. Polar lipids in human meibomian secretions. Curr Eye Res. 2003;26:89–94 [DOI] [PubMed] [Google Scholar]
- 45. Craig J, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci. 1997;74:8–13 [DOI] [PubMed] [Google Scholar]